Abstract
Despite having multiple treatment options, the overall outcomes, including the survival rates of non-small cell lung cancer (NSCLC) patients, remain relatively low, indicating the need to explore new approaches to achieve improved therapeutic responses. To that end, repurposed drugs such as metformin have been evaluated against many cancer types, including NSCLC. Metformin, a widely used oral hypoglycemic drug for type 2 diabetes, exhibits anticancer properties and synergy with several standards of care agents. In this review, we provide a comprehensive overview of the role and anticancer mechanisms of metformin-based combination approaches for the treatment of NSCLC. We logically discussed the experimental evidence from the in vitro and in vivo studies utilizing metformin alone, and then its combination with chemotherapeutic agents, targeted therapy, and immunotherapy. We also present clinical trials that underscore the beneficial and adverse outcomes of metformin use in combination with targeted therapy and chemotherapeutic agents, and emphasize the limitations and challenges for the treatment of diabetic and non-diabetic NSCLC patients. It appears that, regardless of the diverse anticancer mechanisms of this biguanide, the benefits may be confined to a specific patient subgroup, which opens new avenues to be explored for NSCLC treatment.
Keywords: Lung cancer, Drug repurposing, Metformin, Chemotherapy and targeted therapy, Cell signaling pathways
Background
Lung cancer is one of the leading causes of death worldwide, and in the United States, it is responsible for 20 – 21% of all cancer-related deaths [1–5]. Unlike other malignancies that have static or declining rates, a persistent increase in the incidence and prevalence, as well as mortality rates, has been documented with lung cancer [5–7]. The tumor cells begin to grow within the lining of the bronchi, bronchioles, or alveoli. Pathologically, lung cancer is categorized into two types: small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC). Of all lung cancer cases, 10 – 15% represent SCLC, which has a rapid growth rate and is divided into two categories: combined small cell carcinoma and small cell carcinoma, which is also known as oat cell cancer, and most of the SCLC cases are of oat cell cancer [8–10]. The most common type of lung cancer is NSCLC, which accounts for 80 – 85% of cases and is the most prevalent subtype with a poor prognosis [11–13].
NSCLC is classified into 5 types: adenocarcinoma, squamous cell carcinoma, large cell carcinoma, adenosquamous carcinoma, and well-differentiated neuroendocrine tumor [9]. The most common form of NSCLC is adenocarcinoma, accounting for 40% of the cases, and is usually found in the outer regions of the lungs [9]. Squamous cell carcinoma is the second most common form, accounting for 30% of the cases, and originates from the bronchial tubes [13]. The third type is a large cell carcinoma (15%), which generally grows faster and begins to develop in the outside edges of the lungs [9, 14]. About 0.4 – 4.0% of diagnosed NSCLCs are adenosquamous carcinoma, which is comprised of glandular and squamous cells [9, 14]. Neuroendocrine tumors have two subtypes, typical and atypical, of which typical neuroendocrine tumors have a better prognosis and are occasionally associated with neuroendocrine syndrome [9, 14, 15]. In NSCLC, survival rates depend on the tumor site. For example, the rate of survival for localized NSCLC (which does not spread outside of the lung) is 63% and 35% for regional NSCLC (which spreads outside of the lung) [16]. Of several risk factors involved in the development of lung cancer, globally, air pollution is the second leading cause of lung cancer [17]. The other common risk factors include exposure to: 1) cigarette smoking; 2) radon gas, arsenic, chromium, beryllium, and asbestos; 3) alcohol consumption; and 4) family history [18–21].
Given the types of lung cancer and various risk factors involved, to make a treatment decision, factors such as size and location of the tumor, histologic type, margins of surgery, grade of tumor, and role of pleura have been suggested to be considered before. The most common treatment approaches are: 1) surgery with anatomic resection like sublobular resection, segmentectomy, and lobectomy [22, 23]; 2) chemotherapy using platinum and taxol-based drugs such as cisplatin and paclitaxel [24, 25]; 3) radiation therapy, e.g., stereotactic body radiotherapy (SBRT) [26, 27]; 4) immunotherapy, where immune checkpoint inhibitors like programmed cell death protein-1 (PD-1) and cytotoxic T-lymphocyte associated protein 4 are mostly used and have been associated with promising outcomes in a subset of patients [28, 29]; and 5) targeted therapy, a drug treatment focusing on specific tumor cells having aberrated signaling pathway(s). For example, there are FDA-approved treatments (e.g., erlotinib, gefitinib, and Osimertinib) for the receptor tyrosine kinases (RTKs) family members, such as epidermal growth factor receptor (EGFR), which is hyperactivated or mutated in NSCLC patients [30, 31]. In addition, other treatment settings involve both neoadjuvant and adjuvant therapy, where the neoadjuvant phase consists of preoperative chemotherapy or radiotherapy, and the adjuvant phase involves postoperative therapy, during which drugs, including investigational agents such as metformin, may be administered [32, 33].
