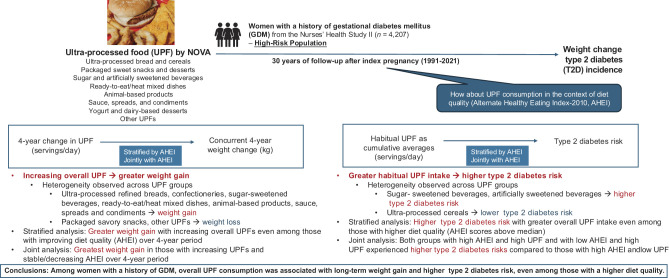
Abstract
OBJECTIVE
We examined the associations of overall and group-specific ultra-processed food (UPF) consumption with long-term weight change and type 2 diabetes (T2D) progression following gestational diabetes mellitus (GDM)-complicated pregnancies.
RESEARCH DESIGN AND METHODS
We included 4,207 women with a history of GDM from the Nurses' Health Study II (1991–2021). UPF intake (servings/day) was assessed via food frequency questionnaires every 4 years and quantified per the NOVA classification. Diet quality was evaluated using the Alternate Healthy Eating Index-2010 (AHEI). Associations between UPF intake changes and weight changes (kg) were assessed using generalized estimating equations (GEE) (n = 3,781). Cox regression models estimated adjusted hazard ratios (HRs) and 95% CIs of habitual UPF intake modeled by time-updated cumulative averages with T2D risk. Joint associations of UPF and AHEI with weight change and T2D risk were examined using the same GEE and Cox regression models, respectively.
RESULTS
T2D developed in 1,040 participants. Increased UPF consumption was associated with greater weight gain (P-trend < 0.0001; quartile 1 [Q1] vs. Q4: 0.52 kg vs. 1.65 kg). Habitual UPF consumption was positively associated with T2D risk: adjusted HRs (95% CIs) for Q1 to Q4 were 1.00 (ref), 1.07 (0.87, 1.32), 1.25 (1.03, 1.53), and 1.20 (0.99, 1.46), respectively (P-trend = 0.04). These associations persisted in women with higher AHEI scores. When modeling UPF and AHEI jointly, only women with stable or decreased UPF intake and increased AHEI achieved 4-year weight maintenance.
CONCLUSIONS
In women with a history of GDM, UPF consumption was associated with weight gain and higher T2D risk, even among those with higher diet quality.
Graphical Abstract
Introduction
Type 2 diabetes (T2D) is a major global health concern due to its high prevalence, rising incidence in several regions and among younger populations, and severe long-term complications (1). T2D is largely preventable through diet, lifestyle, and weight maintenance (2,3), making early intervention crucial, particularly for high-risk groups. Women who experience gestational diabetes mellitus (GDM), a common pregnancy condition (4), face up to 10 times higher T2D risk compared with those without the condition (5). GDM unmasks cardiometabolic vulnerabilities early, highlighting a critical window for early prevention strategies. Adopting and sustaining a healthy diet has been recommended for long-term weight management and T2D prevention in this population (6,7).
Ultra-processed foods (UPFs) are industrially manufactured foods and beverages, often containing additives and other industrial ingredients (8). The prevalence of UPFs is rising globally due to shifts in manufacturing practices resulting in their preferred taste, convenience, durability, and affordability (9). In addition to industrial ingredients, UPFs are typically high in added sugars, sodium, refined starches, and saturated fats, while being low in dietary fiber, contributing to excess energy intake and poor diet quality (8,10). Despite the known adverse relationships of UPF with obesity and cardiometabolic outcomes in the general population (9,11), it is crucial to examine these relationships in women with a history of GDM, who often have distinct metabolic and genetic risk factors (12). Evidence has suggested heterogeneity in the associations between UPF and T2D across different UPF groups (13).
Dietary scores assessing overall diet quality have been associated with T2D risk in this high-risk group (6). Yet, such scores often lack information on specific dietary components, including UPFs, that have emerged as important contributors to T2D risk. Examining the joint associations of UPF and diet quality with obesity and T2D can provide insights into impact of certain UPFs (e.g., packaged sweets, high-fat sauces, and artificially sweetened foods) often overlooked in conventional diet quality metrics, while taking into account of overall diet quality and dietary factors beyond food processing properties (14). This approach can inform more targeted dietary recommendations and preventive strategies for cardiometabolic outcomes, including T2D.
To address research gaps, we investigated changes in UPF consumption in association with concurrent weight changes and habitual UPF consumption with risk of T2D in a longitudinal study of 4,207 predominantly Caucasian women with a history of GDM followed for 30 years. We additionally characterized these associations by UPF groups and in conjunction with diet quality assessed using the Alternate Healthy Eating Index-2010 (AHEI).
Research Design and Methods
Study Population
Our analyses included women with a history of GDM in the Nurses’ Health Study II (NHS II), as part of the Diabetes and Women’s Health Study (15). NHS II is a longitudinal cohort of 116,429 predominantly Caucasian female registered nurses aged 24–44 years at the study initiation in 1989. At enrollment and every 2–4 years thereafter, participants completed regular mailed questionnaires to report updated information on their demographics, lifestyle, and health-related factors, and any new medical conditions or diagnoses. Height was self-reported at enrollment. The study protocol was approved by the Brigham and Women’s Hospital and the Harvard T.H. Chan School of Public Health Institutional Review Boards, with consent implied upon return of baseline questionnaires.
NHS II participants reported their reproductive history in 1989, and all new pregnancies and physician-diagnosed pregnancy complications, including GDM, were self-reported biennially. The last inquiry on incident pregnancies was in 2001, after which most participants were no longer premenopausal. A previous validation study in a subset of NHS II participants indicated a high GDM screening rate in pregnancy and high concordance between self-reported GDM diagnosis and GDM diagnosis confirmed via medical records (94%) (16).
For the present investigation, baseline was defined as the first post-GDM follow-up cycle with available data on diet. At the first eligible questionnaire cycle, we excluded participants who had a missing birthday, with a history of T2D, cardiovascular disease (CVD), or cancer, except nonmelanoma skin cancers, whose age of the first reported GDM diagnosis was <18 years, or who had a multiple-birth pregnancy (6). The final analytic sample included 4,207 individuals with a history of GDM.
