Abstract
An important goal of cancer immunology is the identification of antigens associated with tumor destruction. Vaccination with irradiated tumor cells engineered to secrete granulocyte/macrophage colony-stimulating factor (GM-CSF) generates potent, specific, and long-lasting antitumor immunity in multiple murine tumor models. A phase I clinical trial of this vaccination strategy in patients with advanced melanoma demonstrated the consistent induction of dense CD4+ and CD8+ T lymphocyte and plasma cell infiltrates in distant metastases, resulting in extensive tumor destruction, fibrosis, and edema. Antimelanoma antibody and cytotoxic T cell responses were associated with tumor cell death. To characterize the targets of these responses, we screened an autologous cDNA expression library prepared from a densely infiltrated metastasis with postvaccination sera from a long-term responding patient. High-titer IgG antibodies detected ATP6S1, a putative accessory unit of the vacuolar H+–ATPase complex. A longitudinal analysis of this patient revealed an association between the vaccine-induced increase in antibodies to ATP6S1 and tumor destruction. Three additional vaccinated melanoma patients and three metastatic non-small cell lung carcinoma patients vaccinated with autologous GM-CSF-secreting tumor cells similarly showed a correlation between humoral responses to ATP6S1 and tumor destruction. Moreover, a chronic myelogenous leukemia patient who experienced a complete remission after CD4+ donor lymphocyte infusions also developed high-titer antibodies to ATP6S1. Lastly, vaccination with GM-CSF-secreting B16 melanoma cells stimulated high-titer antibodies to ATPS1 in a murine model. Taken together, these findings demonstrate that potent humoral responses to ATP6S1 are associated with immune-mediated destruction of diverse tumors.
The detailed analysis of immune-mediated tumor destruction provides a powerful approach to identify cancer-rejection antigens (1–5). Vaccination with irradiated tumor cells engineered to secrete granulocyte/macrophage colony-stimulating factor (GM-CSF) stimulates potent, specific, and long-lasting antitumor immunity in multiple murine tumor models (6). Vaccination requires the participation of CD4+ and CD8+ T cells, CD1d-restricted NK1.1+ T cells, and antibodies and likely involves improved tumor-antigen presentation by activated dendritic cells and macrophages (6–9).
We recently reported a phase I clinical trial of vaccination with irradiated, autologous melanoma cells engineered to secrete GM-CSF in patients with metastatic melanoma (10). Immunization sites showed intense infiltrates of dendritic cells, macrophages, eosinophils, and lymphocytes in all 21 evaluable patients. Although metastatic lesions resected before vaccination disclosed minimal immune infiltrates, metastatic lesions resected after vaccination revealed dense infiltrates of CD4+ and CD8+ T lymphocytes and plasma cells in 11 of 16 patients examined. The antitumor immune responses resulted in extensive tumor destruction (at least 80%), fibrosis, and edema. The infiltrating T cells displayed MHC class I-restricted cytotoxicity against autologous tumors and produced both T helper 1 and 2 cytokines. High-titer IgG antibodies against cell-surface and intracellular melanoma determinants were demonstrated by flow cytometry and Western analysis.
These pathologic and laboratory studies showed that GM-CSF-secreting melanoma cell vaccines stimulate a coordinated humoral and cellular antitumor response. To identify the antigens associated with vaccine-induced tumor destruction, we screened an autologous cDNA expression library prepared from a densely infiltrated metastasis with postimmunization sera from a long-term responding patient. High-titer IgG antibodies recognized ATP6S1, a putative accessory unit of the vacuolar H+–ATPase complex (11). Increased reactivity to ATP6S1 as a consequence of vaccination was temporally associated with tumor infiltration and destruction in this patient. Moreover, the development of potent humoral responses to ATP6S1 was correlated with immune-mediated tumor destruction in multiple clinical and experimental systems.
Materials and Methods
Clinical Protocols.
Sera and tumor samples were obtained from patients on Institutional Review Board/Food and Drug Administration/Recombinant DNA Advisory Committee-approved Dana–Farber Partners Cancer Care clinical protocols. The trials of GM-CSF-secreting, autologous melanoma cell vaccines and CD4+ donor lymphocyte infusions (DLIs) for treatment of relapsed chronic myelogenous leukemia (CML) after allogeneic bone marrow transplantation have been described previously (10, 12). The phase I study of vaccination with irradiated, autologous non-small cell lung carcinoma (NSCLC) cells engineered to secrete GM-CSF in patients with metastatic NSCLC will be reported elsewhere. Sera were obtained also from healthy blood-bank donors and hormone refractory advanced cancer patients (kindly provided by Phillip Kantoff) at the Dana–Farber Cancer Institute.
Pathology.
Tissues were fixed in 10% neutral buffered formalin, processed routinely, and embedded in paraffin. Immunohistochemistry was performed by using standard techniques with monoclonal antibodies to CD4, CD8, CD20, and Ig-κ.
Library Construction and Screening.
