Abstract
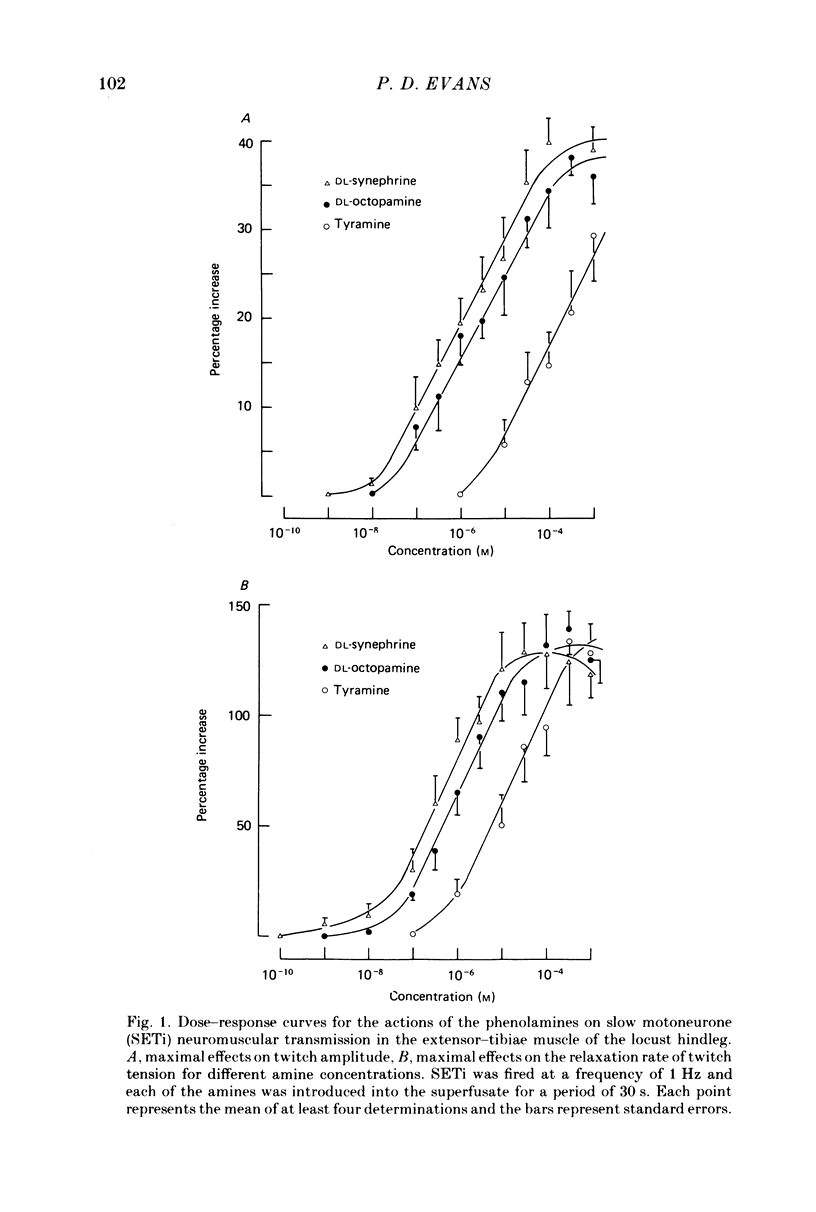
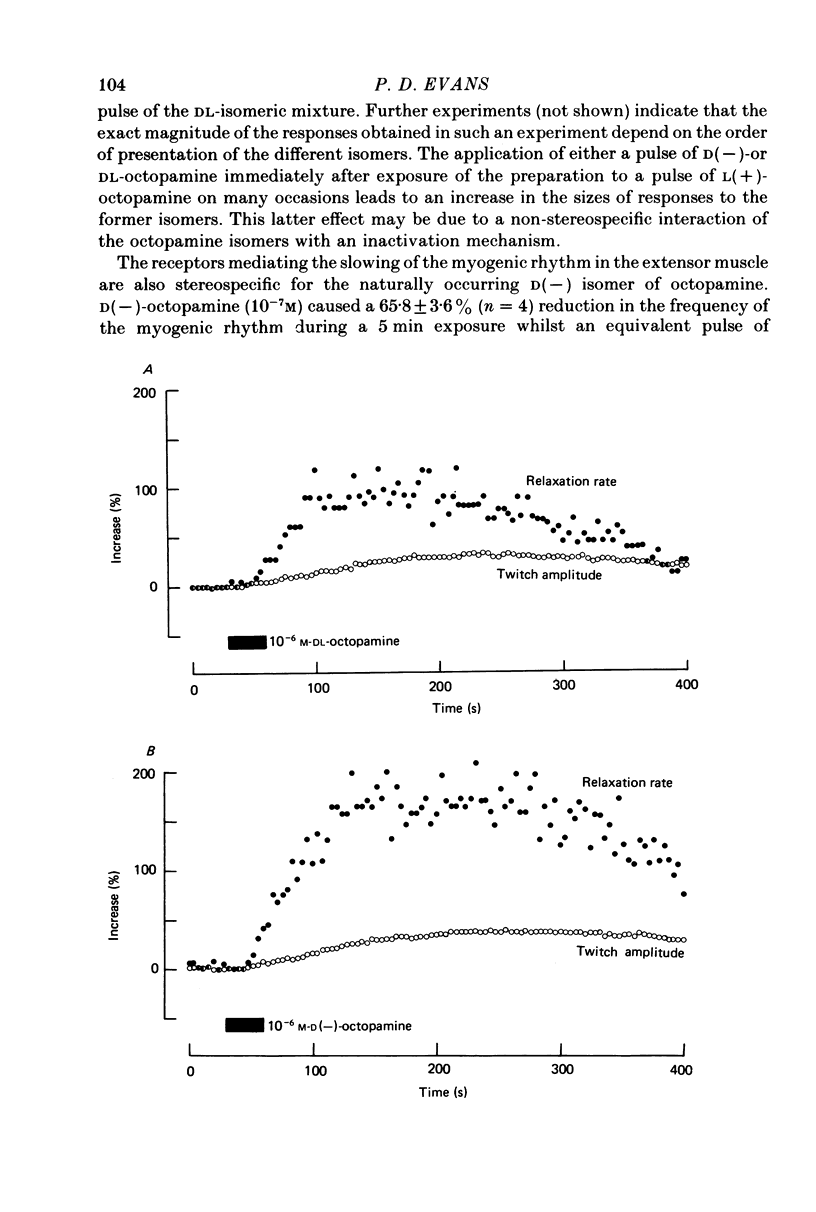
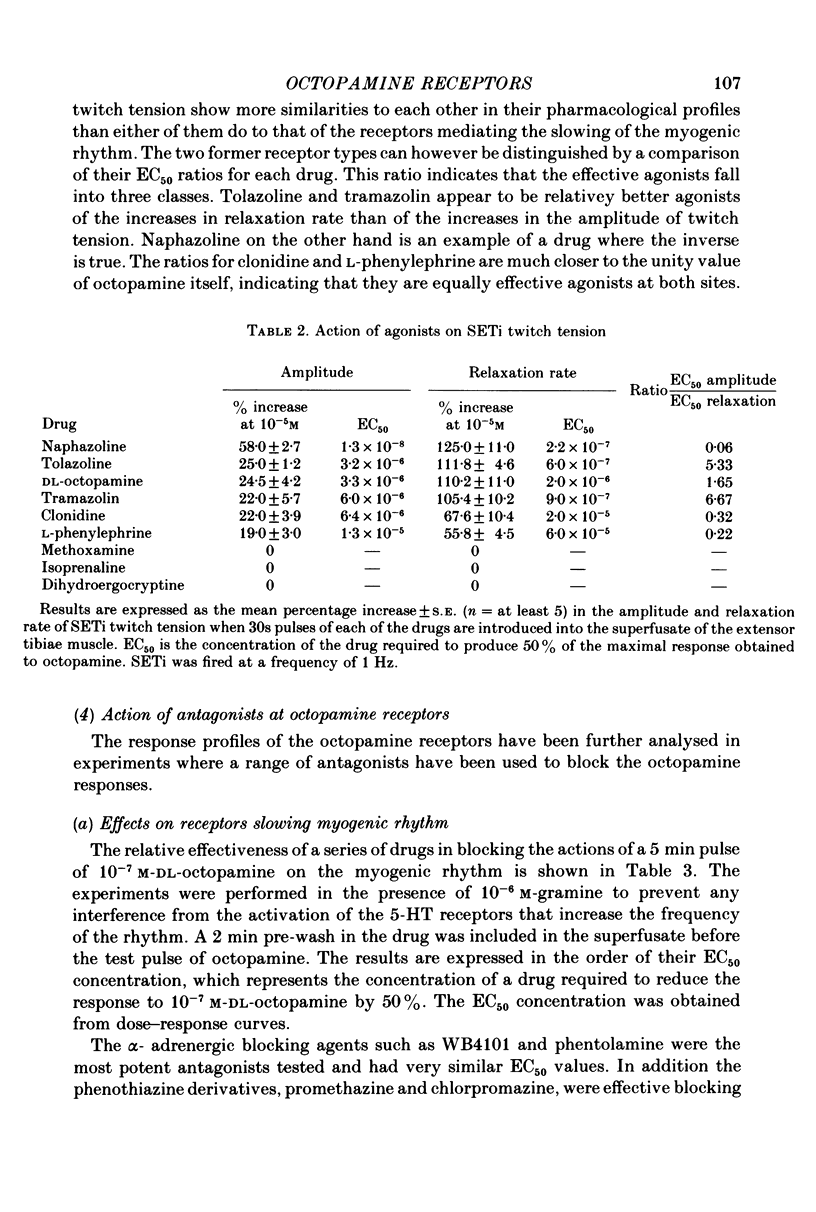
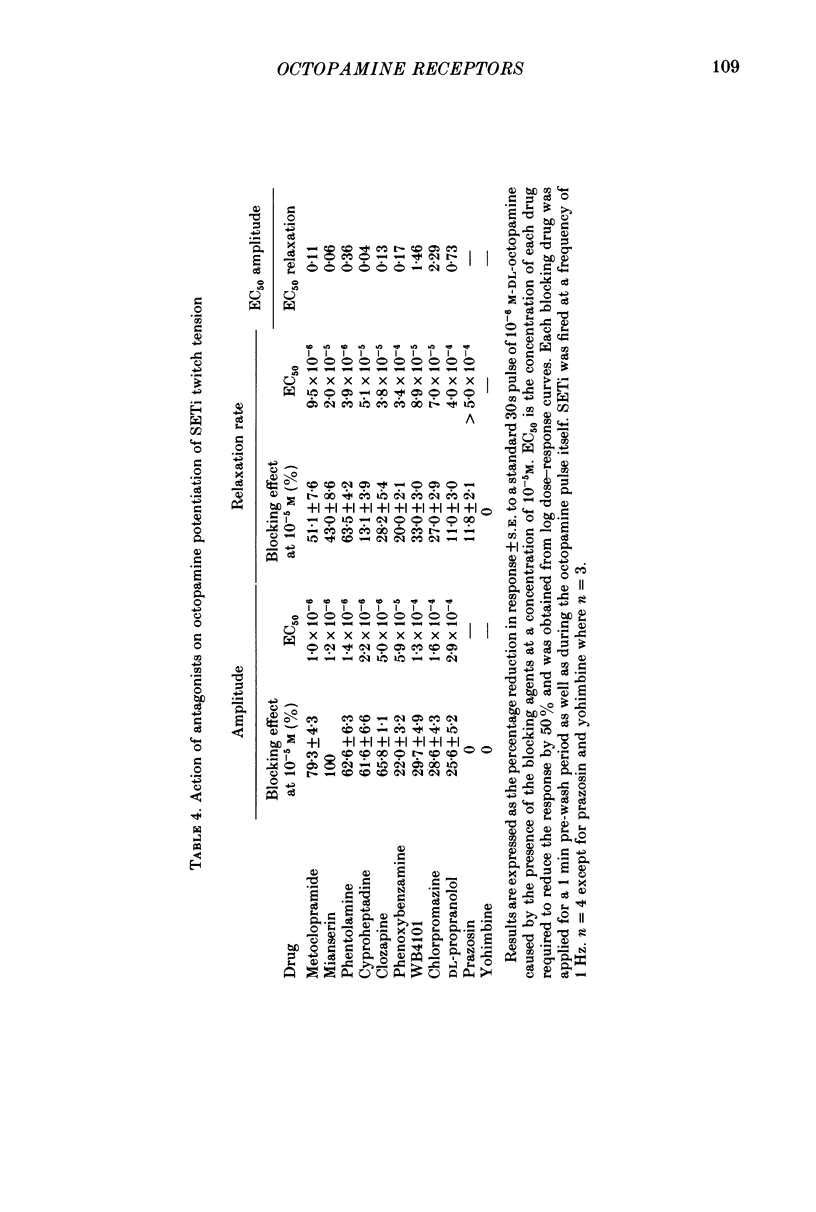
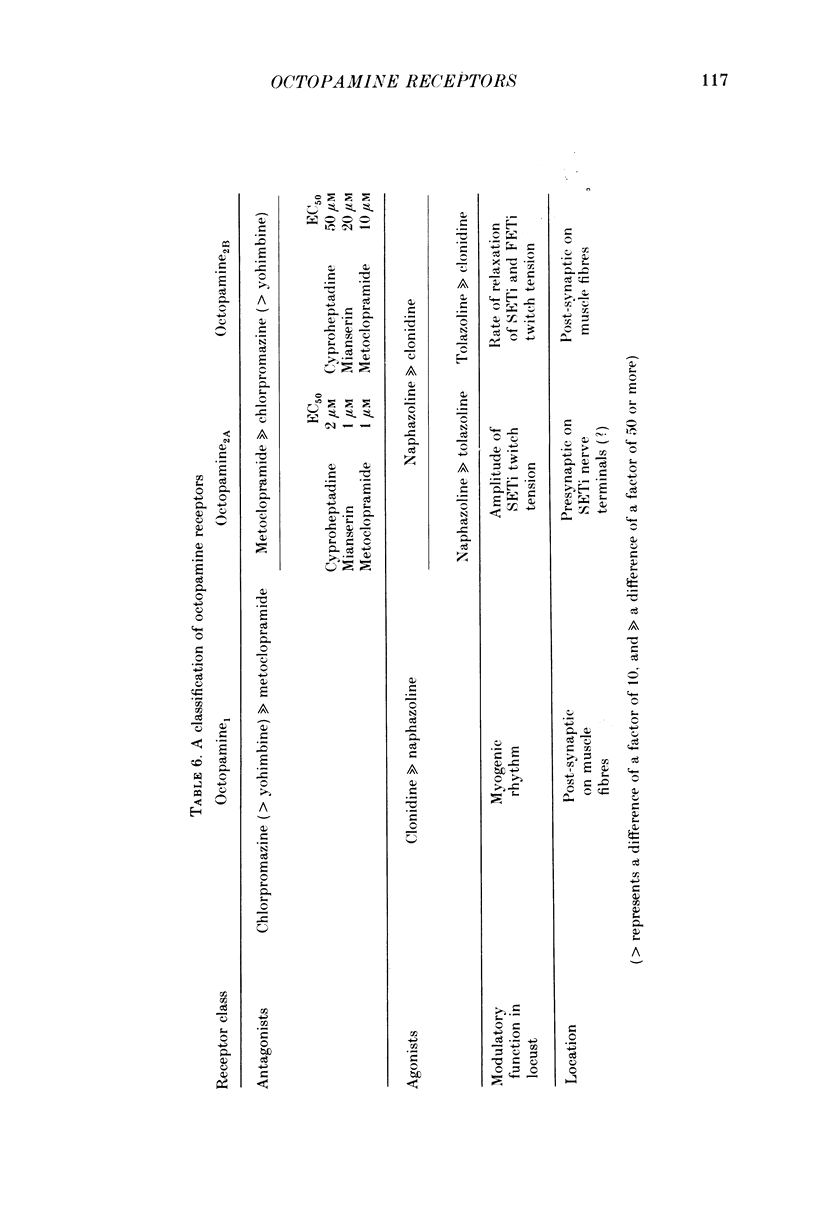
1. Three different pharmacological classes of octopamine receptor mediate the actions of octopamine on the locust extensor-tibiae neuromuscular preparation. A receptor classification scheme is proposed based on the results of detailed studies with agonists and antagonists. 2. Octopamine1 class receptors mediate the slowing of a myogenic rhythm found in a specialized proximal bundle of muscle fibres. Octopamine2A class receptors mediate the increase in amplitude of slow motoneurone twitch tension and octopamine2B class receptors mediate the increase in relaxation rate of twitch tension induced by firing either the fast or the slow motoneurones. 3. Octopamine1 receptors can be distinguished from the 2A and 2B classes since chlorpromazine (and yohimbine) are much better blocking agents than metoclopramide at the former receptors, whereas the converse is true for the latter class. Also clonidine is a more effective agonist than naphazoline for the former receptors and the converse is true for the latter class. 