Assessments of UPF and Other Dietary Factors
Beginning in 1991 and every 4 years thereafter until 2019, NHS II participants reported their diets using a validated semiquantitative food frequency questionnaire (FFQ), detailing typical frequency of consumption for 130 food items in the previous year. UPF classification for each food and beverage item was derived according to the NOVA classification (8,17). UPFs were further divided into nine, mutually exclusive groups: 1) ultra-processed breads and cereals, 2) packaged sweet snacks and desserts, 3) packaged savory snacks, 4) sugar- and artificially sweetened beverages (SSBs, ASBs), 5) ready-to-eat/heat mixed dishes, 6) animal-based products, 7) sauces, spreads, and condiments, 8) yogurt and dairy-based desserts, and 9) other UPFs that could not be categorized into the other eight groups (Supplementary Table 1). Nine food items from the FFQs lacked sufficient details to be considered as UPFs. They were excluded from the primary analysis and reconsidered in sensitivity analysis (17). Total UPFs were the sum of UPF foods and beverages from theses nine groups. UPF consumption in the primary analysis was quantified using servings/day (13). Three UPF units were additionally examined: total calories/day from UPF, percentage of calories/day from UPF, and percentage of grams/day from UPF.
Other dietary factors, including energy intake and overall dietary quality, were derived from the same FFQs. Dietary quality was assessed by the AHEI, a continuous dietary index strongly predictive of chronic diseases, with a higher score indicating a greater adherence to a healthy diet (18). Alcohol was excluded from the AHEI calculation due to its nonlinear relationship with T2D and was separately adjusted for (2). The possible range of AHEI scores was from 0 to 100.
Outcome Ascertainment—Body Weight and T2D
NHS II participants self-reported body weight in the biennial questionnaires. A validation study reported a high correlation (Spearman correlation = 0.97) between self-reported versus staff-measured body weights, with a mean difference of 3.3 lb (1.5 kg) (19).
Since 1989, NHS II participants self-reported physician-diagnosed T2D every 2 years on follow-up questionnaires. For confirmation, a validated supplementary questionnaire on symptoms, diagnostic tests, and diabetes treatment was sent to those who reported a T2D diagnosis (20). Until 1998, the National Diabetes Data Group criteria were used for confirmation (21), and from 1998 onward, the American Diabetes Association criteria were applied (22), consistent with previous NHS II studies. Only confirmed T2D cases were used in the current study.
Statistical Analysis
Change in UPF Consumption in Association With Concurrent Body Weight Change
We assessed the association between UPF consumption and long-term weight change in a subcohort of women with a history of GDM, using methods consistent with previous NHS II analyses (7). Individuals’ change in UPF consumption every 4 years was calculated by subtracting their preceding UPF intake from recent UPF intake; a negative value indicates the amount of UPFs reduced over the past 4 years, while a positive value indicates an increase by that amount. The distribution of UPF intake changes were divided into quartiles (quartile 1 [Q1] as the lowest change, Q2, Q3, and Q4) at each 4-year interval. Changes in servings/day for individual UPF groups were also calculated. Similarly, 4-year weight changes (kg) were calculated as the difference between time points, with positive values indicating a net weight gain. Women began contributing to 4-year follow-up intervals after their first GDM pregnancy. They were skipped from contributing to a given follow-up cycle if they reported a new pregnancy or did not provide FFQ or body weight data (subcohort n = 3,781). All participants were censored from follow-up at age 65, incident cancer diagnosis, or death (6).
To assess the associations between 4-year changes in overall UPF consumption and concurrent weight changes, we used generalized estimating equations (GEEs) to estimate least squares mean of the weight change and its 95% CI for each quartile of UPF change (23). Changes in group-specific UPFs were modeled as continuous variables (servings/day). These models adjusted for follow-up period, age, race and ethnicity, histories of hypertension and hypercholesteremia, family history of diabetes, oral contraceptive use, menopausal status, cycle-specific baseline UPF consumption, and the baseline BMI, as well as the concurrent 4-year changes in alcohol intake, physical activity, and smoking status. All covariates were time-updated except race and ethnicity. We acknowledge that race and ethnicity are social and not biological constructs. However, due to documented racial and ethnic disparities in GDM incidence and management that may have implications for future diabetes risk (24), race and ethnicity were included as a covariate. Given the study’s sample size and the elevated risk of GDM and related complications observed in nearly all racial and ethnic minority groups compared with White women, participants were categorized as White or non-White for adjustment in multivariable models to minimize residual confounding while maintaining model stability.
Changes in UPF Consumption and AHEI in Association With Concurrent Body Weight Change
Given the partial overlap between some UPF groups and food items contributing to AHEI scoring (Pearson correlation between UPF and AHEI: −0.32), we investigated UPF and AHEI jointly with weight change. First, we assessed the associations between UPF changes with 4-year weight changes, stratified by binary groups of AHEI change: stable or decreased (AHEI change ≤0) and increased (AHEI change >0). Heterogeneity between these strata was tested by including cross-product terms in the model and assessing their statistical significance. Second, we examined concurrent changes in UPF consumption and AHEI jointly in relation to weight changes. Binary groups were separately created for changes in AHEI and UPF, resulting in four possible joint categories: 1) stable or decreased UPF and increased AHEI (most favorable), 2) increased UPF and increased AHEI, 3) stable or decrease in both UPF and AHEI, and 4) increased UPF and stable or decreased AHEI. We used GEEs with the same set of covariates adjusted as in the previous models.
UPF Consumption in Association With T2D Risk
We examined the association between habitual UPF consumption and T2D incidence, using cumulative averages updated at each follow-up cycle for dietary factors, including UPFs, to represent long-term dietary patterns and minimize measurement errors (e.g., the 1999 cumulative average was calculated as the average of intake reported in 1991, 1995, and 1999) (6). Levels of UPF consumption were categorized into quartiles. Dietary data from follow-up cycles with reported pregnancies were skipped. Dietary factors were no longer updated if a participant reported a cancer or CVD diagnosis during follow-up, as these conditions could influence diet (6).
