Abstract
1. The recovery of tension in mouse soleus was assayed 1-5 days after crushing the extramuscular nerve in muscles which had been previously either denervated by nerve crush, partly denervated by spinal nerve root section, or paralysed by I.M. injection of botulinum toxin. Recovery of tension following nerve crush in contralateral control muscles from the same mice was also measured. The muscles were then stained with zinc iodide-osmium and examined in the light microscope. 2. Recovery in control muscles began at about 50 hr after crush and was nearly complete by 5 days. Recovery began at about 50 hr after crush and was nearly complete by 5 days. Recovery began about 10 hr earlier and was more rapid in muscles denervated by crushing the muscle nerve 4 days before recrushing at the same site. 3. Paralysis 12 days earlier by intramuscular injection of botulinum toxin did not enhance recovery after nerve crush. The axons remained following partial denervation 6 days before nerve crush also regenerated at a rate similar to controls. 4. It is concluded that (1) nerves regenerate more quickly down a pre-degenerated pathway, (2) chromatolysis does not significantly enhance reinnervation, and (3) each motor axon regenerating after a crush is constrained to follow its own denervated pathway back into the muscle. 5. Histology was consistent with these conclusions, and also showed that end-plates in control muscles reinnervated after short periods of denervation were normal in appearance and possessed little "escaped' nerve growth. This was in contrast to end-plates which had been regenerated in muscle after a preceding nerve crush, botulinum toxin paralysis or partial denervation. This suggests that growth from nerve terminals is controlled locally within a muscle.