Drug repurposing
Drug repurposing or drug repositioning is defined as the identification of a new indication of pre-existing drugs with known mechanisms of action for a defined pathology or disease condition, including cancer [34]. The major advantage of using the repurposed drug is to reduce the timeline of investment and potential risks. For instance, with the drug reuse concept, a new drug can be developed within 3 – 12 years, whereas rudimentary procedures require up to 15 years or more to be fully developed. However, drug repositioning avoids the slow progression and side effects [35, 36]. These drugs are already approved by the regulatory bodies; thus, they have been through clinical trials and approved for human use directly with the portfolio, including dosing regimens, adverse events, drug-drug interactions, known pathways through which drugs are acting, and pharmacokinetics and pharmacodynamics parameters [37].
While several pharmacological agents have been explored for drug repurposing, we review metformin effects, as this is one of the widely used drugs that has been tested against various cancer models, including lung cancer [38]. The first evidence of metformin effects against cancer was reported by Libby et al. [39] in Tayside, Scotland (UK). In the large cohort study, a total of 13,334 diabetes patients with NSCLC were included, and the data demonstrated that metformin treatment resulted in greater cancer-free survival in the patients compared to the other group of patients treated with other drugs [39].
Metformin
It has been more than 60 years since metformin (1, 1-dimethyl biguanide hydrochloride) was first introduced for diabetic treatment, and since then it has become one of the most prescribed hypoglycemic (i.e., glucose-lowering) drugs in the world with its potential role for therapeutic applications in the future [40]. Metformin is a synthetic derivative of guanidine extracted from Galega officinalis, also known as French lilac, goat’s rue, Italian Fitch, Professor Wood, or Spanish sainfoin [41]. Earlier, from 1920 to 1930, guanidine derivatives, including metformin, were used to treat diabetes mellitus, which was later discontinued due to the reported toxicity and availability of insulin injections. In 1940, the drug was rediscovered in search of antimalarial agents, and finally, in 1957, the proper usage of metformin in the treatment of diabetes was first reported by a French physician, Dr. Jean Sterne [42].
Among the common mechanisms, metformin exerts its anti-cancer activity via two mechanisms: indirect and direct (Fig. 1). The indirect mechanisms of metformin involve: 1) reduction of inflammation Due to its ability to decrease the levels of inflammatory markers such as Toll-like receptors 2 and 4, tumor necrosis factor-α, and C-reactive protein [43–45]; 2) improvement in insulin resistance by decreasing the levels of circulating insulin, and glucose levels via inhibiting gluconeogenesis in the liver and stimulating glucose uptake in the muscle [45–47]. In addition, metformin increases the levels of glucagon-like peptide-1 (GLP-1) by enhancing the expression of GLP-1 receptors in the pancreas as well as reducing the breakdown of GLP-1 by inhibiting dipeptidyl peptidase-4 activity [48, 49]; and 3) causing body weight loss via decreasing appetite [50].
Fig. 1.
Antitumor mechanisms of metformin. Schematic representation of the indirect and direct mechanisms of metformin is presented. Akt protein kinase B, AMP adenosine monophosphate, ATP adenosine triphosphate, AMPK AMP-activated protein kinase, Deptor DEP domain-containing mTOR-interacting protein, 4E-BP1 eukaryotic translation initiation factor 4E binding protein 1, EGFR epidermal growth factor receptor, ERK extracellular signal-regulated kinase, FADD Fas-associated death domain, HER2 human epidermal growth factor receptor 2, IGF insulin growth factor, IGF-1R IGF-1 receptor, LKB1 liver kinase B1, MEK mitogen-activated protein kinase kinase, mTORC1 mammalian target of rapamycin complex 1, NF-κB nuclear factor-kappa B, OCT1 organic cation transporter 1, PD-1 programmed cell death protein-1, PD-L1 programmed cell death-ligand 1, PI3K phosphatidylinositol 3-kinase, PRAS40 proline-rich protein kinase B substrate of 40 kD, RAS rat sarcoma viral oncogene homolog, RHEB Ras homolog enriched in brain, S6K1 ribosomal protein S6 kinase B1, SOS son of sevenless, TRAIL tumor necrosis factor-related apoptosis-inducing ligand, TSC1/2 tuberous sclerosis proteins 1 and 2
Among the direct mechanisms in tumor cells, metformin binds with the organic cation transporter 1 (OCT1), which is required for its uptake in tissue. Metformin inhibits the mitochondrial complex I via increasing the ratio of adenosine monophosphate (AMP) to adenosine triphosphate (ATP), and activates liver kinase B1 (LKB1) and the AMP-activated protein kinase (AMPK) pathway, resulting in the inhibition of downstream protein complex, mammalian target of rapamycin complex 1 (mTORC1) [51, 52]. This mTORC1 constitutes the key regulators, such as proline-rich protein kinase B substrate of 40 kD, mammalian target of rapamycin (mTOR), Dishevelled, Egl-10, and Pleckstrin (DEP) domain-containing mTOR-interacting protein (Deptor), Raptor, and mLST8. The inhibition of mTORC1 induces ribosomal protein S6 kinase B1 (S6K1, involved in cell growth, survival) and inhibits eukaryotic translation initiation factor 4E binding protein 1 (4E-BP1, which regulates protein synthesis) [51, 52]. AMPK phosphorylates tuberous sclerosis proteins 1 and 2 (TSC1/2), which act as tumor suppressors and regulate cell growth and division [51, 52]. TSC1/2 inhibits a downstream Ras homolog enriched in brain (RHEB) protein that regulates cell growth, proliferation, and regeneration via the mTOR pathway [51, 52]. Nevertheless, inhibition of the mTOR pathway has been observed with metformin without its effects on LKB1, AMPK, and TSC1/2 signaling, indicating its off-target mechanisms regulating the activation of the phosphatidylinositol 3-kinase (PI3K), protein kinase B (Akt), and mTOR signaling pathways in tumor cells [53, 54].