We calculated eligible person-time years from baseline until the date of T2D diagnosis, death, return of the last biennial questionnaire, or the end of the follow-up (June 2021), whichever came first. We used multivariable Cox regression models to estimate adjusted hazard ratios (HRs) and 95% CIs for UPF quartiles, with the lowest quartile (Q1) as the reference group. Proportional hazards assumption was unlikely to be violated (stratified by age ≤50 and >50 years, P = 0.76). Model 1 was stratified by time-updated age and calendar time period to control potential confounding by age, calendar time, and any possible interactions between these two time scales. Model 2 was additionally adjusted for race and ethnicity, family history of diabetes, baseline BMI, baseline status of hypertension and hypercholesterolemia, parity, oral contraceptive use, menopausal status, smoking status, physical activity, and alcohol intake. All covariates were time-updated except race and ethnicity and baseline-defined variables. We did not use time-updated data for BMI, hypertension, and hypercholesterolemia, because they are likely mediators between UPF and T2D (13). Since UPFs contribute to diet quality and energy intake, model 2 was considered primary. We further adjusted for AHEI in model 3 and total energy intake (both continuous) in model 4. Additionally, to formally evaluate the role of body weight on linking UPF consumption to T2D risk, we conducted mediation analysis to quantify the proportion of the association between UPF consumption and T2D explained by time-updated BMI (kg/m2) (25).
UPF Consumption and Status of AHEI in Association With T2D Risk
We examined habitual UPF intake with T2D risk in the context of overall diet quality as measured by AHEI. First, we stratified the T2D associations by binary AHEI status based on the sample’s AHEI distributions (low vs. high by AHEI median: 47.6). Second, we explored the joint associations of UPF intake and AHEI with T2D risk. Binary groups (low vs. high) were created by the median values for UPF (6.6 servings/day) and AHEI separately, forming four joint groups: low UPF and high AHEI (reference, most favorable), low UPF and low AHEI, high UPF and high AHEI, and high UPF and low AHEI.
We further performed stratified analyses to evaluate whether the associations between UPF and T2D may differ by major T2D risk factor status: BMI (<25.0 kg/m2 vs. ≥25.0 kg/m2), leisure-time physical activity (below vs. above the median of 11.4 MET-h/week), and family history of diabetes (yes vs. no). All the analyses were performed using SAS 9.4 software, with a two-sided P value <0.05 as the statistical significance level.
Data and Resource Availability
The statistical analysis codes used for the present analysis can be made available on a case-by-case basis with approval from the senior author of this manuscript. Data described in the manuscript will not be made publicly available. Further information including the procedures for obtaining and accessing data from the Nurses’ Health Studies II is described online (https://www.nurseshealthstudy.org/researchers; email: nhsaccess@channing.harvard.edu).
Results
Among the 4,207 individuals with a history of GDM, average baseline UPF consumption was 3.6 servings/day in the lowest quartile (Q1) and 11.6 servings/day in the highest quartile (Q4), which contributed to 27.5% and 41.0% of daily energy intake, respectively (Table 1). The baseline BMI in 48.1% (n = 2,022) was BMI ≥ 25 kg/m2 (overall mean: 26.3 kg/m2). Compared with individuals in the lowest UPF quartile, those in the highest quartile were more likely to be White, to have a higher BMI and a history of hypertension or hypercholesteremia, to be less physically active, and tended to have a lower diet quality as indicated by a lower AHEI and a higher energy intake.
Table 1.
Baseline population characteristics by consumption of UPFs among individuals with a history of GDM, NHS II (n = 4,207)
| Total UPF intake | ||||
|---|---|---|---|---|
| Q1 (n = 1,011) | Q2 (n = 963) | Q3 (n = 1,015) | Q4 (n = 1,218) | |
| UPF intake, servings/day | 3.6 (0.9) | 5.7 (0.5) | 7.6 (0.6) | 11.6 (2.5) |
| UPF intake, total calories/day | 402 (134) | 585 (160) | 727 (210) | 1,005 (333) |
| UPF intake, % calories/day | 27.5 (9.3) | 32.7 (8.6) | 36.0 (8.8) | 41.0 (9.9) |
| UPF intake, % g/day | 15 (10.9) | 20.5 (12.3) | 26.3 (14.7) | 33.5 (16.4) |
| Age, years | 39.1 (6.1) | 38.5 (5.6) | 38.2 (5.2) | 38.1 (5.1) |
| Age at GDM incidence, years | 30.6 (5.2) | 30.3 (5.2) | 30.5 (5.1) | 30.5 (5.1) |
| White race and ethnicity, n (%) | 867 (85.8) | 903 (93.8) | 956 (94.2) | 1137 (93.3) |
| BMI, kg/m2 | 25.3 (5.4) | 25.8 (5.5) | 26 (5.9) | 27.6 (6.5) |
| <25.0, n (%) | 596 (59.0) | 528 (54.8) | 554 (54.6) | 507 (41.6) |
| 25.0–29.9, n (%) | 241 (23.8) | 252 (26.2) | 248 (24.4) | 342 (28.1) |
| ≥30.0, n (%) | 174 (17.2) | 183 (19.0) | 213 (21.0) | 369 (30.3) |
| Age at first birth, years | 27.8 (5.0) | 27.2 (4.7) | 27.5 (4.7) | 27.4 (4.8) |
| Parity, median (Q25, Q75), n | 2 (2, 3) | 2 (2, 3) | 2 (2, 3) | 2 (2, 3) |
| Status of oral contraceptive use | ||||
| Current user, n (%) | 154 (15.2) | 123 (12.8) | 137 (13.5) | 154 (12.6) |
| Past user, n (%) | 787 (77.8) | 759 (78.8) | 800 (78.8) | 976 (80.1) |
| Never use, n (%) | 70 (6.9) | 81 (8.4) | 78 (7.7) | 88 (7.2) |
| Family history of diabetes, n (%) | 478 (47.3) | 450 (46.7) | 475 (46.8) | 619 (50.8) |
| Ever had hypertension, n (%) | 77 (7.6) | 65 (6.7) | 83 (8.2) | 113 (9.3) |
| Ever had high cholesterol, n (%) | 165 (16.3) | 168 (17.4) | 204 (20.1) | 263 (21.6) |
| Menopausal status | ||||
| Premenopausal, n (%) | 953 (94.3) | 917 (95.2) | 973 (95.9) | 1,168 (95.9) |
| Postmenopausal, n (%) | 58 (5.7) | 46 (4.8) | 42 (4.1) | 50 (4.1) |
| Physical activity, MET-h/week* | 18.3 (23.0) | 17.7 (25.4) | 18 (27.2) | 16.1 (21.6) |
| Smoking status | ||||
| Never smoker, n (%) | 667 (66.0) | 639 (66.4) | 676 (66.6) | 814 (66.8) |
