Abstract
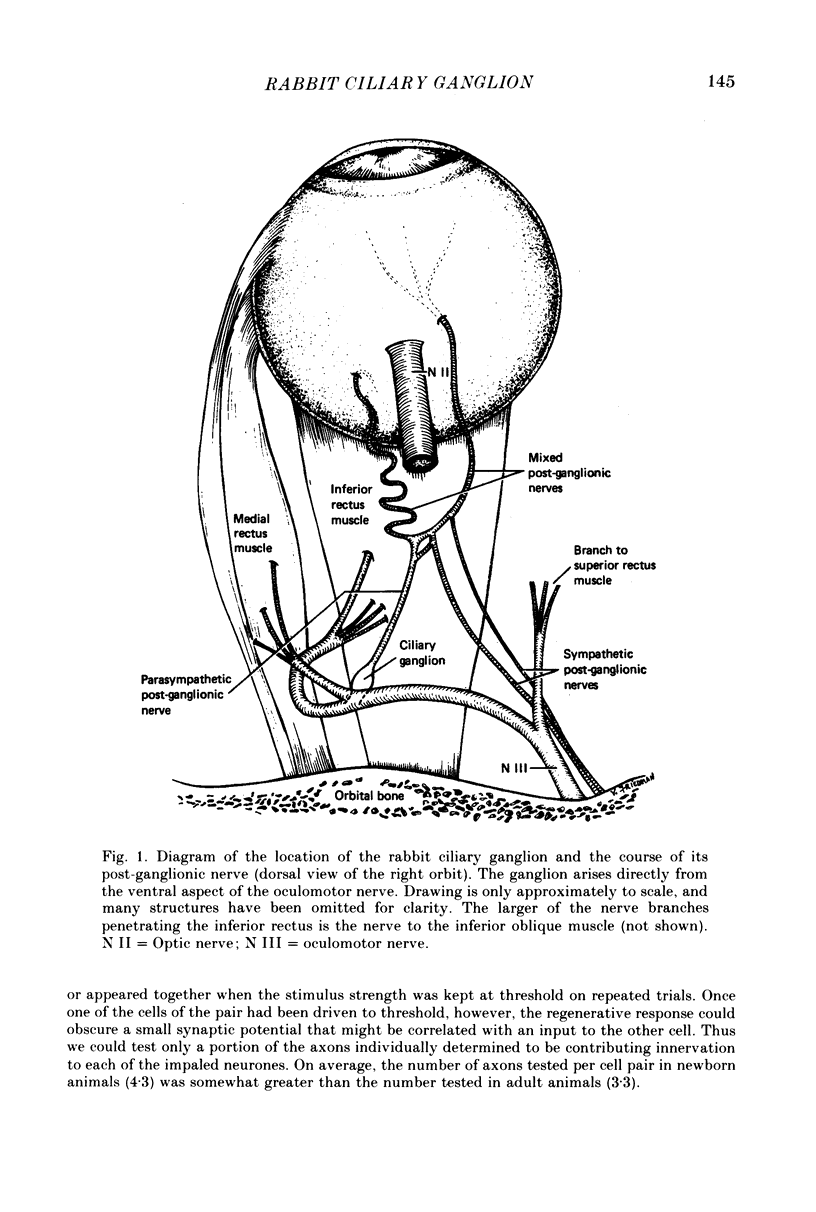
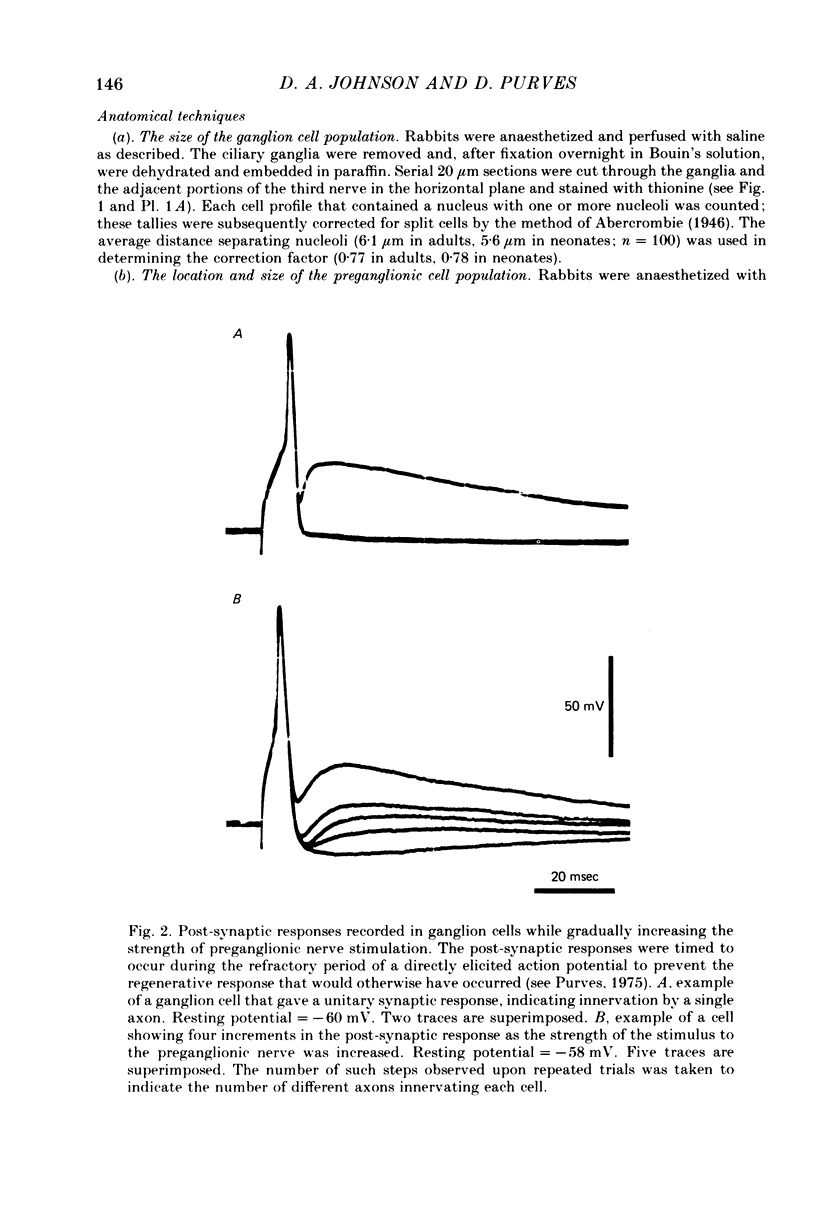
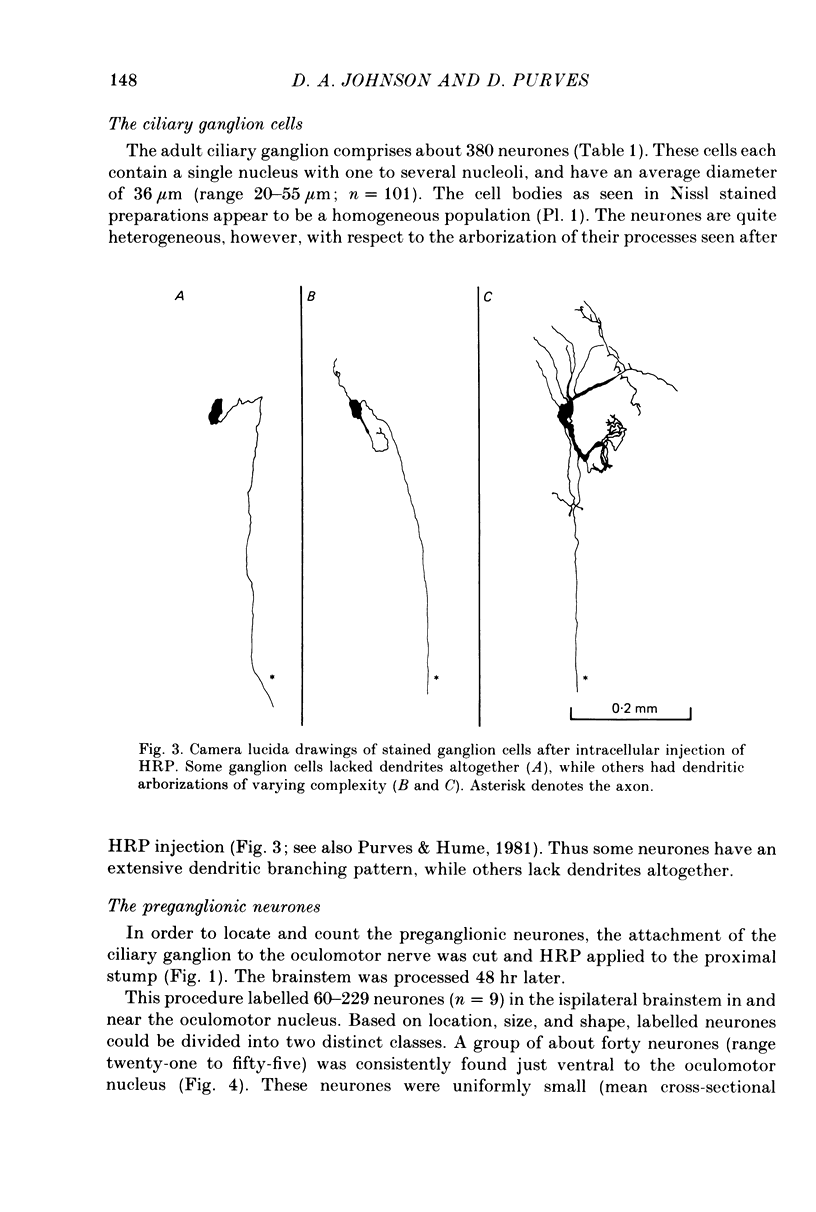
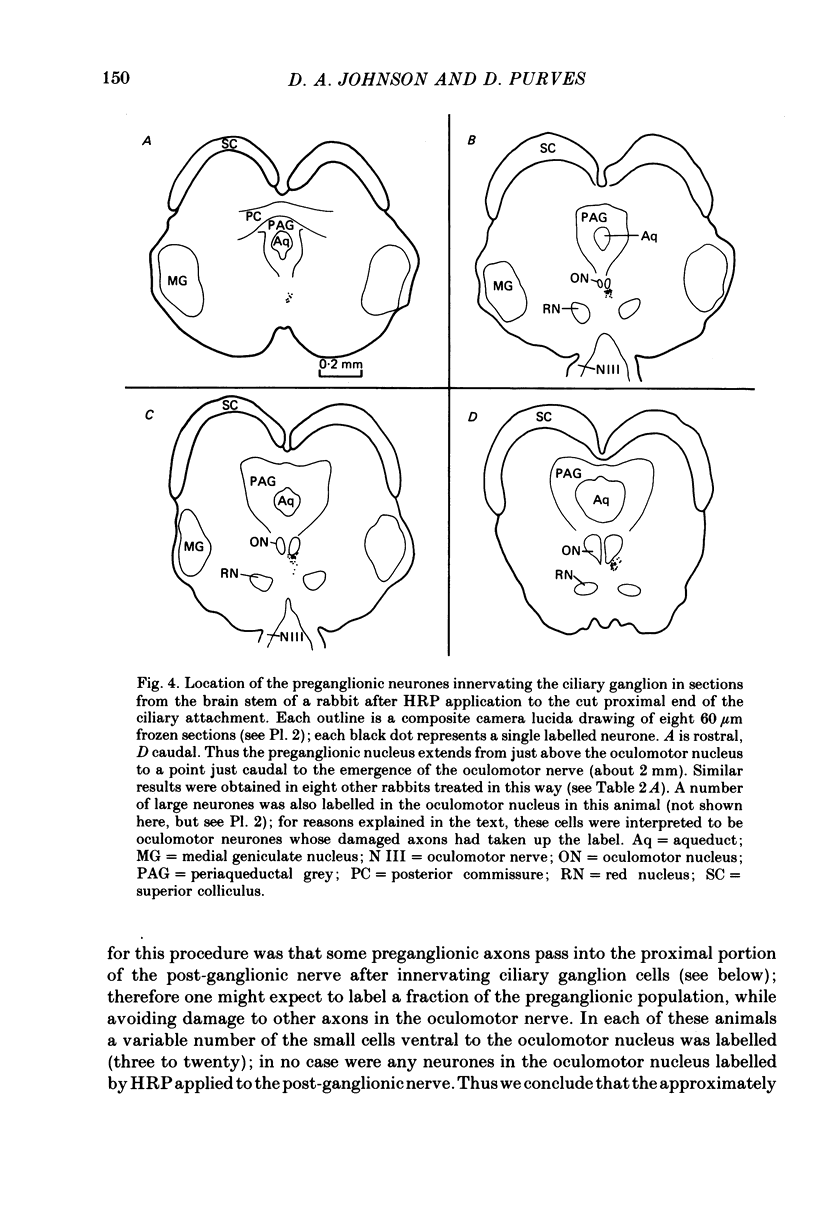
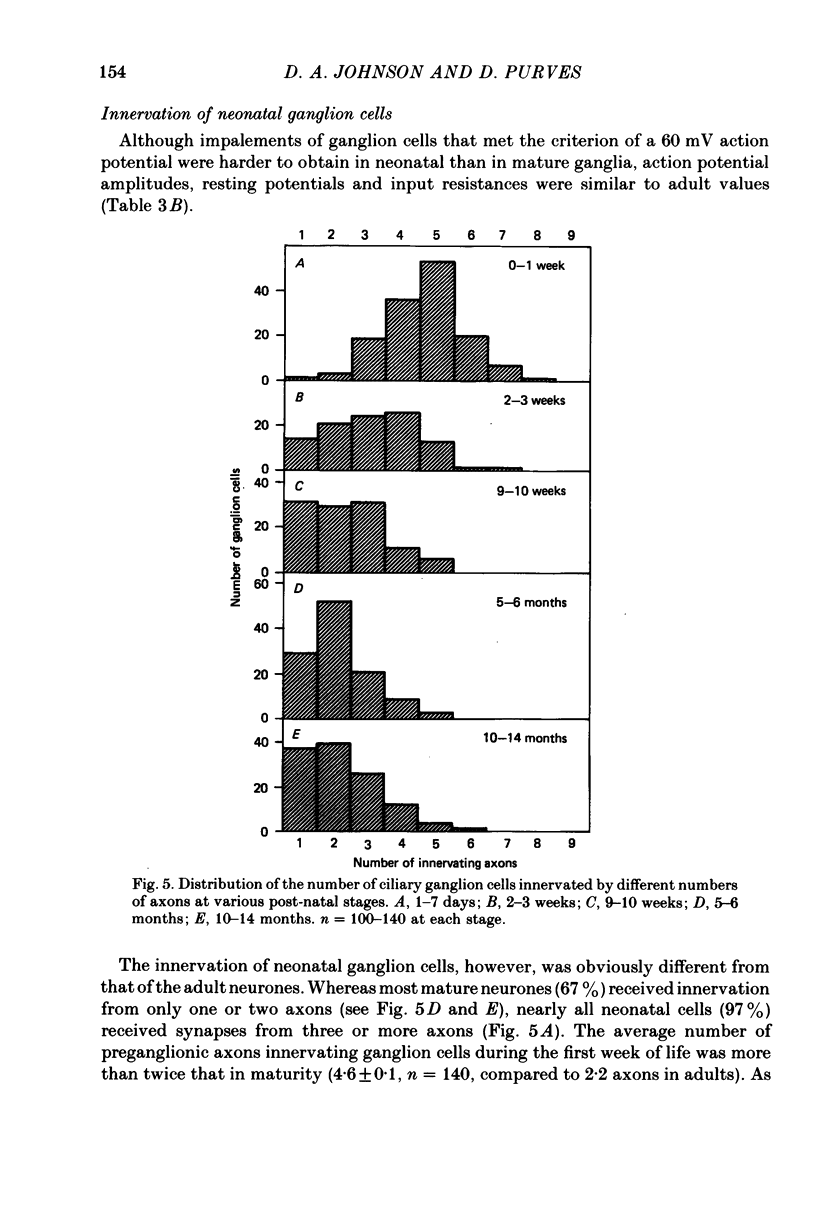
We have studied the innervation of adult and neonatal ciliary ganglia in the rabbit to determine the average number of ganglion cells innervated by each preganglionic neurone at different stages of development. 1. The adult ciliary ganglion comprises about 380 ganglion cells which are innervated by about forty preganglionic neurones. 2. Ciliary ganglion cells in adult rabbits are on average innervated by 2.2 different axons; in contrast, neonatal ganglion cells are on average innervated by 4.6 different axons. The transition to the adult pattern of innervation occurs gradually during the first few post-natal weeks. 