Total RNA was isolated from the melanoma cell line K008 (10) by using guanidine isothiocyanate, and the mRNA was selected with two rounds of oligo(dT) cellulose. A cDNA expression library was constructed in the Lambda Zap vector by using a commercial cDNA library kit (Stratagene) according to the manufacturer's procedures. Plaques (1 × 106) were screened with precleared (against Escherichia coli and λ phage lysates) postvaccination sera from patient K008 at a 1:1,000 dilution in TBS/0.1% Tween-20/2% nonfat dried milk (NFDM). Positive plaques were detected with an alkaline phosphatase-conjugated goat anti-human IgG antibody (Jackson ImmunoResearch) diluted 1:2,000 in TBST (50 mM Tris/138 mM NaCl/2.7 mM KCl/0.05% Tween 20, pH 8.0). Reactive clones were plaque-purified, and the excised phagemids were sequenced.
Immunoblotting and ELISA.
Glutathione S-transferase (GST)-APT6S1 and GST-BRAP2 full-length recombinant proteins were produced with the PGEX 5X-3 vector (Amersham Pharmacia) according to the manufacturer's procedures. One microgram of the recombinant proteins was electrophoresed on an 8% polyacrylamide/SDS gel and visualized with Coomassie staining. For Western analysis, the gel was transferred to a poly(vinylidene difluoride) membrane (Millipore), blocked in 5% NFDM/PBS, washed in TBST, and incubated with a 1:500 dilution of patient sera at 4°C overnight. A goat anti-human IgG conjugated to horseradish peroxidase (Zymed) was added at room temperature, and the blot was developed with a chemiluminescence kit (NEN).
ELISA plates (Costar) were coated overnight at 4°C with 500 ng of GST-ATP6S1 or GST protein or, in some experiments, with mumps (Aventis Pasteur, Lyon, France) or candida (Miles) antigens in a carbonate buffer, pH 9.6. The wells were blocked overnight with 2% NFDM/PBS, washed, and incubated in duplicate with 100 μl of patient sera diluted 1:500 in 2% NFDM/PBS overnight at 4°C. A goat anti-human IgG conjugated to horseradish peroxidase (Zymed) was added at room temperature, and the plate was developed with a one-step 3,3′,5,5′-tetramethylbenzidine/peroxide reagent (Dako). The values reported were the mean absorbance at 450 nm of GST-ATP6S1–GST. A one-sided Wilcoxon rank sum test was used to compare values among the patient groups.
Northern Analysis.
Multitissue and human tumor mRNA blots (Stratagene) were blocked in QuikHyb (Stratagene) and probed with labeled ATP6S1 cDNA spanning the ORF. Membranes were washed in SSC (0.15 M sodium chloride/0.015 M sodium citrate, pH 7.0)/SDS and developed by autoradiography.
Murine Studies.
Female C57BL/6 mice (8–12 weeks old; Taconic Farms) were immunized s.c. on the abdominal wall with 5 × 105 irradiated (35 Gy) GM-CSF-secreting B16 cells at weekly intervals up to 10 times as described (6) and bled by cardiac puncture. A 1:300 dilution of sera (kindly provided by Joosang Park) and a sheep anti-mouse IgG coupled to horseradish peroxidase (Amersham Pharmacia) were used for the Western analysis. All animal experiments were approved and conducted under Institutional Animal Care and Use Committee guidelines.
Results
Serologic Detection of ATP6S1.
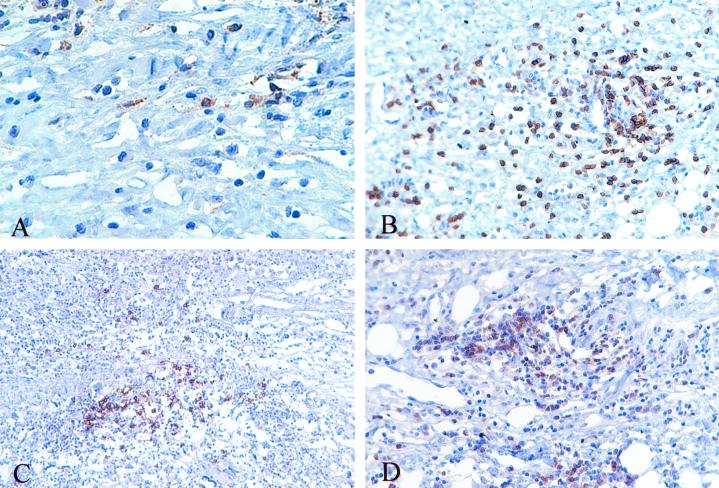
To identify cancer antigens associated with tumor destruction, we initially studied a metastatic melanoma patient (K008) who manifested a long-term response to immunization with irradiated, autologous tumor cells engineered to secrete GM-CSF. A pulmonary metastasis was used to manufacture six vaccines that were administered at 2-week intervals. After the fifth treatment, a s.c. metastasis became erythematous and hemorrhagic. The mass was resected 6 weeks after completion of therapy, and pathologic examination revealed an intense infiltrate of CD4+ and CD8+ T lymphocytes and CD20+ B cells producing Ig (Fig. 1 A–D). This coordinated immune response resulted in extensive tumor destruction, and the patient remains free of detectable disease 6 years after vaccination.