| Past smoker, n (%) | 235 (23.2) | 221 (22.9) | 237 (23.3) | 280 (23.0) |
| Current smoker, n (%) | 109 (10.8) | 103 (10.7) | 102 (10.0) | 124 (10.2) |
| AHEI score | 47.7 (10.7) | 44.4 (10.7) | 42.4 (10) | 39.8 (9.9) |
| Total energy intake, kcal/day | 1,462 (414) | 1,769 (439) | 1,976 (461) | 2,355 (544) |
| Alcohol intake, g/day | 2.4 (5.0) | 2.4 (4.6) | 2.4 (4.5) | 2.4 (5.1) |
| Group-specific UPFs, servings/day | ||||
| UPF breads and cereals | ||||
| UPF cereals | 0.2 (0.3) | 0.4 (0.4) | 0.4 (0.4) | 0.5 (0.5) |
| UPF dark breads and whole-grain breads | 0.4 (0.4) | 0.6 (0.5) | 0.8 (0.8) | 1.3 (1.2) |
| UPF refined breads | 0.3 (0.4) | 0.5 (0.6) | 0.7 (0.8) | 0.9 (1.1) |
| Packaged sweet snacks and desserts | ||||
| Confectioneries | 0.2 (0.2) | 0.3 (0.3) | 0.3 (0.4) | 0.5 (0.7) |
| Packaged sweet snacks | 0.3 (0.3) | 0.5 (0.4) | 0.6 (0.6) | 0.9 (1) |
| Fruit-based products | 0.1 (0.2) | 0.2 (0.2) | 0.2 (0.3) | 0.3 (0.4) |
| ASBs and SSBs | ||||
| SSBs | 0.2 (0.4) | 0.4 (0.6) | 0.5 (0.9) | 0.7 (1.3) |
| ASBs | 0.4 (0.5) | 0.7 (0.9) | 1.1 (1.3) | 1.9 (2.0) |
| Animal-based products | 0.2 (0.2) | 0.3 (0.3) | 0.4 (0.3) | 0.5 (0.4) |
| Ready-to-eat/heat mixed dishes | 0.2 (0.1) | 0.3 (0.2) | 0.3 (0.2) | 0.4 (0.2) |
| Packaged savory snacks | 0.1 (0.2) | 0.2 (0.2) | 0.2 (0.3) | 0.3 (0.5) |
| Sauces, spreads, and condiments | 0.7 (0.5) | 1.2 (0.8) | 1.6 (1.1) | 2.4 (1.6) |
| Yogurt and dairy-based desserts | 0.2 (0.2) | 0.2 (0.2) | 0.3 (0.3) | 0.4 (0.4) |
| Other UPFs | 0 (0.2) | 0.1 (0.3) | 0.1 (0.4) | 0.4 (1.1) |
Data are presented as mean (SD) unless otherwise stated.
*Metabolic equivalents from leisure-time physical activities of moderate or vigorous intensity were summed.
Change in UPF Consumption in Association With Concurrent Body Weight Change
A subset of 3,781 of the 4,207 individuals contributed at least one 4-year interval to the weight change analyses (Supplementary Table 2). After adjusting for covariates, including baseline UPF consumption and concurrent changes in other lifestyle factors, increasing UPF consumption was associated with a greater weight gain across quartiles of UPF changes (P-trend < 0.0001) (Fig. 1). The adjusted mean 4-year weight change was 0.52 kg (95% CI 0.21–0.83) for Q1, 0.97 kg (95% CI 0.68, 1.26) for Q2, 1.27 kg (95% CI 1.02, 1.55) for Q3, and 1.65 kg (95% CI 1.36–1.95) for Q4.
Figure 1.
Four-year change in consumption of UPFs in association with concurrent 4-year weight change among individuals with a history of gestational diabetes mellitus, NHS II (n = 3,781, follow-up between 1991 and 2019). Changes in UPF (servings/day) between each 4-year period were calculated and categorized into quartiles; participants in the lowest quartile group had the smallest increase in UPF consumption. Least squares means of the concurrent 4-year weight change (kg) were modeled in the multivariable marginal models with GEEs adjusting for follow-up period, race and ethnicity (White, non-White), history of hypertension (yes, no), history of hypercholesteremia (yes, no), family history of diabetes (yes, no), age (years), baseline BMI (kg/m2), oral contraceptive use (current, past, never), menopausal status (premenopausal, postmenopausal), baseline UPF intake (servings/day), and concurrent changes in the other lifestyle factors (alcohol intake g/day by quartiles, physical activities MET-h/week by quartiles, and categorical change in smoking status). Mean 4-year weight change: 1.20 (SD 7.57) kg. The black circles indicate UPF quartile-specific 4-year weight change (kg) with corresponding 95% CI.
Changes in UPF Consumption and AHEI Score in Association With Concurrent Body Weight Change
Despite evidence of heterogeneity by binary status of AHEI change (P-heterogeneity [P-het] = 0.04), increasing intakes of UPFs were associated with greater weight changes in both groups with stable or decreased AHEI (P-trend = 0.0002) and increased AHEI (P-trend = 0.003) (Fig. 2A). When the joint concurrent changes of UPF and AHEI categories were examined, the group with stable or decreased UPF and increased AHEI did not show significant weight change over 4 years (0.20 kg; 95% CI −0.07, 0.48). In contrast, all of the other three joint groups experienced weight gain, with the group having increased UPF and stable or decreased AHEI showing the greatest gain (2.48 kg; 95% CI 2.15, 2.80) (Fig. 2B).
Figure 2.
Four-year changes in consumption of UPFs and AHEI-2010 in association with concurrent 4-year weight change among individuals with a history of GDM, NHS II (n = 3,781, follow-up between 1991and 2019). A: Changes in UPF and the concurrent 4-year weight change, stratified by binary concurrent changes in AHEI modeled as stable or decreased, and increased (P-trend = 0.0002 in stratum of stable or decreased AHEI, P-trend = 0.003 in stratum of increased AHEI); overall P-het = 0.04. B: Joint changes in UPF consumption and AHEI and the concurrent 4-year weight change. Four categories of joint changes in UPF and AHEI were categorized such that stable or decreased UPF and increased AHEI was the most favorable category, whereas increased UPF and stable or decreased AHEI was the least favorable category. Least squares means of 4-year weight change (kg) were modeled in the multivariable marginal models with GEEs adjusting for follow-up period, race and ethnicity (White, non-White), history of hypertension (yes, no), history of hypercholesteremia (yes, no), family history of diabetes (yes, no), age (years), baseline BMI (kg/m2), oral contraceptive use (current, past, never), menopausal status (premenopausal, postmenopausal), baseline UPF intake (continuous, servings/day), and concurrent changes in the other lifestyle factors (alcohol intake g/day by quartiles, physical activities MET-h/week by quartiles, and categorical change in smoking status). The black circles indicate UPF quartile-specific 4-year weight change (kg) with corresponding 95% CI.