3. The numbers of ganglion cells and preganglionic neurones do not change appreciably after birth. Accordingly, the loss of some innervation to individual neurones during post-natal development indicates that each preganglionic axon innervates progressively fewer ciliary ganglion cells. 4. The number of synaptic boutons found in ganglia at birth, however, is less than the number of synaptic boutons found in adult ganglia. 5. We conclude that synaptic connexions in this ganglion age gradually rearranged in early post-natal life such that each preganglionic neurone focuses an increasing number of synaptic contacts on a progressively smaller subset of the ganglion cell population.
Full text
PDF










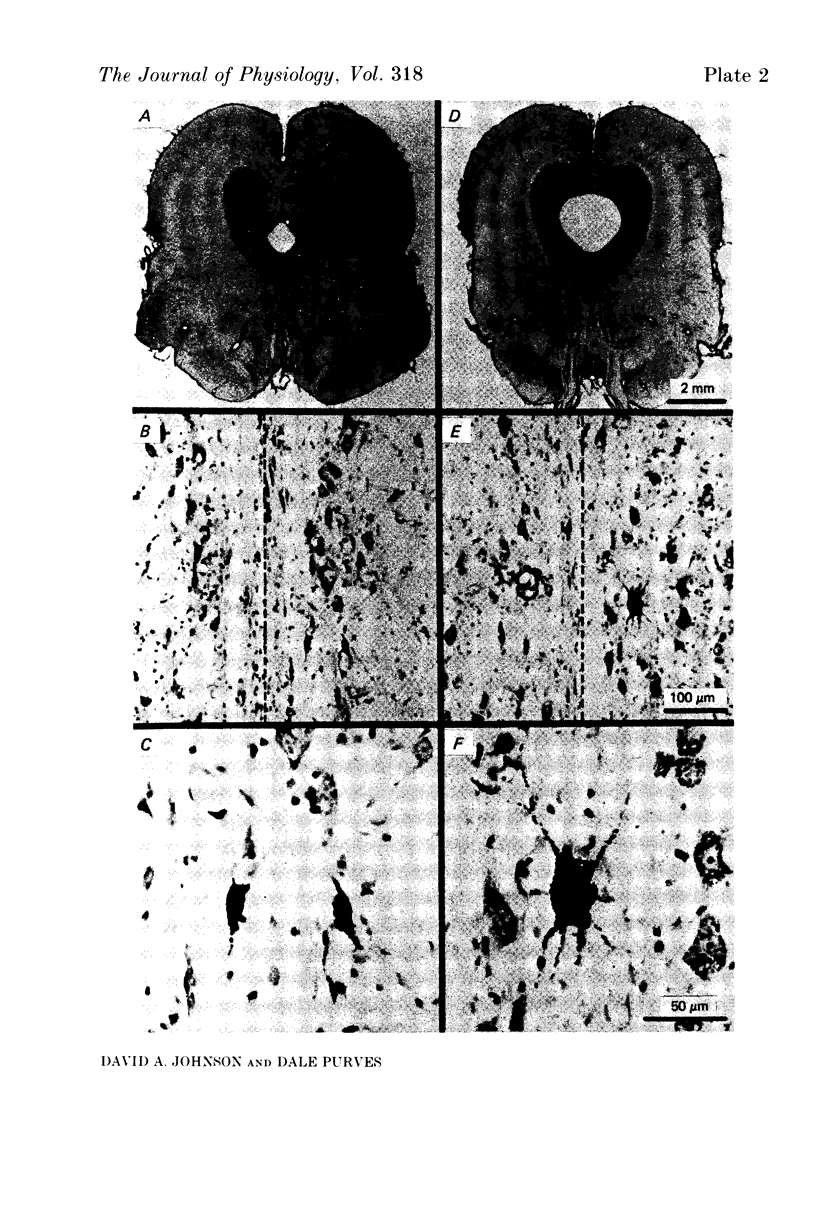
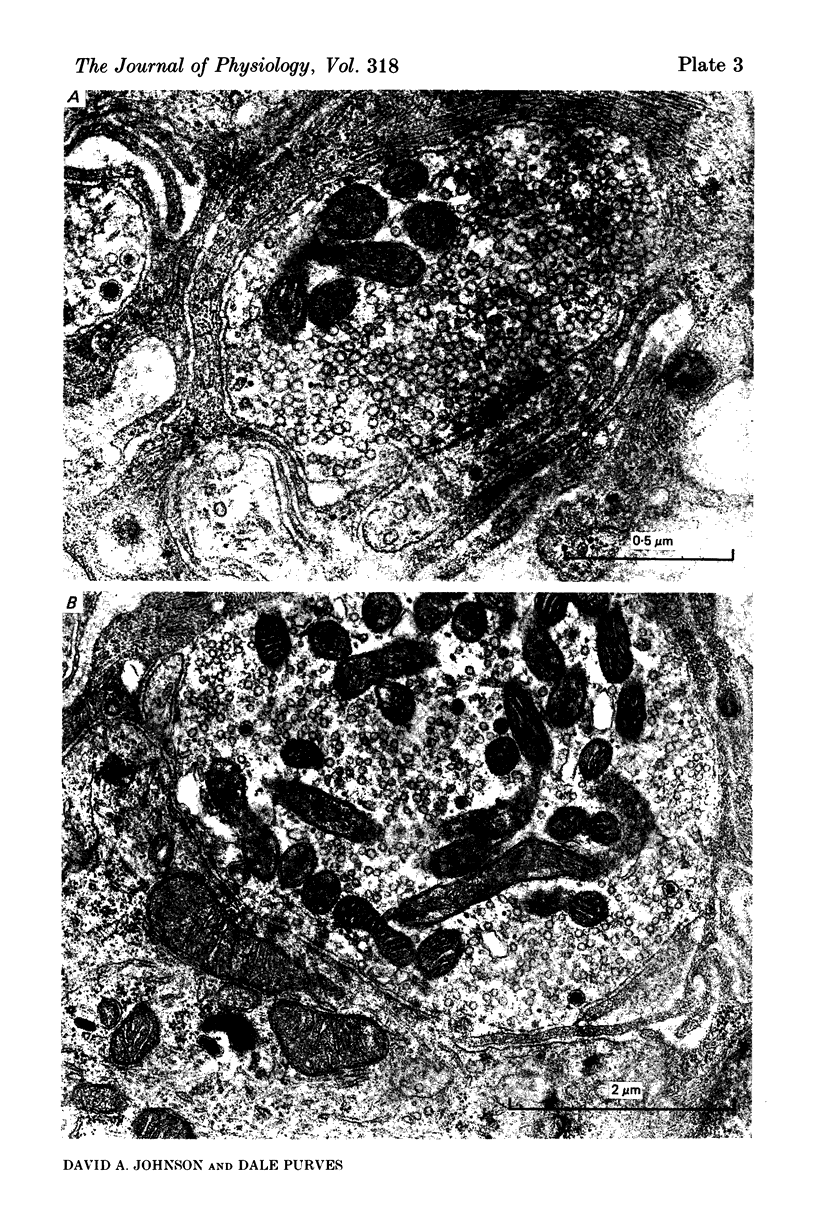

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akert K., Glicksman M. A., Lang W., Grob P., Huber A. The Edinger-Westphal nucleus in the monkey. A retrograde tracer study. Brain Res. 1980 Feb 24;184(2):491–498. doi: 10.1016/0006-8993(80)90816-1. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Jansen J. K., Van Essen D. Polyneuronal innervation of skeletal muscle in new-born rats and its elimination during maturation. J Physiol. 1976 Oct;261(2):387–422. doi: 10.1113/jphysiol.1976.sp011565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burde R. M., Loewy A. D. Central origin of oculomotor parasympathetic neurons in