APMK activation decreases the expression level of programmed cell death-ligand 1 (PD-L1, which acts as a brake to prevent T cells via binding to an immune checkpoint protein, PD-1, expressed on T cells), which induces cytotoxic T-cell-mediated tumor cell death [51, 55]. Moreover, other signaling cascades such as EGFR/human epidermal growth factor receptor 2 (HER2), PI3K/Akt, insulin growth factor (IGF)/IGF-1 receptor (IGF-1R, which also activates PI3K/Akt), nuclear factor-kappa B (NF-κB), EGFR/HER3, rat sarcoma viral oncogene homolog (RAS)/rapidly accelerated fibrosarcoma (RAF)/mitogen-activated protein kinase (MEK)/extracellular signal-regulated kinase (ERK), and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), Fas-associated death domain (FADD) pathways, which play critical roles in regulating cell proliferation, cell cycle, and caspase-mediated apoptosis, are identified as the common targets of metformin in cancer chemoprevention, including in NSCLC models [56–59].
Importantly, metformin’s mechanisms of action are multifaceted and differ across different histologic subtypes of lung cancer with distinct pathological and genomic contexts. For example, in EGFR-mutant lung adenocarcinoma cells, metformin inhibits mitochondrial electron transport complex 1 to induce AMPK activation, leading to the inhibition of the mTOR pathway, which causes G0/G1 cell cycle arrest, mitochondrial apoptosis, and reduction of tumor growth [60, 61]. However, in LKB1-deficient NSCLC cells, metformin restores the inactive AMPK signaling despite the absence of the canonical upstream kinase, likely through engaging an alternative pathway [53]. In tumor protein p53 gene (TP53) mutant squamous cell carcinomas, metformin exploits metabolic dependencies created by higher glycolytic activity due to increased expression of glucose transporter 1 and hexokinase 2, for its anti-cancer mechanism [62]. Unlike NSCLC, in SCLC, metformin inhibits the PI3K/Akt pathway, but paradoxically activates the MEK/ERK pathway [63]. This indicates that the limited efficacy of metformin in SCLC could be enhanced by the combination of MEK/ERK pathway inhibitors.
Experimental evidence from the in vitro and in vivo studies with metformin or its combination with chemotherapy, targeted therapy, and immunotherapy
Metformin
MicroRNAs (miRNAs) are single-stranded small (21 – 22 nucleotides) non-coding RNA molecules, which regulate gene expression by recruiting messenger RNAs to the miRNA-induced silencing complex, resulting in diminishing mRNA 3’ translation region through base pairing [64, 65]. Among various miRNAs, miR-7 is identified as a potential tumor suppressor via its ability to target the EGFR pathway in glioblastoma and plays a critical role in regulating such disease conditions. Considering the anti-cancer properties of metformin and its ability to target miRNAs [66, 67], Dong et. al. [68] determined the effect of metformin on miR-7 regulation using the NSCLC model. The authors demonstrated that treatment with metformin or miR-7 mimic resulted in the inhibition of cell viability, cell migration, and cell invasion in a dose and/or time-dependent manner in the A549 cell line. In addition, it was found that miR-7 expression was upregulated by metformin through the AMPK pathway, as an AMPK inhibitor significantly reduced metformin and miR-7 mimic induced increased miR-7 expression [68]. These findings suggest that a decreased level of AMPK can downregulate the level of miR-7. Interestingly, metformin and miR-7 mimic reduced protein expression of p-Akt, p-mTOR, p-ERK1/2, and p-p65, which suggests that the combination treatment would exert an enhanced effect in downregulating NF-κB, mitogen-activated protein kinase (MAPK)/ERK, and Akt/mTOR pathways. These findings suggest that metformin upregulates miR-7 through AMPK and regulates different signaling pathways to exert its anti-cancer effects [68]. The summary of in vitro and in vivo studies with metformin or its combination with other drugs is shown in Table 1 [59, 68–78].
Table 1.