Change in UPF Consumption by Groups in Association With Concurrent Body Weight Change
Among the group-specific UPFs (Supplementary Fig. 1), increases in the consumption of ultra-processed refined breads, confectioneries, SSBs, ready-to-eat/heat mixed dishes, animal-based products, and sauces, spreads, and condiments were associated with greater weight gain. Increases in packaged savory snacks and the group of other UPFs were associated with a negative weight change. No significant weight change was observed with ultra-processed cereals or dark breads and whole-grain breads, packaged sweet snacks, fruit-based products, ASBs, or yogurt and dairy-based products.
UPF Consumption in Association With T2D Risk
Over 30 years of follow-up, we documented 1,040 incident T2D cases over a total of 86,159.4 person-years, equivalent to 12.1 cases per 1,000 person-years. After adjusting for covariates in model 2, consumption of UPF was positively associated with a higher risk of T2D (P-trend = 0.04) (Table 2). Compared with the reference group (Q1), the adjusted HRs (95% CI) were 1.07 (0.87, 1.32) for Q2, 1.25 (1.03, 1.53) for Q3, and 1.20 (0.99, 1.46) for Q4. Additional adjustment for AHEI (P-trend = 0.14) or energy intake (P-trend = 0.28) attenuated the associations. Mediation analysis showed that BMI explained 38.6% (95% CI 14.0, 70.9%; P < 0.0001) of the association between UPF consumption and T2D risk (Table 2).
Table 2.
Consumption of UPF and risk of subsequent T2D among individuals with a history of GDM, NHS II (n = 4,207, 1,040 events, 86,159.4 person-years)
| T2D risk, HR (95% CI) | |||||||
|---|---|---|---|---|---|---|---|
| Case per 1,000 | Model 1* | Model 2† | Model 3‡ | Model 4§ | |||
| Cases, n | Person-time | person-years | Age-adjusted | MV-adjusted | Model 2 + AHEI | Model 2 + TEI | |
| UPF, servings/day | |||||||
| Q1 | 198 | 20,896.2 | 9.5 | REF (HR = 1.00) | REF (HR = 1.00) | REF (HR = 1.00) | REF (HR = 1.00) |
| Q2 | 227 | 21,547.6 | 10.5 | 1.13 (0.92, 1.37) | 1.07 (0.87, 1.32) | 1.05 (0.85, 1.30) | 1.05 (0.85, 1.30) |
| Q3 | 278 | 21,796.1 | 12.8 | 1.34 (1.11, 1.63) | 1.25 (1.03, 1.53) | 1.22 (0.99, 1.49) | 1.21 (0.98, 1.50) |
| Q4 | 337 | 21,919.6 | 15.4 | 1.57 (1.31, 1.90) | 1.20 (0.99, 1.46) | 1.15 (0.94, 1.40) | 1.14 (0.90, 1.44) |
| P-trendǁ | <0.0001 | 0.04 | 0.14 | 0.28 | |||
| Association mediated through BMI, %¶ | 38.6 (14.0–70.9) | ||||||
| Below AHEI median (47.6) | Above AHEI median (47.6) | ||||||
| Cases/person- time, n | Case per 1,000 person-years | Model 2† MV-adjusted | Cases/person-time, n | Case per 1,000 person-years | Model 2† MV-adjusted | ||
| UPF, servings/day | |||||||
| Q1 | 79/7,334.1 | 10.8 | REF (HR = 1.00) | 119/13,562.08 | 8.8 | REF (HR = 1.00) | |
| Q2 | 124/10,278.8 | 12.1 | 0.97 (0.70, 1.35) | 103/11,268.75 | 9.1 | 1.04 (0.76, 1.41) | |
| Q3 | 158/12,108.7 | 13.0 | 1.10 (0.81, 1.51) | 120/9,687.42 | 12.4 | 1.36 (1.00, 1.84) | |
| Q4 | 224/14,936.9 | 15.0 | 1.02 (0.76, 1.37) | 113/6,982.67 | 16.2 | 1.50 (1.10, 2.05) | |
| P-trend | 0.85 | 0.003 | |||||
| P-het# | 0.14 | ||||||
| Joint UPF and AHEI** | Cases/person-time, n | Case per 1,000 person-years | Model 2† MV-adjusted | ||||
| Low UPF and high AHEI | 222/24,830.8 | 8.9 | REF (HR = 1.00) | ||||
| Low UPF and low AHEI | 203/17,612.9 | 11.5 | 1.17 (0.94, 1.44) | ||||
| High UPF and high AHEI | 233/16,670.1 | 14.0 | 1.40 (1.14, 1.71) | ||||
| High UPF and low AHEI | 382/27,045.6 | 14.1 | 1.21 (1.00, 1.45) | ||||
HR, hazard ratio, MV, multivariable; TEI, total energy intake.
*Model 1 was stratified by age (years) and calendar time period (indicators). †Model 2 was additionally adjusted for race and ethnicity (White, non-White), family history of diabetes (yes, no), baseline BMI (continuous, kg/m2), baseline status of hypertension (yes, no), baseline status of hypercholesterolemia (yes, no), parity (1, 2+), oral contraceptive use (current, past, never), menopausal status (premenopausal, postmenopausal), smoking (never, current, past), physical activity (MET-h/week), and alcohol intake (0 g/day, 0.1–4.9 g/day, 5.0–14.9 g/day, ≥15.0 g/day). ‡AHEI (continuous) was additionally adjusted for in the model. §Total energy intake (kcal/day; continuous) was additionally adjusted for in the model. ǁMedium values for each UPF category were entered into the model to estimate P value for trend. ¶Mediation analysis was conducted to assess the proportion of the associations (model 2) mediated through cycle-updated BMI (kg/m2). #P-het was calculated by including an interaction term in the model and testing for statistical significance with the Wald test. **UPF and AHEI were separately categorized as binary variables according to their median values (6.6 servings/day for UPF, 47.6 for AHEI), and then joint categories were created.