the monkey. Brain Res. 1980 Oct 6;198(2):434–439. doi: 10.1016/0006-8993(80)90757-x. [DOI] [PubMed] [Google Scholar]
- Hanker J. S., Yates P. E., Metz C. B., Rustioni A. A new specific, sensitive and non-carcinogenic reagent for the demonstration of horseradish peroxidase. Histochem J. 1977 Nov;9(6):789–792. doi: 10.1007/BF01003075. [DOI] [PubMed] [Google Scholar]
- Lichtman J. W. On the predominantly single innervation of submandibular ganglion cells in the rat. J Physiol. 1980 May;302:121–130. doi: 10.1113/jphysiol.1980.sp013233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman J. W., Purves D. The elimination of redundant preganglionic innervation to hamster sympathetic ganglion cells in early post-natal life. J Physiol. 1980 Apr;301:213–228. doi: 10.1113/jphysiol.1980.sp013200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman J. W., Purves D., Yip J. W. Innervation of sympathetic neurones in the guinea-pig thoracic chain. J Physiol. 1980 Jan;298:285–299. doi: 10.1113/jphysiol.1980.sp013081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman J. W. The reorganization of synaptic connexions in the rat submandibular ganglion during post-natal development. J Physiol. 1977 Dec;273(1):155–177. doi: 10.1113/jphysiol.1977.sp012087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D. Functional and structural changes in mammalian sympathetic neurones following interruption of their axons. J Physiol. 