Summary of in vitro and in vivo studies with metformin, or its combination with anti-cancer agents
| Study type | Mouse model | Cell line(s) | Therapeutic agent(s) | Targets/ Pathways |
Findings | References |
|---|---|---|---|---|---|---|
| In vitro | – | A549 | Metformin | miR-7, AMPK, NF-κB, Akt/mTOR, MAPK/ERK | Decreased cell viability, migration, and invasion | [68] |
|
In vitro, in vivo |
Athymic nude mice |
H460, A549, H1650 |
Metformin | TRAIL | Increased apoptosis and decreased cell proliferation, and tumor growth | [59] |
| In vitro | – | A549 | Metformin + 2-dDG | Glycolytic pathway, p38, and AMPK | Increased cell cytotoxicity, ROS generation, mitochondrial membrane potential, and apoptosis | [69] |
| In vitro, in vivo | PDX73 and PDX111 models in SCID mice | A549, H1299 | Metformin + Cisplatin | KRAS/LKB1 | Decreased tumor volume, increased apoptosis, and inhibition of drug resistance | [70] |
|
In vitro, in vivo |
BALB/c male mice | Cisplatin resistant A459, H838 cell lines | Metformin + Cisplatin |
Nrf2 and HO-1 |
Overcomes chemoresistance, induces apoptosis, and decreases cell proliferation | [71] |
| In vitro, in vivo | Athymic nude mice |
A549, H1299 |
Metformin + Celecoxib | p53, p21, RAF/MEK/ERK and PI3K/Akt | Decreased cell proliferation, cell migration, and increased cell apoptosis and cell cycle arrest | [72] |
| In vitro | – | Ncl-H2087 | Metformin + Trametinib |
ERK, AMPK, PI3K/Akt/ mTOR |
Biphasic anti-tumor effect | [73] |
| In vitro | – | PC9R, PC9R/OS | Metformin + Gefitinib/Osimertinib | EGFR-TKIs resistance | Increased cell apoptosis and sensitized EGFR-TKIs-resistant NSCLC cells | [74] |
|
In vitro, in vivo |
Female BALB/c | A549, H460 | Metformin + Anlotinib |
AMPK/ mTOR/ACC/ HIF-1α and MAPK |
Decreased cell proliferation and intracellular ATP levels, and increased cell apoptosis | [75] |
| In vitro, in vivo, ex vivo | Male BALB/cAnN, Cg-Foxnlnu/crlNARC | A549, H1975, HCC827 | Metformin + Pemetrexed | AMPK, CDKN1B, Akt and cyclin D1 | Decreased cell proliferation and angiogenesis, induced cell cycle arrest, increased apoptosis, and decreased tumor growth | [76] |
|
In vitro, in vivo |
PBMCs-CDx mouse | A549, H460 | Metformin + Pembrolizumab | STING, TBK1 and TRF3 pathways | Overcome primary resistance to PD-1 inhibitors and reduce tumor growth | [77] |
| In vitro, in vivo | SPF nude C57BL/6 J mice | A549, H1299 | Metformin + Atezolizumab | AMPK-CEBPB-PD-L1 | Inhibited cell proliferation and tumor growth | [78] |
2-dDG 2-deoxy D-glucose, ATP adenosine triphosphate, miR-7 microRNA 7, AMPK AMP-activated protein kinase, NF-κB nuclear factor kappa-B, Akt protein kinase B, mTOR mammalian target of rapamycin, MAPK mitogen-activated protein kinase, ERK extracellular signal-regulated kinase, TRAIL tumor necrosis factor-related apoptosis-inducing ligand, LKB1 liver kinase B1, KRAS Kirsten rat sarcoma viral oncogene homolog, Nrf2 nuclear factor erythroid-2 related factor 2, HO-1 heme oxygenase-1, RAF rapidly accelerated fibrosarcoma, MEK mitogen-activated protein kinase kinase, EGFR-TKIs epidermal growth factor receptor-tyrosine kinase inhibitors, HIF-1α hypoxia-inducible factor-1α, CDKN1B cyclin-dependent kinase inhibitor 1B, STING stimulator of interferon genes, TBK1 TANK-binding kinase 1, CEBPB CCAAT-enhancer-binding protein beta, PBMCs peripheral blood mononuclear cells, PD-L1 programmed cell death-ligand 1, PDX patient-derived xenograft, PI3K phosphatidylinositol 3-kinase, TRF3 TATA-box-binding protein-related factor 3
As TRAIL exhibits an anti-cancer activity [79, 80], a study by Liu et al. [59] determined the effects of metformin on TRAIL-mediated effects using in vitro and in vivo models of NSCLC. The authors demonstrated that metformin inhibits the proliferation and survival of H460, A549, and H1650 NSCLC cell lines in a dose-dependent manner via inducing apoptosis as measured by increased cleavage of poly (ADP-ribose) polymerase (PARP), a nuclear enzyme involved in multiple cellular processes, including apoptosis, caspase-8, and caspase-3. To investigate the involvement of the TRAIL-death receptor pathway, metformin was found to induce the protein levels of TRAIL without altering the expression of TRAIL receptors, DR4 and DR5. This contradicts the hypothesis regarding the role of DR4 and DR5 in metformin-mediated apoptosis. As metformin induces TRAIL secretion, it was found that TRAIL-R2 Fc chimera protein, which is a human recombinant protein containing an extracellular domain and shortened intracellular domain of DR5 and Fc fragment of immunoglobulin G, neutralizes TRAIL’s ability to induce cell death. These findings indicate that metformin increases endogenous TRAIL expression in lung cancer, which is secreted into a conditioned medium to trigger autocrine stimulation and apoptosis via a death receptor [59]. Similar results were obtained in animal studies where reductions in tumor size and tumor growth were documented. Overall, these findings indicated that the activation of the TRAIL death receptor pathway is essential to promote the anti-cancer activity of metformin.