4. Octopamine 2A can be distinguished for octopamine 2B receptors since metoclopramide, mianserin and cyproheptadine show a strong preference for blocking the former class. Also naphazoline is a much better agonist than tolazoline at the former receptors and tolazoline is a much better agonist than clonidine at a latter. 5. The results are discussed in terms of the location of the various classes of octopamine receptors, their possible relationship to vertebrate alpha-adrenoreceptors, and the significance of the results for studies on octopamine receptors in the vertebrate central nervous system.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arbilla S., Langer S. Z. Differences between presynaptic and postsynaptic alpha-adrenoceptors in the isolated nictitating membrane of the cat: effects of metanephrine and tolazoline. Br J Pharmacol. 1978 Oct;64(2):259–264. doi: 10.1111/j.1476-5381.1978.tb17298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batta S., Walker R. J., Woodruff G. N. Pharmacological studies on Helix neuron octopamine receptors. Comp Biochem Physiol C. 1979;64(1):43–51. doi: 10.1016/0306-4492(79)90027-3. [DOI] [PubMed] [Google Scholar]
- Battelle B. A., Kravitz E. A. Targets of octopamine action in the lobster: cyclic nucleotide changes and physiological effects in hemolymph, heart and exoskeletal muscle. J Pharmacol Exp Ther. 1978 May;205(2):438–448. [PubMed] [Google Scholar]