1975 Nov;252(2):429–463. doi: 10.1113/jphysiol.1975.sp011151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D., Lichtman J. W. Elimination of synapses in the developing nervous system. Science. 1980 Oct 10;210(4466):153–157. doi: 10.1126/science.7414326. [DOI] [PubMed] [Google Scholar]
- Purves D., Lichtman J. W. Formation and maintenance of synaptic connections in autonomic ganglia. Physiol Rev. 1978 Oct;58(4):821–862. doi: 10.1152/physrev.1978.58.4.821. [DOI] [PubMed] [Google Scholar]
- Rubin E., Purves D. Segmental organization of sympathetic preganglionic neurons in the mammalian spinal cord. J Comp Neurol. 1980 Jul 1;192(1):163–174. doi: 10.1002/cne.901920111. [DOI] [PubMed] [Google Scholar]
- Smolen A., Raisman G. Synapse formation in the rat superior cervical ganglion during normal development and after neonatal deafferentation. Brain Res. 1980 Jan 13;181(2):315–323. doi: 10.1016/0006-8993(80)90615-0. [DOI] [PubMed] [Google Scholar]
- Toyoshima K., Kawana E., Sakai H. On the neuronal origin of the afferents to the ciliary ganglion in cat. Brain Res. 1980 Mar 3;185(1):67–76. doi: 10.1016/0006-8993(80)90671-x. [DOI] [PubMed] [Google Scholar]
- WARWICK R. The ocular parasympathetic nerve supply and its mesencephalic sources. J Anat. 1954 Jan;88(1):71–93. [PMC free article] [PubMed] [Google Scholar]
- de Olmos J., Hardy H., Heimer L. The afferent connections of the main and the accessory olfactory bulb formations in the rat: an experimental HRP-study. J Comp Neurol. 1978 Sep 15;181(2):213–244. doi: 10.1002/cne.901810202. [DOI] [PubMed] [Google Scholar]