Full text
PDF


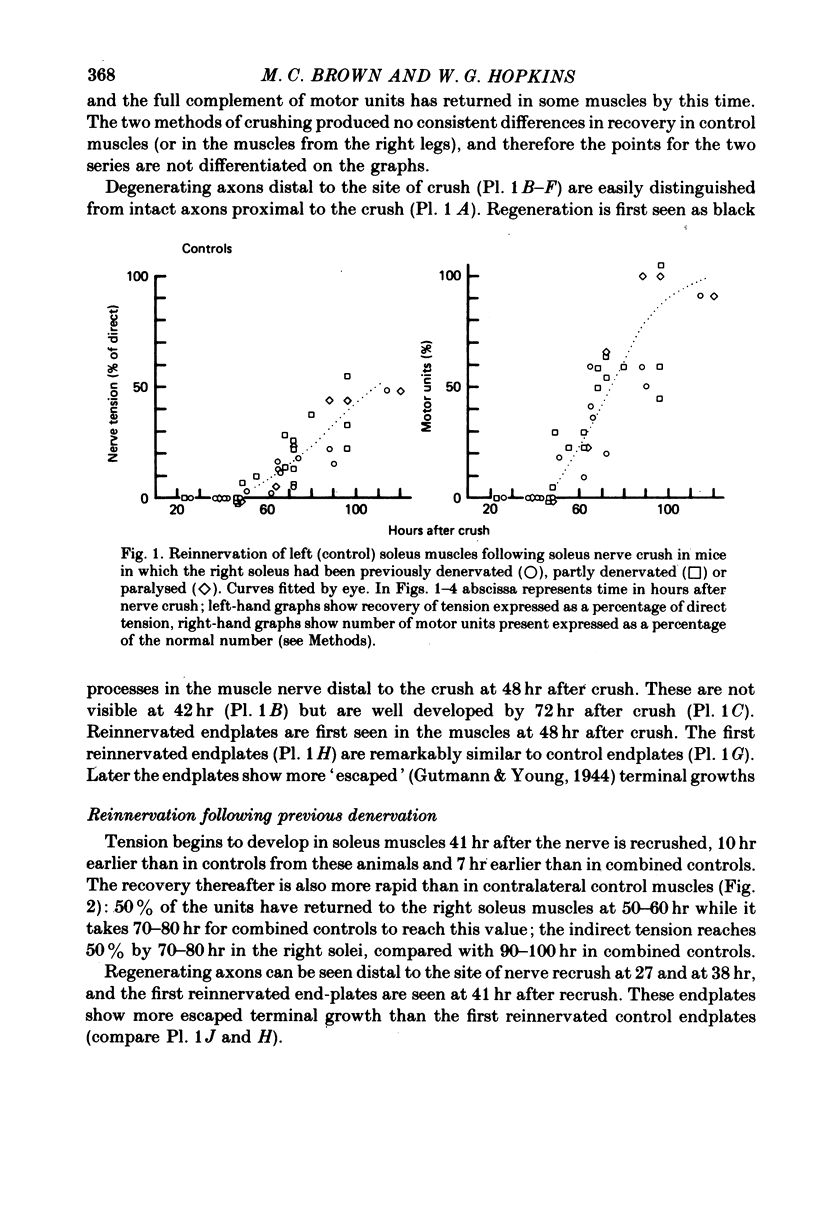
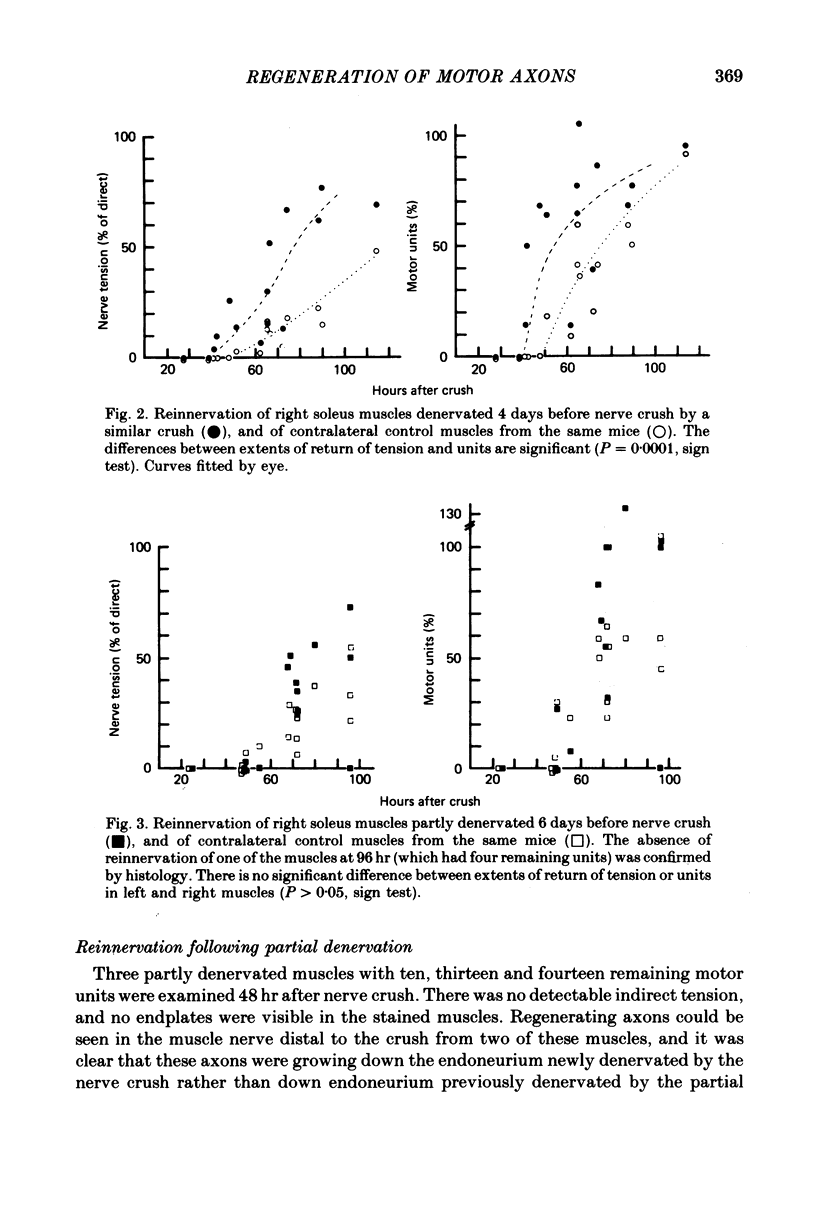





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agranoff B. W., Field P., Gaze R. M. Neurite outgrowth from explanted Xenopus retina: an effect of prior optic nerve section. Brain Res. 1976 Aug 27;113(2):225–234. doi: 10.1016/0006-8993(76)90938-0. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Holland R. L., Ironton R. Nodal and terminal sprouting from motor nerves in fast and slow muscles of the mouse. J Physiol. 1980 Sep;306:493–510. doi: 10.1113/jphysiol.1980.sp013410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Ironton R. Sprouting and regression of neuromuscular synapses in partially denervated mammalian muscles. J Physiol. 1978 May;278:325–348. doi: 10.1113/jphysiol.1978.sp012307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen L. W., Strich S. J. The effects of botulinum toxin on the pattern of innervation of skeletal muscle in the mouse. Q J Exp Physiol Cogn Med Sci. 1968 Jan;53(1):84–89. doi: 10.1113/expphysiol.1968.sp001948. [DOI] [PubMed] [Google Scholar]
- Gutmann E., Young J. Z. The re-innervation of muscle after various periods of atrophy. J Anat. 1944 Jan;78(Pt 1-2):15–43. [PMC free article] [PubMed] [Google Scholar]
- HOFFMAN H. Fate of interrupted nerve-fibres regenerating into partially denervated muscles. Aust J Exp Biol Med Sci. 1951 May;29(3):211–219. doi: 10.1038/icb.1951.25. [DOI] [PubMed] [Google Scholar]
- Hall-Craggs E. C., Brand P. Effect of previous nerve injury on the regeneration of free autogenous muscle grafts. Exp Neurol. 1977 Oct;57(1):275–281. doi: 10.1016/0014-4886(77)90063-2. [DOI] [PubMed] [Google Scholar]