- Berthelsen S., Pettinger W. A. A functional basis for classification of alpha-adrenergic receptors. Life Sci. 1977 Sep 1;21(5):595–606. doi: 10.1016/0024-3205(77)90066-2. [DOI] [PubMed] [Google Scholar]
- Bowser-Riley F., House C. R., Smith R. K. Competitive antagonism by phentolamine of responses to biogenic amines and the transmitter at a neuroglandular junction. J Physiol. 1978 Jun;279:473–489. doi: 10.1113/jphysiol.1978.sp012357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson A. D. Effect of drugs on luminescence in larval fireflies. J Exp Biol. 1968 Aug;49(1):195–199. doi: 10.1242/jeb.49.1.195. [DOI] [PubMed] [Google Scholar]
- Dao W. P., Walker R. J. Effect of cyproheptadine on the octopamine-induced responses in the mammalian central nervous system. Experientia. 1980 May 15;36(5):584–585. doi: 10.1007/BF01965816. [DOI] [PubMed] [Google Scholar]
- Dougan D. F., Wade D. N. Action of octopamine agonists and stereo isomers at a specific ostopamine receptor. Clin Exp Pharmacol Physiol. 1978 Jul-Aug;5(4):333–339. doi: 10.1111/j.1440-1681.1978.tb00682.x. [DOI] [PubMed] [Google Scholar]
- Dougan D. F., Wade D. N. Differential blockade of octopamine and dopamine receptors by analogues of clozapine and metoclopramide. Clin Exp Pharmacol Physiol. 1978 Jul-Aug;5(4):341–349. doi: 10.1111/j.1440-1681.1978.tb00683.x. [DOI] [PubMed] [Google Scholar]