Figure 1.
Infiltrated metastasis with extensive tumor destruction after vaccination. (A) CD4+ T cells. (B) CD8+ T cells. (C) CD20+ B cells. (D) Ig-κ-producing cells.
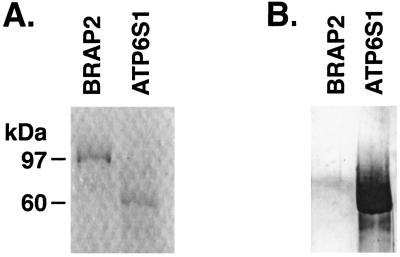
A melanoma cell line (K008) was generated from the infiltrated metastasis and used to construct a cDNA expression library in the Lambda Zap phage vector. The library was screened with a 1:1,000 dilution of sera obtained 2 months after beginning vaccination, and positive clones were identified with an anti-human IgG secondary antibody. One of the purified clones contained the ORFs for two gene products, BRAP2 and ATP6S1 (13, 14). However, the presence of a long series of adenines followed by an EcoR1 site (one of the adaptors used in library construction) between the two coding sequences raised the possibility that the fusion was a library artifact. Consistent with this idea, a putative transcript spanning the two genes could not be amplified by reverse transcription–PCR from K008-derived cDNA. These findings suggested that BRAP2, ATP6S1, or both gene products might be the targets for K008 antibodies. Indeed, previous work showed that some renal cell carcinoma patients mount humoral responses to BRAP2, a candidate retinoblastoma-binding protein (15). To determine which gene products encoded by the phage clone were immunogenic for patient K008, we produced full-length recombinant BRAP2 and ATP6S1 proteins in bacteria. Surprisingly, postvaccination sera detected ATP6S1 but not BRAP2 in Western analysis (Fig. 2). Thus, patient K008 developed a high-titer IgG antibody response to ATP6S1.
Figure 2.
K008 antibodies recognize ATP6S1. (A) Coomassie stain of GST-BRAP2 and GST-ATP6S1 proteins. (B) Immunoblotting with K008 postvaccination sera (1:500 dilution) and an anti-human IgG secondary antibody.
ATP6S1 Is Broadly Immunogenic.
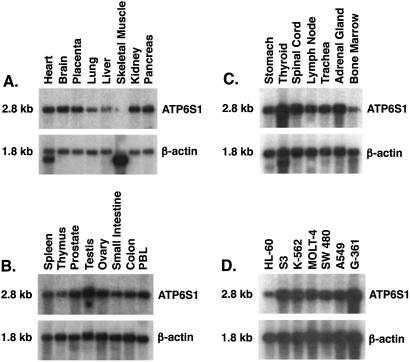
ATP6S1 is the human homolog of a protein first characterized as a putative accessory subunit of the vacuolar H+–ATPase complex in bovine chromaffin granules (11). Consistent with the conserved role of this complex in regulating pH throughout the secretory and endocytic pathways (16), the human protein is related closely to the corresponding molecules from the cow (92% similarity), rat (92% similarity), mouse (90% similarity), and Xenopus (77% similarity) (11, 14, 17–20). A single 2.8-kb transcript was detected by Northern analysis in most human tissues, with slightly increased levels in endocrine organs (Fig. 3 A–C). Diverse human tumors similarly were strongly positive (Fig. 3D), suggesting that maintenance of ATP6S1 function may be important for cancer development.
Figure 3.
ATP6S1 is widely expressed. (A–C) A full-length ATP6S1 cDNA probe was used for Northern analyses of normal human tissues. PBL, peripheral blood lymphocyte. (D) Diverse human tumors.