- MAILLET M. [Modification of the Champy osmium tetroxide-potassium iodide technic. Results of its application to the study of nerve fibers]. C R Seances Soc Biol Fil. 1959;153:939–940. [PubMed] [Google Scholar]
- McQuarrie I. G., Grafstein B. Axon outgrowth enhanced by a previous nerve injury. Arch Neurol. 1973 Jul;29(1):53–55. doi: 10.1001/archneur.1973.00490250071008. [DOI] [PubMed] [Google Scholar]
- McQuarrie I. G., Grafstein B., Dreyfus C. F., Gershon M. D. Regeneration of adrenergic axons in rat sciatic nerve: effect of a conditioning lesion. Brain Res. 1978 Feb 3;141(1):21–34. doi: 10.1016/0006-8993(78)90614-5. [DOI] [PubMed] [Google Scholar]
- McQuarrie I. G. The effect of a conditioning lesion on the regeneration of motor axons. Brain Res. 1978 Sep 8;152(3):597–602. doi: 10.1016/0006-8993(78)91116-2. [DOI] [PubMed] [Google Scholar]
- Scheff S., Benardo I., Cotman C. Progressive brain damage accelerates axon sprouting in the adult rat. Science. 1977 Aug 19;197(4305):795–797. doi: 10.1126/science.887924. [DOI] [PubMed] [Google Scholar]
- Slack J. R., Hopkins W. G., Williams M. N. Nerve sheaths and motoneurone collateral sprouting. Nature. 1979 Nov 29;282(5738):506–507. doi: 10.1038/282506a0. [DOI] [PubMed] [Google Scholar]
- THOMAS P. K. The connective tissue of peripheral nerve: an electron microscope study. J Anat. 1963 Jan;97:35–44. [PMC free article] [PubMed] [Google Scholar]
- Watson W. E. The response of motor neurones to intramuscular injection of botulinum toxin. J Physiol. 1969 Jun;202(3):611–630. doi: 10.1113/jphysiol.1969.sp008830. [DOI] [PMC free article] [PubMed] [Google Scholar]