- ERSPAMER V. Identification of octopamine as l-p-hydroxyphenylethanolamine. Nature. 1952 Mar 1;169(4296):375–376. doi: 10.1038/169375b0. [DOI] [PubMed] [Google Scholar]
- Evans P. D., Kravitz E. A., Talamo B. R. Octopamine release at two points along lobster nerve trunks. J Physiol. 1976 Oct;262(1):71–89. doi: 10.1113/jphysiol.1976.sp011586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans P. D., Kravitz E. A., Talamo B. R., Wallace B. G. The association of octopamine with specific neurones along lobster nerve trunks. J Physiol. 1976 Oct;262(1):51–70. doi: 10.1113/jphysiol.1976.sp011585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans P. D., O'Shea M. An octopaminergic neurone modulates neuromuscular transmission in the locust. Nature. 1977 Nov 17;270(5634):257–259. doi: 10.1038/270257a0. [DOI] [PubMed] [Google Scholar]
- Evans P. D., O'Shea M. The identification of an octopaminergic neurone and the modulation of a myogenic rhythm in the locust. J Exp Biol. 1978 Apr;73:235–260. doi: 10.1242/jeb.73.1.235. [DOI] [PubMed] [Google Scholar]
- Evans P. D. Octopamine: a high-affinity uptake mechanism in the nervous system of the cockroach. J Neurochem. 1978 May;30(5):1015–1022. doi: 10.1111/j.1471-4159.1978.tb12394.x. [DOI] [PubMed] [Google Scholar]