Metformin and chemotherapy
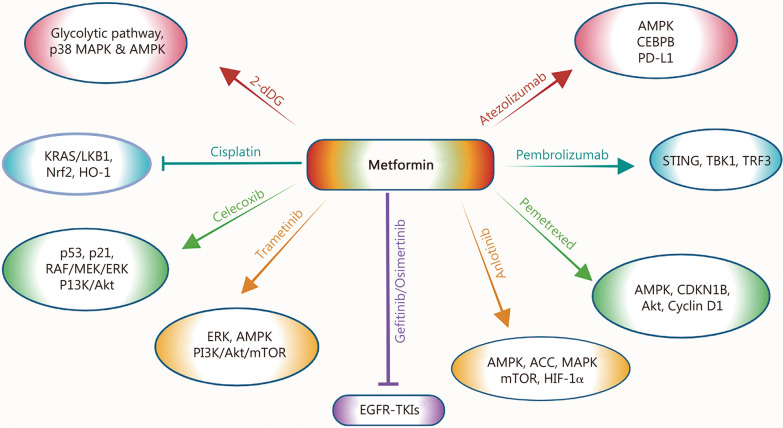
In addition to its use as a single agent, metformin has also been explored in combination with different chemotherapeutic agents [69–76]. The schematic representation of metformin mechanisms in combination with therapeutic agents is shown in Fig. 2.
Fig. 2.
The cellular mechanisms of metformin and its combination with chemotherapy, targeted therapy, and immunotherapy for NSCLC treatment are presented. Agents with anticancer properties, 2-dDG, cisplatin, celecoxib, gefitinib/osimertinib, anlotinib, pemetrexed, pembrolizumab and atezolizumab target diverse signaling cascades (shown above) to inhibit multiple cellular properties and/or overcome resistance mechanisms (as mentioned in Table 1). 2-dDG 2-deoxy D-glucose, Akt protein kinase B, AMP adenosine monophosphate, AMPK AMP-activated protein kinase, ASS acetyl-coA carboxylase, CDKN1B, cyclin-dependent kinase inhibitor 1B, CEBPB CCAAT/enhancer-binding protein beta, EGFR epidermal growth factor receptor, ERK extracellular signal-regulated kinase, HIF-1α hypoxia-inducible factor-1α, HO-1 heme oxygenase-1, KRAS Kirsten rat sarcoma viral oncogene homolog, LKB1 liver kinase B1, MAPK mitogen-activated protein kinase, MEK mitogen-activated protein kinase, mTORC1 mammalian target of rapamycin complex 1, Nrf2 nuclear factor erythroid-2 related factor 2, PD-L1 programmed cell death-ligand 1, PI3K phosphatidylinositol 3-kinase, RAF rapidly accelerated fibrosarcoma, STING stimulator of interferon genes, TBK1 TANK-binding kinase 1, TRF3 TATA-box-binding protein-related factor 3, TKIs tyrosine kinase inhibitors
Metformin and 2-deoxy D-glucose (2-dDG)
A study conducted by Hou et al. [69] investigated the relevance of targeting intracellular metabolism as an alternative approach to NSCLC treatment. To that end, considering the antiproliferative properties of 2-dDG that are mediated through the glycolytic pathway, the authors demonstrated that metformin and 2-dDG combination reduces the viability of A549 cells, which resulted in the induction of lactose dehydrogenase (LDH) release [69]. LDH is a cytoplasmic enzyme that is released upon damage to the plasma membrane. Besides, this combination resulted in dramatically increased levels of thiobarbituric acid, a marker of lipid peroxidation, indicating the degeneration of cell membrane lipids and induction of cell damage [69]. Additionally, compared to individual treatments, metformin and 2-dDG combination induced an increased cell death via inducing reactive oxygen species (ROS) generation and alterations in the mitochondrial membrane potential. Importantly, metformin and the 2-dDG combination resulted in the suppression of antioxidant enzymes such as superoxide dismutase, glutathione, and catalase, which process blocked by the pretreatment of antioxidant, N-acetyl cysteine [69]. While evaluating the effects of both drugs on DNA adducts and DNA damage, it was found that combination treatment induced DNA damage by increasing tail length and DNA adduct formation, as confirmed by immunohistochemical studies demonstrating the nuclear localization of 8-hydroxy-2’-deoxyguanosine (8-OHdg) [69]. Moreover, it was observed that combination therapy caused increased induction of early/late apoptosis as confirmed by caspase-3 activity, as well as increased activation of p38-MAPK and AMPK signaling pathways [69]. Overall, these findings provided the rationale for further exploration of metformin and 2-dDG combination for the treatment of NSCLC (Fig. 2).