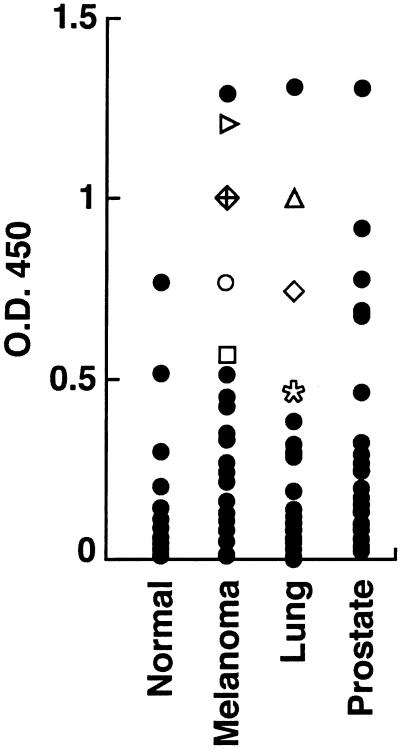
The broad expression in normal tissues raised the possibility that tolerance might preclude the frequent development of immunity to ATP6S1 (21). To investigate this idea further, we established an ELISA with recombinant ATP6S1 protein and analyzed sera from a variety of advanced cancer patients including 26 metastatic melanoma patients immunized with autologous GM-CSF-secreting tumor cell vaccines, 26 metastatic NSCLC patients immunized with autologous GM-CSF-secreting tumor cell vaccines, 29 nonimmunized metastatic prostate carcinoma patients, and 29 healthy blood-bank donors (Fig. 4). The median absorbance at 450 nm was determined for each population to compare the reactivity between groups while accounting for the skewed populations. The medians (and ranges) were: normal controls, 0.06 (0.005–0.770); metastatic melanoma patients, 0.238 (0.012–1.29); metastatic NSCLC patients, 0.118 (0.000–1.31); and metastatic prostate carcinoma patients, 0.182 (0.022–1.30). A one-sided Wilcoxon rank sum test showed that the melanoma, NSCLC, and prostate cancer patients all were significantly more reactive than the controls (P values: 0.003 for the comparison with melanoma, 0.025 for NSCLC, and 0.001 for prostate). Thus, humoral responses to ATP6S1 are relatively common in cancer patients but infrequent in healthy donors.
Figure 4.
ATP6S1 is broadly immunogenic. Sera samples (1:500 dilution) from healthy controls (n = 29) or patients with metastatic melanoma (n = 26), NSCLC (n = 26), and prostate carcinoma (n = 29) were evaluated in an ELISA for reactivity to ATP6S1 (anti-human IgG secondary antibody). The symbols denote patients that showed increased antibody titers as a function of vaccination.
Vaccination Augments Immunity to ATP6S1.
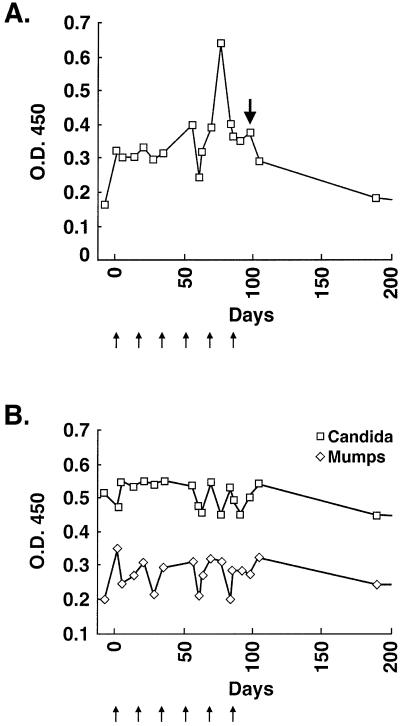
The serologic data indicated that a proportion of vaccinated and nonvaccinated cancer patients generate antibodies to ATP6S1. To examine whether immunization with autologous GM-CSF-secreting tumor cells influenced reactivity to ATP6S1, we initially performed a longitudinal analysis of patient K008 (Fig. 5A). IgG antibodies to ATP6S1 were demonstrable before the beginning of therapy, suggesting that tumor development may have provoked a nascent immune response. Nonetheless, a distinct augmentation in antibody titers was evident after the fifth vaccination (12-fold increase), whereas levels gradually returned to baseline thereafter (Table 1). Antibodies against mumps and candida antigens, in contrast, were not affected consistently by therapy (Fig. 5B). Intriguingly, the peak reactivity to ATP6S1 was temporally related to the development of erythema and hemorrhage in the s.c. metastasis.
Figure 5.
Vaccination stimulated K008 humoral immunity to ATP6S1. (A) Longitudinal analysis of K008 reactivity to ATP6S1 by ELISA (sera 1:500 dilution; anti-human IgG secondary antibody). The small arrows denote vaccinations, and the bold arrow indicates time of s.c. metastasis resection (pathology shown in Fig. 1). (B) Reactivity to mumps and candida antigens (sera 1:1,000 dilution).
Table 1.
Antibodies to ATP6S1 increase in response to immunotherapy
| Patient | Fold increase |
|---|---|
| Melanoma | |
| K007 | 3 |
| K008 | 12 |
| K023 | 3 |
| K030 | 8 |
| NSCLC | |
| L1 | 4 |
| L11 | 4 |
| L33 | 3 |
| CML | |
| C1 | 6 |
The vaccinated patients who did not show treatment-induced increases had detectable antibodies at 1:500–1:2,000 dilutions.