- Harmar A. J., Horn A. S. Octopamine-sensitive adenylate cyclase in cockroach brain: effects of agonists, antagonists, and guanylyl nucleotides. Mol Pharmacol. 1977 May;13(3):512–520. [PubMed] [Google Scholar]
- Hicks T. P., McLennan H. Actions of octopamine upon dorsal horn neurones of the spinal cord. Brain Res. 1978 Nov 24;157(2):402–406. doi: 10.1016/0006-8993(78)90050-1. [DOI] [PubMed] [Google Scholar]
- Hicks T. P. The possible role of octopamine as a synaptic transmitter: a review. Can J Physiol Pharmacol. 1977 Apr;55(2):137–152. doi: 10.1139/y77-022. [DOI] [PubMed] [Google Scholar]
- Hoyle G., Burrows M. Neural mechanisms underlying behavior in the locust Schistocerca gregaria. I. Physiology of identified motorneurons in the metathoracic ganglion. J Neurobiol. 1973;4(1):3–41. doi: 10.1002/neu.480040104. [DOI] [PubMed] [Google Scholar]
- Hoyle G. Distributions of nerve and muscle fibre types in locust jumping muscle. J Exp Biol. 1978 Apr;73:205–233. doi: 10.1242/jeb.73.1.205. [DOI] [PubMed] [Google Scholar]
- Hoyle G. Evidence that insect dorsal unpaired medican (DUM) neurons are octopaminergic. J Exp Zool. 1975 Sep;193(3):425–431. doi: 10.1002/jez.1401930321. [DOI] [PubMed] [Google Scholar]
- Jenner P., Marsden C. D., Peringer E. Proceedings: Behavioural and biochemical evidence for cerebral dopamine receptor blockade by metoclopramide in rodents. Br J Pharmacol. 1975 Jun;54(2):275P–276P. [PMC free article] [PubMed] [Google Scholar]