Metformin and cisplatin
Notably, Kirsten rat sarcoma viral oncogene homolog (KRAS) is one of the frequently altered genes in NSCLC, and KRAS-activating mutations exhibit other concurrent mutations in genes, including LKB1 [81, 82]. While the exact roles of various KRAS mutations remained unclear, LKB1 functions to suppress tumor cell survival, tumor growth, and metabolic activity by targeting the AMPKα1 pathway, as AMPKα1 gets activated under metabolic stress to spare energy units and nutrients [83]. As metformin induces metabolic stress, Moro et al. [70] determined the effects of metformin to selectively target KRAS/LKB1 co-mutated NSCLC cells. The data demonstrated that metformin treatment resulted in significantly greater inhibition of cell growth and induction of apoptosis in A549 cells harboring KRAS/LKB1 commutation as compared to H1299 cells having wildtype KRASWT/LKB1WT, in a concentration-dependent manner [70]. While metformin caused higher apoptosis in A549 cells than in H1299 cells, G1 cell cycle arrest was specifically seen in H1299. To determine the differential sensitivity of metformin-induced apoptosis in A549 vs. H1299 cells and its dependence on the KRAS and LKB1 gene mutation status, as well as to evaluate metformin’s effect with cisplatin combination, the authors generated A549 LKB1WT and H1299 cells with different KRAS and LKB1 status, including KRASWT/LKB1WT, KRASWT/LKB1del, KRASG12C/LKB1WT, and KRASG12C/LKB1del. The data demonstrated that metformin and cisplatin caused increased apoptosis in A549 LKB1WT and H1299WT/WT cells with enhanced effects in cells having double mutation [70].
The in vivo studies utilized 2 patient-derived xenograft (PDX) models, PDX111 harboring KRASG12V/LKB1WT and PDX73 harboring KRASG12V/LKB1K287X mutations [70]. The data demonstrated that metformin inhibited the growth of PDX111 tumors at a lower dose, but had no effect on the growth of PDX73 tumors. However, at higher metformin dosage, tumor growth suppression was observed in both the PDXs, and that synergistic response was observed with cisplatin [70]. Importantly, the metformin and cisplatin combination targets CD133+ cancer stem cells in PDXs with KRAS/LKB1 co-mutation to prevent resistance to cisplatin. Taken together, metformin enhanced the apoptosis and anti-cancer activity of cisplatin in NSCLC with KRAS/LKB1 mutations and prevented resistance to cisplatin.
The efficacy of chemotherapeutic agents is limited due to drug resistance, mediated in part via ROS-induced oxidative stress [84–86]. Notably, hyperactivation of nuclear factor erythroid-2 related factor 2 (Nrf2), a master regulator of antioxidant defense response, has been shown to promote NSCLC development and enhance chemoresistance [87, 88]. To that end, Huang et al. [71] determined the mechanism of metformin and Nrf2 in cisplatin-induced ROS generation and chemoresistance in NSCLC cells. The data demonstrated that metformin and cisplatin combination synergistically reduced cell proliferation, enhanced apoptosis, and decreased the expression of Nrf2 and heme oxygenase-1 (HO-1), one of the antioxidant phase II detoxifying enzymes, in normal NSCLC H838 cell line, and cisplatin-resistant A549 cell line A549/DDP [71]. Further studies demonstrated that shRNA-mediated Nrf2 knockdown increased mitochondrial ROS generation and cisplatin sensitivity and cytotoxicity of the NSCLC cells; whereas overexpression of Nrf2 and HO-1 decreased the level of intracellular ROS by metformin and cisplatin [71]. These studies suggested that the detoxification of Nrf2 and HO-1 is essential for tumor cells to acquire cisplatin drug resistance [71].
Mechanistically, cisplatin-mediated increased expression of Nrf2 and HO-1 proteins, as well as phosphorylation of Raf and ERK1/2, were reduced by metformin treatment in both H838 and A549/DDP cell lines [71]. These findings indicated that the inactivation of the RAF-ERK pathway plays a critical role in the metformin-induced decreased expression of the Nrf2/HO-1 axis via ubiquitin proteasome-mediated degradation of the Nrf2 protein. Importantly, decreased tumor growth, reduced protein expression of the Nrf2/HO-1 axis, and increased apoptosis were observed in A549/DDP tumor xenografts by cisplatin and metformin treatment compared to monotherapy [71]. Moreover, Nrf2 expression on patients’ survival after neo-adjuvant chemotherapy was evaluated using tumor samples from the NSCLC patients. Patients were classified into 2 groups: one with decreased Nrf2 expression, including patients who exhibited low Nrf2 score after neo-adjuvant chemotherapy, and the second group with non-decreased Nrf2 expression, including patients exhibiting unchanged or higher Nrf2 score. In the non-decreased Nrf2 group, the majority of NSCLC patients were associated with poor survival and chemoresistance as compared to the patients in the Nrf2 decreased group, indicating that Nrf2 expression could be used as an important clinical marker to predict the prognosis of patients undergoing cisplatin-based neoadjuvant chemotherapy [71]. Taken together, cisplatin and metformin combination reduced Nrf2 protein expression by suppressing the RAS/RAF and ERK pathways to eliminate detoxification and induce ROS-mediated apoptosis (Fig. 2).