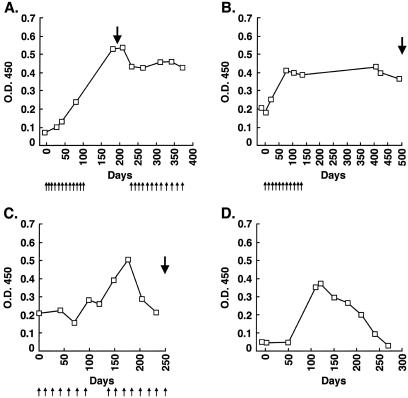
These findings raised the possibility that vaccine-enhanced reactivity to ATP6S1 generally might be associated with tumor destruction. To explore this potential correlation, we studied seven additional melanoma patients with antibodies to ATP6S1 who were immunized with autologous GM-CSF-secreting tumor cells. Three of the patients mounted clear increases in antibody titers (3–8-fold) as a result of therapy (Table 1). (Patients showing changes are indicated with special symbols in Fig. 4.) Fig. 6A illustrates the longitudinal analysis for K030, a patient who achieved an objective partial response and a 4.5-year survival after vaccination. The two other melanoma patients with heightened reactivity to ATP6S1 similarly developed dense T lymphocyte and plasma cell infiltrates in distant metastases that effectuated extensive tumor destruction.
Figure 6.
Augmented humoral responses to ATP6S1 in vaccinated metastatic melanoma and NSCLC patients and a CML patient treated with CD4+ DLI. Patient sera were used in an ELISA at a 1:500 dilution and developed with an anti-human IgG secondary antibody. The small arrows denote vaccinations, and the bold arrows indicate resections of distant metastases. (A) Melanoma patient K030. (B) NSCLC patient L1. (C) NSCLC patient L33. (D) CML patient C1. DLI was administered on day 0, and clinical, cytogenetic, and molecular responses began at day 80.
To examine further the generality of these findings, we studied four NSCLC patients with antibodies to ATP6S1 who were immunized with autologous GM-CSF-secreting tumor cells. Three of the patients developed augmented antibody titers to ATP6S1 (3–4-fold) as a consequence of vaccination (Table 1). The longitudinal analyses for two of the cases are depicted in Fig. 6 B and C. Intriguingly, two of the responding patients displayed lymphocyte infiltrates associated with tumor destruction in distant metastases, and the third manifested attenuated disease progression (no postimmunization tumor sample was available for pathologic review). Together, these studies indicate that vaccine-enhanced reactivity to ATP6S1 is correlated with tumor destruction in patients with either metastatic NSCLC or metastatic melanoma.
DLI Stimulates Immunity to ATP6S1.
To determine whether other forms of immunotherapy similarly modulate reactivity to ATP6S1, we studied 14 patients with relapsed CML who received CD4+ DLI after allogeneic bone marrow transplantation. This treatment strategy induces major clinical responses in most patients during the chronic phase of the disease (12). The antitumor effects of CD4+ DLI are associated with the generation of antibodies to diverse CML-associated antigens (22). Some of the target molecules also are expressed in solid tumors and elicit humoral responses in a proportion of metastatic melanoma, NSCLC, and prostate cancer patients (23). Together, these findings raised the possibility that immunity to ATP6S1 might correlate with DLI-stimulated leukemic destruction. Consistent with this idea, one of the CML patients developed high-titer IgG antibodies to ATP6S1 3 months after DLI, whereas levels gradually declined thereafter (Fig. 6D, Table 1). The increase in humoral reactivity was temporally related to a cytogenetic (loss of Ph+ chromosome), molecular (PCR negativity for bcr-abl transcripts), and clinical antileukemic response. Importantly, only low titers of antibodies were detectable before DLI despite the earlier bone marrow transplantation, suggesting that ATP6S1 was not functioning primarily as an allogeneic target. This patient remains in a complete remission 6 years after DLI without significant graft-versus-host disease.
ATP6S1 Is Immunogenic in Mice.
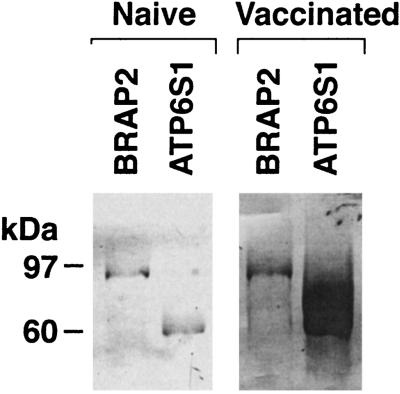
The investigation of diverse patients undergoing immunotherapy revealed an association between augmented humoral immunity to ATP6S1 and tumor destruction. To delineate whether a similar relationship was demonstrable in murine tumor models, we studied C57BL/6 mice vaccinated with irradiated GM-CSF-secreting B16 melanoma cells. This immunization strategy stimulates potent, specific, and long-lasting tumor protection (6). Vaccinated but not naive mice generated high-titer IgG antibodies to ATP6S1, as assessed by Western analysis (Fig. 7). The GST-ATP6S1 protein used in these experiments retains 90% sequence similarity with the murine homolog. Thus, the correlation between increased humoral immunity to ATP6S1 and tumor destruction is conserved between human and murine systems.
Figure 7.
ATP6S1 is immunogenic in murine melanoma. Sera from C57BL/6 mice vaccinated with irradiated GM-CSF-secreting B16 cells or from naive controls were evaluated by Western analysis (1:300 dilution) against GST-ATP6S1 (human) and GST-BRAP2 (human) (anti-mouse IgG secondary antibody). Murine ATP6S1 shows 90% and murine BRAP2 shows 95% sequence similarity to the respective human homologs. (A) Naive. (B) Vaccinated.