- Konishi S., Kravitz E. A. The physiological properties of amine-containing neurones in the lobster nervous system. J Physiol. 1978 Jun;279:215–229. doi: 10.1113/jphysiol.1978.sp012341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathanson J. A. Octopamine receptors, adenosine 3',5'-monophosphate, and neural control of firefly flashing. Science. 1979 Jan 5;203(4375):65–68. doi: 10.1126/science.214856. [DOI] [PubMed] [Google Scholar]
- Pearson K. G., Bergman S. J. Common inhibitory motoneurones in insects. J Exp Biol. 1969 Apr;50(2):445–471. doi: 10.1242/jeb.50.2.445. [DOI] [PubMed] [Google Scholar]
- Robertson H. A., Carlson A. D. Octopamine: presence in firefly lantern suggests a transmitter role. J Exp Zool. 1976 Jan;195(1):159–164. doi: 10.1002/jez.1401950116. [DOI] [PubMed] [Google Scholar]
- USHERWOOD P. N., GRUNDFEST H. PERIPHERAL INHIBITION IN SKELETAL MUSCLE OF INSECTS. J Neurophysiol. 1965 May;28:497–518. doi: 10.1152/jn.1965.28.3.497. [DOI] [PubMed] [Google Scholar]
- Williams L. T., Mullikin D., Lefkowitz R. J. Identification of alpha-adrenergic receptors in uterine smooth muscle membranes by [3H]dihydroergocryptine binding. J Biol Chem. 1976 Nov 25;251(22):6915–6923. [PubMed] [Google Scholar]