Metformin and celecoxib
Along similar lines, Cao et al. [72] determined the effects of celecoxib, a nonsteroidal anti-inflammatory drug (NSAID) with anti-proliferative properties with metformin to overcome drug resistance and side effects induced by NSAIDs. The data demonstrated that the metformin and celecoxib combination resulted in decreased cell viability and lower DNA proliferation rates in A549 and H1299 cell lines, indicating that the suppression of cellular DNA synthesis inhibits cell proliferation and induces cytotoxicity, as confirmed by low LDH secretion as compared with metformin and celecoxib alone [72]. Furthermore, the combination treatment induced cell apoptosis as measured by increased cleavage of caspase-3, -7, -8, -9, and PARP as well as upregulation of pro-apoptotic proteins, Bad and Bax, and reduced expression of anti-apoptotic proteins, B-cell lymphoma-extra large (Bcl-xl) and B-cell lymphoma 2 protein (Bcl-2), indicating the activation of both endogenous and exogenous pathways in eliciting apoptotic activity [72].
To validate intracellular metabolism changes, combined treatment was found to increase the level of intracellular ROS [72]. As excessive ROS can induce DNA damage, metformin and celecoxib treatments were found to increase the levels of gamma histone H2AX (γ-H2AX), a marker of DNA damage. These Changes promoted DNA injury resulting in increased activation of ataxia-telangiectasia mutated and checkpoint kinase 2, as well as G1 phase cell cycle arrest via induced expression of p53 and p21 signaling cascades [72]. Mechanistically, inhibition of the RAF/MEK/ERK and PI3K/Akt pathways by metformin and celecoxib was found to be involved in the induction of apoptosis [72]. Moreover, the metformin and celecoxib combination inhibited cell migration and invasion by reducing the expression of N-cadherin, p-FAK, and matrix metalloproteinase 9. Furthermore, the combination treatment caused decreased growth of A549 tumor xenografts without exerting a deleterious effect on the mice’s bodyweight, as well as induced tumor necrosis and tumor apoptosis [72]. Overall, these findings indicated that the metformin and celecoxib combination exerts promising antitumor effects via their ability to target multiple cell signaling pathways (Fig. 2).
Metformin and targeted therapy
Metformin and trametinib
The RAS/RAF/MEK/ERK pathway plays a central role in tumor cell proliferation and signals through receptor tyrosine kinases. A study by Ko et al. [73] determined the combined effect of metformin and trametinib, a MEK inhibitor, using NCI-H2087 NSCLC cells harboring neuroblastoma RAS viral oncogene homolog (NRAS) and v-RAF murine sarcoma viral oncogene homolog B (BRAF) mutations. These studies demonstrated that metformin treatment resulted in a dose-dependent increase in activation of ERK1/2 and AMPK and suppression of the PI3K/Akt/mTOR pathway. However, trametinib treatment exerted an opposing effect on ERK1/2 and AMPK activations, but did not substantially affect the PI3K/Akt/mTOR pathway. Interestingly, the combination of metformin and trametinib resulted in synergistic attenuation of metformin-induced increased ERK1/2 and AMPK activation as well as inhibition of cell viability and colony formation at lower doses compared to individual treatments [73]. However, at high doses, this combination caused an antagonistic effect and induced cell survival and proliferation, indicating that such a discrepancy could be attributed to the differences in the mutational status of NSCLC cells [73]. Overall, these findings indicated that a combination of metformin and trametinib results in a biphasic antitumor effect, which could be dependent on the alterations of signaling cascades, including ERK activity and mutational status of lung cancer cells.
Metformin and osimertinib
RTKs belong to the growth receptor family members and are responsible for tumor cell growth and angiogenesis. Thus, various inhibitors targeting RTKs [i.e., tyrosine kinase inhibitors (TKIs)] have been approved for the treatment of lung cancer [89, 90]. As drug resistance remained one of the major hurdles in limiting the efficacy of EGFR-TKIs for NSCLC treatment, a study by Han et al. [74] determined the effect of metformin in overcoming EGFR-TKI resistance using in vitro models and retrospective clinical data. For in vitro studies, the osimertinib-resistant PC9R/OR cell line was generated from gefitinib-resistant PC9R cells. Metformin treatment exerted synergistic effects and re-sensitized PC9R cells to gefitinib, and PC9R/OS cells to osimertinib. These findings indicate that metformin can overcome EGFR-TKIs resistance by sensitizing EGFR-TKI-resistant cells to EGFR-TKIs via lowering their median inhibition concentration (IC50) values. Besides, metformin treatment caused synergistic apoptosis induction when combined with both EGFR-TKIs [74] (Fig. 2).