Discussion
These experiments were undertaken in an effort to identify cancer antigens associated with immune-mediated tumor destruction. Although genetic and biochemical techniques have uncovered an impressive repertoire of gene products that evoke immune recognition in cancer patients (24), the biologic importance of these responses in most cases is poorly understood. Although a few antigens mediate tumor regressions in the context of specific vaccination schemes or adoptive cellular therapies (25–28), the possible role of immunity to most antigens in cancer diagnosis, prognosis, or treatment remains to be established.
One strategy for delineating cancer antigens that elicit clinically significant immune recognition involves the analysis of patients who achieve durable benefits as a consequence of immunotherapy. Previous investigations of a long-term surviving melanoma patient defined the first tumor antigens recognized by cytotoxic T lymphocytes (1, 29). Comparable studies of patients in which ex vivo expanded tumor-infiltrating lymphocytes effectuated tumor regressions further revealed several immunogenic melanosomal protein targets (2, 3). Thus, we characterized the immune response of a melanoma patient who, despite pulmonary and s.c. metastases upon protocol entry, remains disease-free 6 years after completing vaccination with irradiated, autologous tumor cells engineered to secrete GM-CSF.
Because our pathologic and laboratory analyses disclosed a coordinated CD4+ and CD8+ T lymphocyte and plasma cell reaction in distant metastases, we identified candidate antigens by probing a cDNA expression library derived from an infiltrated metastasis with postvaccination sera. One of the gene products detected in the screen was ATP6S1, a putative subunit of the vacuolar H+–ATPase complex (11, 14, 17–19). The immunogenicity of this gene product was surprising, given its widespread expression in normal tissues and the absence of tumor-associated mutations or increased mRNA expression (data not shown). Nonetheless, an increasing number of cancer antigens fail to show tumor-restricted expression (15, 30); an elegant recent study defined actin as one target of the oligoclonal B cell reaction to medullary breast carcinoma (31).
Despite the broad tissue expression, ATP6S1 stimulated humoral responses in a significant proportion of cancer patients without provoking clinical autoimmunity. Both vaccinated and untreated patients generated antibodies, indicating that tumor growth sometimes may elicit a nascent immune reaction. Nonetheless, longitudinal analyses of the immunized patients harboring antibodies revealed that four of eight metastatic melanoma and three of four metastatic NSCLC patients developed augmented titers as a function of treatment. Remarkably, these heightened humoral responses were correlated in six of the patients with the induction of T and B cell infiltrates and tumor destruction in distant metastases; the remaining NSCLC patient (from whom tissue was not available) manifested attenuated disease progression. Three of the four melanoma patients who did not mount increases in antibody titers generated T and B cell infiltrates with tumor destruction as well, raising the possibility that humoral responses to ATP6S1 might serve as a marker for identifying patients likely to benefit from GM-CSF-secreting autologous tumor cell vaccines or other therapies. Augmented immunity to ATP6S1 also was found in one CML patient who experienced a complete remission after CD4+ DLI, illustrating that this antigen is a target for adoptive T cell therapy. Lastly, the immunogenicity of ATP6S1 was conserved between human and murine systems.
The potential role of antibodies to ATP6S1 in tumor destruction remains to be clarified. Interestingly, the Xenopus ATP6S1 localizes to the cell membrane and a juxtanuclear vacuolar compartment after ectopic expression in mammalian cells (14). An internalization signal in the cytoplasmic tail (17 of 26 amino acid identities with the human protein) directs trafficking to the endocytic system when fused in a heterologous construct to a truncated human IL-2Rα cDNA (14). Moreover, the Xenopus ATP6S1 is up-regulated with proopiomelanocortin in response to dark adaptation, suggesting a possible function in the secretory pathway (17). Although analogous studies with human ATP6S1 remain to be performed, these findings raise the idea that high-titer antibodies might mediate antitumor effects through cell surface interactions, as has been shown for tyrosinase-related proteins 1 and 2 (32–34). Alternatively, antibodies may be stimulated after tumor destruction through antigen processing by scavenging dendritic cells and macrophages.
Additional experiments are required to determine whether ATP6S1 provokes T cell recognition. The generation of high-titer IgG antibodies suggests that cognate CD4+ T cells likely are produced. Indeed, recent investigations indicate that patients with antibodies to the cancer-testis antigen NY-ESO-1 concurrently mount CD4+ T cells, particularly in the context of a prevalent HLA DP4 allele (35). NY-ESO-1-specific CD8+ T cells are elicited also, demonstrating that cellular and humoral antitumor immunity may be induced coordinately (36). Consistent with these findings, we have delineated a combined cytotoxic T cell and antibody reaction to an inhibitor of apoptosis protein in a long-term surviving melanoma patient vaccinated with autologous GM-CSF-secreting tumor cells (J.C.S., R. H. Vonderheide, and G.D., unpublished data).