UPF Consumption and AHEI Score in Association With T2D Risk
Among individuals with AHEI scores above the median, UPF consumption was significantly associated with a higher risk of T2D (P-trend = 0.003; adjusted HR for Q4 vs. Q1 1.50 [95% CI 1.10, 2.05]) (Table 2). Although a similar positive trend was observed among those with lower AHEI scores, the associations did not reach statistical significance (P-trend = 0.85). Test of heterogeneity by AHEI status (above or below AHEI median) was not statistically significant (P-het = 0.14). When AHEI and UPF were modeled as a joint exposure, compared with the group with low UPF and high AHEI, the high UPF and high AHEI group, and the high UPF and low AHEI group both experienced elevated T2D risks (adjusted HR 1.40 [95% CI 1.14, 1.71] and 1.21 [95% CI 1.00, 1.45], respectively) (Table 2).
Additional Analyses on UPF Consumption With T2D Risk
When we evaluated the group-specific UPFs (Supplementary Table 3), after multivariable adjustment (model 2), each additional serving of SSBs and ASBs per day was significantly associated with a 7% (HR 1.07; 95% CI 1.00, 1.16) and 8% (HR 1.08; 95% CI 1.03, 1.13) higher risk of T2D, whereas ultra-processed cereals were associated with a lower risk (HR 0.80; 95% CI 0.64, 0.98). Elevated risks were seen for each additional serving of ultra-processed dark breads and whole-grain breads, refined breads, and other UPFs, and decreased risks for fruit-based products, although these were not statistically significant. In stratified analyses by major T2D risk factors (Supplementary Table 4), the positive associations between UPF consumption and T2D risk did not vary materially by physical activity or family history of diabetes (P-het > 0.05). When stratifying by baseline BMI, positive associations were seen only among those with the BMI ≥25.0 kg/m2; no apparent associations were observed in those with a BMI <25 kg/m2 (P-het < 0.0001).
A series of sensitivity analyses were conducted. In the weight change analyses, additional adjustment of 4-year change in energy intake or diet quality (AHEI) did not materially alter the associations (results not shown). In the T2D analyses, after classifying the nine undetermined food items as UPFs, the positive associations of UPFs with T2D risk became stronger (P-trend = 0.004) and persisted after additional adjustment for AHEI or energy intake (P-trend < 0.05) (Supplementary Table 5). In the analysis characterizing UPF by total calories/day, percentage of calories/day, or percentage of grams/day (Supplementary Table 6), incidence rates of T2D were consistently higher with greater UPF quartiles for all three UPF metrics; the positive associations of UPFs assessed by percentage of grams/day were significant even after additional adjustment for AHEI or energy intake (P-trend < 0.05). We additionally adjusted for energy intake using the nutrient residual method and observed similar findings to those from the model adjusting for energy intake as a covariate (Supplementary Table 6).
Conclusions
Based on a prospective cohort of women with a history of GDM, increasing UPF consumption was associated with greater weight gain, and greater habitual UPF consumption was associated with a higher risk of T2D. These associations persisted even among those with a higher AHEI score. Our findings suggest overall UPFs contribute to midlife weight gain and T2D development in this at-risk population. Heterogeneity in UPF groups in relation to weight change and T2D outcomes was observed. To our knowledge, this study represents the first prospective and longest investigation into UPF consumption following GDM-complicated pregnancies on long-term weight change and subsequent progression to T2D.
With repeated measures of dietary factors and weight, we examined changes in UPF in relation to concurrent weight changes at 4-year intervals, as changes in dietary behaviors over time may be more relevant in terms of their physiological effects on weight change and their translation in preventive strategies. Among these women who experienced modest yet steady midlife weight gain, increased UPF consumption was associated with greater weight gain, independent of other lifestyle factors and baseline UPF intake. The associations were particularly strong among groups of ultra-processed refined breads, confectioneries, SSBs, ready-to-eat/heat mixed dishes, and animal-based products. Evidence from randomized (26) and observational studies in the general population consistently links UPF to overweight and obesity (27). Considering obesity as a significant risk factor for chronic diseases such as T2D and CVD (28), the clinical implications of excessive UPF consumption, if sustained over the long term, can be substantial.
In contrast, for T2D risk, we modeled habitual UPF intake using cumulative averages. This approach is appropriate given the relatively long latency period and the sustained influence of dietary factors underlying T2D development. Among these high-risk women, habitual UPF consumption was positively associated with an increased T2D risk. A 2023 meta-analysis that pooled five studies (n = 415,554) characterizing UPFs by the NOVA classification reported an overall risk ratio of 1.40 (95% CI 1.23, 1.59) when comparing the highest to the lowest UPF consumption quantile (percentage of grams from UPF/day) (13). Another meta-analysis (>1 million individuals) that included studies evaluating UPFs without limiting to those using the NOVA classification reported consistent findings (29). Consumption of UPFs was predominantly characterized by grams/day in past studies, accounting for UPFs that do not contribute to energy intake (30). In our analysis, UPF consumption was characterized by servings/day for clear public health implications, consistent with earlier work in the general population of NHS II (13). Associations between UPFs in grams/day and T2D risk remained significant even after adjusting for diet quality or energy intake. Collectively, the relationship between overall UPFs and increased risk of T2D has been confirmed across diverse populations, including this high-risk group. Given the established role of BMI as a key risk factor for T2D (3), our study suggests that UPFs contribute to the progression from GDM to T2D through pathways involving weight gain. Our analyses on group-specific UPFs confirmed increased risks of T2D with SSBs and ASBs and reduced risk with ultra-processed cereals, though the ASB finding may partly reflect reverse causation. Potential heterogeneity across UPF groups regarding weight change and T2D risk was noted, similar to previous findings (11,13), although some results for certain UPF groups were likely underpowered due to relatively low intake levels in this cohort.