In conclusion, potent humoral responses to ATP6S1 are associated with cancer destruction in diverse clinical and experimental tumor systems. The long-term survival and lack of autoimmunity in patients with high-titer antibodies to ATP6S1 suggest that this tumor-associated antigen may prove useful for cancer immunotherapy. Intriguingly, recent experiments in murine models illustrate that the incorporation of gene products identified by antibodies into vaccine protocols augments CD8+ T cell responses and tumor protection (37).
Acknowledgments
We thank the Connell-O'Reilly Laboratory for excellent processing of patient material, Christine Sheehan and Esther Brisson (Albany Medical College) for excellent help with the histological specimens, and Phillip Kantoff and Joosang Park (Dana–Farber Cancer Institute) for sera samples. This study was supported by the Berlex Oncology Foundation, National Institutes of Health Grants CA78880 (to F.S.H.), CA74886, and CA39542 (to G.D.), the Leukemia and Lymphoma Society Score Award in acute myeloid leukemia (to G.D. and J.R.), the Cancer Research Institute/Partridge Foundation Clinical Investigator Award, and the Cancer Research Institute Melanoma Initiative (to G.D.). E.P.A. is a Special Fellow of the Leukemia and Lymphoma Society. G.D. and R.S. are Clinical Scholars of the Leukemia and Lymphoma Society.
Abbreviations
- GM-CSF
granulocyte/macrophage colony-stimulating factor
- DLI
donor lymphocyte infusion
- CML
chronic myelogenous leukemia
- NSCLC
non-small cell lung carcinoma
- GST
glutathione S-transferase
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, Knuth A, Boon T. Science. 1991;254:1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 2.Kawakami Y, Eliyahu S, Delgado C H, Robbins P F, Sakaguchi K, Appella E, Yannelli J R, Adema G J, Miki T, Rosenberg S A. Proc Natl Acad Sci USA. 1994;91:6458–6462. doi: 10.1073/pnas.91.14.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakker A, Schreurs M, de Boer A, Kawakami Y, Rosenberg S, Adema G, Figdor C. J Exp Med. 1994;179:1005–1009. doi: 10.1084/jem.179.3.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zorn E, Hercend T. Eur J Immunol. 1999;29:592–601. doi: 10.1002/(SICI)1521-4141(199902)29:02<592::AID-IMMU592>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 5.Zorn E, Hercend T. Eur J Immunol. 1999;29:602–607. doi: 10.1002/(SICI)1521-4141(199902)29:02<602::AID-IMMU602>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 6.Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan R C. Proc Natl Acad Sci USA. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang A Y, Golumbek P, Ahmadzadeh M, Jaffee E, Pardoll D, Levitsky H. Science. 1994;264:961–965. doi: 10.1126/science.7513904. [DOI] [PubMed] [Google Scholar]
- 8.Mach N, Gillessen S, Wilson S B, Sheehan C, Mihm M, Dranoff G. Cancer Res. 2000;60:3239–3246. [PubMed] [Google Scholar]
- 9.Reilly R, Machiels J-P, Emens L, Ercolini A, Okoye F, Lei R, Weintraub D, Jaffee E. Cancer Res. 2001;61:880–883. [PubMed] [Google Scholar]
- 10.Soiffer R, Lynch T, Mihm M, Jung K, Rhuda C, Schmollinger J, Hodi F, Liebster L, Lam P, Mentzer S, et al. Proc Natl Acad Sci USA. 1998;95:13141–13146. doi: 10.1073/pnas.95.22.13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Supek F, Supekova L, Mandiyan S, Pan Y C, Nelson H, Nelson N. J Biol Chem. 1994;269:24102–24106. [PubMed] [Google Scholar]
- 12.Alyea E, Soiffer R, Canning C, Neuberg D, Schlossman R, Pickett P, Collins H, Wang Y, Anderson K, Ritz J. Blood. 1998;91:3671–3680. [PubMed] [Google Scholar]
- 13.Li S, Ku C Y, Farmer A A, Cong Y S, Chen C F, Lee W H. J Biol Chem. 1998;273:6183–6189. doi: 10.1074/jbc.273.11.6183. [DOI] [PubMed] [Google Scholar]
- 14.Jansen E J, Holthuis J C, McGrouther C, Burbach J P, Martens G J. J Cell Sci. 1998;111:2999–3006. doi: 10.1242/jcs.111.20.2999. [DOI] [PubMed] [Google Scholar]
- 15.Scanlan M, Gordon J, Williamson B, Stockert E, Bander N, Jongeneel V, Gure A, Jager D, Jager E, Knuth A, Chen Y-T, Old L. Int J Cancer. 1999;83:456–464. doi: 10.1002/(sici)1097-0215(19991112)83:4<456::aid-ijc4>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 16.Schoonderwoert V T, Holthuis J C, Tanaka S, Tooze S A, Martens G J. Eur J Biochem. 2000;267:5646–5654. doi: 10.1046/j.1432-1327.2000.01648.x. [DOI] [PubMed] [Google Scholar]
- 17.Holthuis J C, Jansen E J, van Riel M C, Martens G J. J Cell Sci. 1995;108:3295–3305. doi: 10.1242/jcs.108.10.3295. [DOI] [PubMed] [Google Scholar]
- 18.Getlawi F, Laslop A, Schagger H, Ludwig J, Haywood J, Apps D. Neurosci Lett. 1996;219:13–16. doi: 10.1016/s0304-3940(96)13151-7. [DOI] [PubMed] [Google Scholar]
- 19.Holthuis J C, Jansen E J, Schoonderwoert V T, Burbach J P, Martens G J. Eur J Biochem. 1999;262:484–491. doi: 10.1046/j.1432-1327.1999.00396.x. [DOI] [PubMed] [Google Scholar]
- 20.Kutsche R, Brown C J. Genomics. 2000;65:9–15. doi: 10.1006/geno.2000.6153. [DOI] [PubMed] [Google Scholar]
- 21.Houghton A. J Exp Med. 1994;180:1–4. doi: 10.1084/jem.180.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu C, Yang X-F, McLaughlin S, Neuberg D, Canning C, Stein B, Alyea E, Soiffer R, Dranoff G, Ritz J. J Clin Invest. 2000;106:705–714. doi: 10.1172/JCI10196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang X F, Wu C J, McLaughlin S, Chillemi A, Wang K S, Canning C, Alyea E P, Kantoff P, Soiffer R J, Dranoff G, Ritz J. Proc Natl Acad Sci USA. 2001;98:7492–7497. doi: 10.1073/pnas.131590998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilboa E. Immunity. 1999;11:263–270. doi: 10.1016/s1074-7613(00)80101-6. [DOI] [PubMed] [Google Scholar]
- 25.Marchand M, van Baren N, Weynants P, Brichard V, Dreno B, Tessier M-H, Rankin E, Parmiani G, Arienti F, Humblet Y, et al. Int J Cancer. 1999;80:219–230. doi: 10.1002/(sici)1097-0215(19990118)80:2<219::aid-ijc10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 26.Rosenberg S, Yang J, Schwartzentruber D, Hwu P, Marincola F, Topalian S, Restifo N, Dudley M, Schwarz S, Spiess P, et al. Nat Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jäger E, Gnjatic S, Nagata Y, Stockert E, Jäger D, Karbach J, Neumann A, Rieckenberg J, Chen Y-T, Ritter G, et al. Proc Natl Acad Sci USA. 2000;97:12198–12203. doi: 10.1073/pnas.220413497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yee C, Thompson J, Roche P, Byrd D, Lee P, Piepkorn M, Kenyon K, Davis M, Riddell S, Greenberg P. J Exp Med. 2000;192:1637–1643. doi: 10.1084/jem.192.11.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boon T, van der Bruggen P. J Exp Med. 1996;183:725–729. doi: 10.1084/jem.183.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scanlan M, Chen Y-T, Williamson B, Gure A, Stockert E, Gordan J, Tureci O, Sahin U, Pfreundschuh M, Old L. Int J Cancer. 1998;76:652–658. doi: 10.1002/(sici)1097-0215(19980529)76:5<652::aid-ijc7>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 31.Hansen M H, Nielsen H, Ditzel H J. Proc Natl Acad Sci USA. 2001;98:12659–12664. doi: 10.1073/pnas.171460798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naftzger C, Takechi Y, Kohda H, Hara I, Vijayasaradhi S, Houghton A. Proc Natl Acad Sci USA. 1996;93:14809–14814. doi: 10.1073/pnas.93.25.14809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clynes R, Takechi Y, Moroi Y, Houghton A, Ravetch J. Proc Natl Acad Sci USA. 1998;95:652–656. doi: 10.1073/pnas.95.2.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bowne W, Srinivasan R, Wolchok J, Hawkins W, Blachere N, Dyall R, Lewis J, Houghton A. J Exp Med. 1999;190:1717–1722. doi: 10.1084/jem.190.11.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeng G, Wang X, Robbins P, Rosenberg S, Wang R-F. Proc Natl Acad Sci USA. 2001;98:3964–3969. doi: 10.1073/pnas.061507398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jager E, Nagata Y, Gnjatic S, Wada H, Stockert E, Karbach J, Dunbar P, Lee S, Jungbluth A, Jager D, et al. Proc Natl Acad Sci USA. 2000;97:4760–4765. doi: 10.1073/pnas.97.9.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishikawa H, Tanida K, Ikeda H, Sakakura M, Miyahara Y, Aota T, Mukai K, Watanabe M, Kuribayashi K, Old L J, Shiku H. Proc Natl Acad Sci USA. 2001;98:14571–14576. doi: 10.1073/pnas.251547298. [DOI] [PMC free article] [PubMed] [Google Scholar]