A novel contribution of our study is the examination of UPF consumption while simultaneously considering overall diet quality using AHEI. The AHEI scoring, excluding alcohol, reflects optimal intakes of fruits and vegetables, whole grains, nuts and legumes, red meats and processed meats, SSBs, sodium, and trans- and polyunsaturated fats (18), directly or indirectly capturing some UPF groups. Our joint analysis of UPF and AHEI changes revealed that increasing diet quality and not increasing UPFs supports weight maintenance, a key message for midlife chronic disease prevention, given obesity’s role as a major risk factor. In stratified analysis by AHEI, significant associations between UPF intake and increased T2D risk were only observed among individuals with higher AHEI scores. In the joint analysis, the adjusted HR for T2D appeared higher among individuals with both high UPF and high AHEI compared with those with high UPF and low AHEI. Based on additional analyses of food group intakes, individuals with high AHEI and high UPF intake showed a mix of healthy and unhealthy dietary behaviors, making it difficult to isolate specific food groups driving the elevated risk. Moreover, incidence rates were nearly identical between the two groups, suggesting that differences in the adjusted HRs may reflect variation in covariate distributions rather than true differences in absolute risk. Reverse causation may also play a role, whereby individuals experiencing early T2D symptoms adopt healthier dietary patterns without necessarily changing UPF intakes. A high UPF, high AHEI diet may include ASBs, foods with processed sauces and dressings, ultra-processed whole-grain breads, or “less unhealthy” UPFs, such as fat-free popcorn, low-fat cookies, artificial sweeteners, and low-fat mayonnaise, as captured in the FFQs. Sugar-containing foods, including those with artificial sugars and high-fat sauces or foods, are linked to obesity and metabolic disturbance (11,31–33). In contrast, an example of a low UPF, high AHEI diet resembles the Mediterranean diet, with minimally processed foods. Overall, these findings underscore the complexity of UPF as food sources and highlight UPF consumption as a critical factor in assessing diet quality for optimal cardiometabolic health, extending beyond conventional food groups or properties considered in a relatively high-quality diet. Reducing UPF intake might be considered as a critical part of dietary counseling in diabetes prevention programs.
Although the precise mechanisms remain unclear, our findings are biologically plausible. Common UPFs contain high levels of sodium, sugar, fat, energy, and additives, while lacking natural ingredients such as fiber and nutrients (26). These properties influence satiety and digestibility (26), contributing to obesity, inflammation (34), increased glycemic index and insulin resistance (35), and disrupted gut microbiota (36), all of which increase susceptibility to cardiometabolic diseases, including T2D. UPF consumption also displaces unprocessed or less processed foods, limiting intakes of natural foods and those high in plant-based bioactive compounds (14). Additionally, the nature of UPFs and their processing and packaging, often with plastic products, introduce added chemical compounds (e.g., emulsifiers, acrylamide), contaminants, and chemicals, such as endocrine disruptors. Emerging evidence suggests both individual and “cocktail” effects of additives and chemicals on gut microbiota and T2D development (37,38). These features and components of UPFs are not directly captured in the FFQs but warrant exploration in future research.
This study has several notable strengths, including a focus on a high-risk group, a prospective design with 30 years of follow-up, detailed, repeated assessments of exposure, including information on UPF groups, covariates, and outcomes, and a joint consideration of UPF and diet quality. Several limitations merit discussion. First, body weight and diet were self-reported by participants, potentially introducing errors. Given the prospective design, we assume that exposure misclassification would be nondifferential regarding the outcomes. We acknowledge that the FFQs may not fully capture all UPFs or information on foods and beverages necessary for accurate NOVA classification. Residual confounding is possible given the observational nature of this study, including the inability to control for all concurrent changes in diet and lifestyle that may also affect weight change and T2D risk over time, although we have attempted to adjust for the most significant ones, including diet and physical activity. The homogenous nature of this cohort, predominantly comprising female U.S. nurses, helped mitigate confounding by socioeconomic factors to some degree. Lastly, findings from this study should be replicated in other populations to confirm generalizability.
In conclusion, among U.S. women with a history of GDM, increased overall UPF consumption over time was associated with greater weight gain in midlife. Habitual higher UPF intake was related to a higher T2D risk. These significant associations persisted even among those with higher diet quality. Well-designed studies are warranted to investigate the underlying mechanisms and impacts of UPF groups on individuals at high risk.
This article contains supplementary material online at https://doi.org/10.2337/figshare.29657063.
Article Information
Acknowledgments. The authors thank all the participants of the Nurses’ Health Study II for their contributions and the Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, as the home of the Nurses’ Health Study I and II. The Nurses’ Health Study II thanks the following state cancer registries for their help: Alabama, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Idaho, Illinois, Indiana, Iowa, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Nebraska, New Hampshire, New Jersey, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, Tennessee, Texas, Virginia, Washington, and Wyoming. F.B.H. and C.Z. are editors of Diabetes Care but were not involved in any of the decisions regarding review of the manuscript or its acceptance.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. J.Y. conducted the data analysis and interpretation and wrote the initial draft of the manuscript. J.Y. and C.Z. conceived and designed the study. C.Z. obtained funding and supervised the study. All authors contributed to the interpretation of the results and revision of the manuscript for important intellectual content and approved the final version of the manuscript. C.Z. and J.Y. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented as an abstract during the American Society for Nutrition NUTRITION 2023 Conference, Boston, MA, 22–25 July, 2023.
Handling Editors. The journal editor responsible for overseeing the review of the manuscript was Mark A. Atkinson.
Funding Statement
This study was supported by contract HHSN275201000020C from the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (NIH). The Nurses’ Health Study II was funded by research grants UM1 CA186107, DK58845, CA50385, P30 DK46200, U01 CA176726, R01 CA67262, and U01 HL145386 from the from the National Cancer Institute, National Institute of Diabetes and Digestive and Kidney Diseases, and National Heart, Lung, and Blood Institute, NIH. D.K.T. reported receiving research grants from the American Diabetes Association. J.E.C. reported receiving grants from the NIH paid to the institution during the conduct of the study and grants from the NIH, U.S. Food and Drug Administration, and U.S. Centers for Disease Control and Prevention paid to the institution outside the submitted work.
Supporting information
References
- 1. Ahmad E, Lim S, Lamptey R, Webb DR, Davies MJ.. Type 2 diabetes. Lancet 2022;400:1803–1820 [DOI] [PubMed] [Google Scholar]
- 2. Yang J, Qian F, Chavarro JE, et al. Modifiable risk factors and long term risk of type 2 diabetes among individuals with a history of gestational diabetes mellitus: prospective cohort study. BMJ 2022;378:e070312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hu FB, Manson JE, Stampfer MJ, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med 2001;345:790–797 [DOI] [PubMed] [Google Scholar]
- 4. Zhu Y, Zhang C.. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: a global perspective. Curr Diab Rep 2016;16:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vounzoulaki E, Khunti K, Abner SC, Tan BK, Davies MJ, Gillies CL.. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ 2020;369:m1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tobias DK, Hu FB, Chavarro J, Rosner B, Mozaffarian D, Zhang C.. Healthful dietary patterns and type 2 diabetes mellitus risk among women with a history of gestational diabetes mellitus. Arch Intern Med 2012;172:1566–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang J, Zhang C, Chavarro JE, et al. Lifestyle changes and long-term weight gain in women with and without a history of gestational diabetes mellitus: a prospective study of 54,062 women in the Nurses' Health Study II. Diabetes Care 2022;45:348–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Monteiro CA, Cannon G, Levy RB, et al. Ultra-processed foods: what they are and how to identify them. Public Health Nutr 2019;22:936–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lane MM, Gamage E, Du S, et al. Ultra-processed food exposure and adverse health outcomes: umbrella review of epidemiological meta-analyses. BMJ 2024;384:e077310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Juul F, Parekh N, Martinez-Steele E, Monteiro CA, Chang VW.. Ultra-processed food consumption among US adults from 2001 to 2018. Am J Clin Nutr 2022;115:211–221 [DOI] [PubMed] [Google Scholar]
- 11. Mendoza K, Smith-Warner SA, Rossato SL, et al. Ultra-processed foods and cardiovascular disease: analysis of three large US prospective cohorts and a systematic review and meta-analysis of prospective cohort studies. Lancet Reg Health Am 2024;37:100859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Elliott A, Walters RK, Pirinen M, et al. ; FinnGen . Distinct and shared genetic architectures of gestational diabetes mellitus and type 2 diabetes. Nat Genet 2024;56:377–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen Z, Khandpur N, Desjardins C, et al. Ultra-processed food consumption and risk of type 2 diabetes: three large prospective U.S. cohort studies. Diabetes Care 2023;46:1335–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Touvier M, da Costa Louzada ML, Mozaffarian D, Baker P, Juul F, Srour B.. Ultra-processed foods and cardiometabolic health: public health policies to reduce consumption cannot wait. BMJ 2023;383:e075294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang C, Olsen SF, Hinkle SN, et al. ; Diabetes & Women’s Health Study team . Diabetes & Women’s Health (DWH) study: an observational study of long-term health consequences of gestational diabetes, their determinants and underlying mechanisms in the USA and Denmark. BMJ Open 2019;9:e025517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Solomon CG, Willett WC, Carey VJ, et al. A prospective study of pregravid determinants of gestational diabetes mellitus. JAMA 1997;278:1078–1083 [PubMed] [Google Scholar]
- 17. Khandpur N, Rossato S, Drouin-Chartier J-P, et al. Categorising ultra-processed foods in large-scale cohort studies: evidence from the Nurses' Health Studies, the Health Professionals Follow-up Study, and the Growing Up Today Study. J Nutr Sci 2021;10:e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chiuve SE, Fung TT, Rimm EB, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr 2012;142:1009–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC.. Validity of self-reported waist and hip circumferences in men and women. Epidemiology 1990;1:466–473 [DOI] [PubMed] [Google Scholar]
- 20. Manson JE, Rimm EB, Stampfer MJ, et al. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet 1991;338:774–778 [DOI] [PubMed] [Google Scholar]
- 21. National Diabetes Data Group . Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979;28:1039–1057 [DOI] [PubMed] [Google Scholar]
- 22. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus . Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20:1183–1197 [DOI] [PubMed] [Google Scholar]
- 23. Fitzmaurice GM, Laird NM, Ware JH.. Applied Longitudinal Analysis (Wiley Series in Probability and Statistics). New York, John Wiley & Sons, 2012 [Google Scholar]
- 24. Chen L, Zhu Y.. Gestational diabetes mellitus and subsequent risks of diabetes and cardiovascular diseases: the life course perspective and implications of racial disparities. Curr Diab Rep 2024;24:244–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jun H-J, Austin SB, Wylie SA, et al. The mediating effect of childhood abuse in sexual orientation disparities in tobacco and alcohol use during adolescence: results from the Nurses' Health Study II. Cancer Causes Control 2010;21:1817–1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hall KD, Ayuketah A, Brychta R, et al. Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metab 2019;30:67–77.e3 e63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rauber F, Chang K, Vamos EP, et al. Ultra-processed food consumption and risk of obesity: a prospective cohort study of UK Biobank. Eur J Nutr 2021;60:2169–2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol 2019;15:288–298 [DOI] [PubMed] [Google Scholar]
- 29. Delpino FM, Figueiredo LM, Bielemann RM, et al. Ultra-processed food and risk of type 2 diabetes: a systematic review and meta-analysis of longitudinal studies. Int J Epidemiol 2022;51:1120–1141 [DOI] [PubMed] [Google Scholar]
- 30. Srour B, Fezeu LK, Kesse-Guyot E, et al. Ultraprocessed food consumption and risk of type 2 diabetes among participants of the NutriNet-Santé Prospective Cohort. JAMA Intern Med 2020;180:283–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sheludiakova A, Rooney K, Boakes RA.. Metabolic and behavioural effects of sucrose and fructose/glucose drinks in the rat. Eur J Nutr 2012;51:445–454 [DOI] [PubMed] [Google Scholar]
- 32. World Health Organization . Use of non-sugar sweeteners: WHO guideline summary. 2023. Accessed 24 March 2025. Available from https://iris.who.int/handle/10665/375565 [PubMed]
- 33. Salmerón J, Hu FB, Manson JE, et al. Dietary fat intake and risk of type 2 diabetes in women. Am J Clin Nutr 2001;73:1019–1026 [DOI] [PubMed] [Google Scholar]
- 34. Chassaing B, Koren O, Goodrich JK, et al. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015;519:92–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fardet A. Minimally processed foods are more satiating and less hyperglycemic than ultra-processed foods: a preliminary study with 98 ready-to-eat foods. Food Funct 2016;7:2338–2346 [DOI] [PubMed] [Google Scholar]
- 36. Singh RK, Chang H-W, Yan D, et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med 2017;15:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lin C-Y, Chen P-C, Lin Y-C, Lin L-Y.. Association among serum perfluoroalkyl chemicals, glucose homeostasis, and metabolic syndrome in adolescents and adults. Diabetes Care 2009;32:702–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lind PM, Lind L.. Endocrine-disrupting chemicals and risk of diabetes: an evidence-based review. Diabetologia 2018;61:1495–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.