Metformin and anlotinib
Similarly, Zhu et al. [75] determined the anti-tumor efficacy of metformin with anlotinib, an oral RTK inhibitor that targets vascular endothelial growth factor receptor (VEGFR), fibroblast growth factor receptor, platelet-derived growth factor receptor, and C-Kit [89, 91]. Metformin combination with anlotinib was found to potentiate the inhibition of cell viability and colony formation ability of A549 and H460 NSCLC cell lines compared to individual drug treatment [75]. In a xenograft model, the combined treatment also decreased tumor growth without eliciting any change in the body weight of mice. Furthermore, the combination treatment caused increased cell apoptosis via inducing BCL2-associated X protein (Bax) and decreasing Bcl-2 expression, as well as increasing caspase-3 and PARP cleavage as compared to monotherapy [75]. Moreover, metformin also induced AMPK activation and suppressed mTOR phosphorylation.
As acetyl-coA carboxylase (ACC) plays an important role in the biosynthesis and oxidation of fatty acids, the combination treatment was found to induce ACC activation, downregulate hypoxia-inducible factor-1α (HIF-1α) expression, and decrease intracellular ATP levels, resulting in the inhibition of ATP production [75]. Since HIF-1α promotes glycolysis to induce ATP production, these studies indicate that metformin and anlotinib combination targets the AMPK/mTOR/ACC/HIF-1α axis. Given that ROS plays an important role in cell apoptosis, and the oxidized nicotinamide adenine dinucleotide phosphate (NADP+)/reduced nicotinamide adenine dinucleotide phosphate (NADPH) ratio is considered a redox couple of oxidative stress, this combination treatment increased the intracellular NADP+/NADPH ratio to regulate homeostasis and promote oxidative stress [75]. This ROS-mediated cell death was found to be regulated by the MAPK pathway as metformin and anlotinib combination increased the expressions of MAPK family members, including p38, c-jun N-terminal kinase, and ERK1/2 [75]. Taken together, metformin and anlotinib combination exerts anti-cancer effects by activating AMPK/mTOR and MAPK/ERK pathways, and metformin sensitizes NSCLC to anlotinib; thus, this combination has the potential to be explored in clinical settings for malignancies, including NSCLC (Fig. 2).
Metformin and pemetrexed
Given that chemotherapy is the first choice of treatment when none of the driver mutations is found to be activated in NSCLC [92, 93], studies by Wang et al. [76] determined the anti-cancer effects of metformin and pemetrexed combination in NSCLC models. The data demonstrated that the metformin and pemetrexed combination significantly decreased the proliferation and colony formation ability of A549, H1975, and HCC827 cell lines. Besides, the combination treatment resulted in cell cycle arrest at the S phase and increased apoptosis, whereas a low cytotoxic effect was documented on normal Lung cells. Mechanistically, increased activation and expression of AMPK and cyclin-dependent kinase inhibitor 1B (CDKMB), as well as decreased activation and expression of Akt and cyclin D1, were involved in the regulation of cell cycle arrest [76]. In a xenograft model of BALB/cAnN mice, combination therapy reduced blood vessel density along with the length of the blood vessel network and blood vessel segments, suggesting the suppression of vessel growth and inhibition of angiogenesis [76]. Importantly, combination therapy resulted in additive inhibition of A549 orthotopic tumor growth as well as the percentage of endothelial cell markers, such as VEGF-positive area and platelet endothelial cell adhesion molecule-1 (PECAM-1)-positive area, compared to monotherapy. Moreover, reduced expression of tumor angiogenic markers such as endoglin, VEGFR, and carcinoembryonic antigen was documented by metformin and pemetrexed treatment [76]. Overall, these studies indicated that the metformin & pemetrexed combination exerts promising antitumor and antiangiogenic effects against NSCLC (Fig. 2).
Metformin and immunotherapy
Metformin and pembrolizumab
Most of the NSCLC harbor mutations in a tumor suppressor serine/threonine kinase 11 (STK11), also known as the LKB1 gene, one of the potential factors responsible for resistance to immune checkpoint inhibitors, such as PD-1 therapy [94, 95]. To investigate whether metformin could enhance the efficacy of immune checkpoint inhibitors in NSCLC harboring STK11 mutation, Wang et al. [77] conducted studies with metformin and pembrolizumab, a PD-1 inhibitor. Metformin treatment resulted in reduced cell viability and cell proliferation via increasing IFN-γ secretion by activated T cells in STK11 mutant A549 and H460 NSCLC cells. In a mouse model, a combination of metformin and pembrolizumab treatment significantly reduced the growth of H460 tumor xenografts and enhanced CD8+ T-cell infiltration [77]. Besides, T-cell-mediated tumor cell death by metformin was found to be mediated via increased activation of stimulator of interferon genes (STING) and downstream TANK-binding kinase 1 (TBK1) and TRF3 pathways.
Notably, AXIN-1, a protein encoded by the AXIN-1 gene, was found to be necessary for STING activation and served as a platform for several protein functions required for binding with STING [77]. Besides, metformin combination with pembrolizumab decreased cell viability and cell proliferation in AXIN-1+/+ H460 cells, indicating the specific role of axis inhibition protein 1 (AZIN-1) in the stabilization of STING to induce cell death. The key amino acid of AXIN-1 is Lys 107 (K107), which binds with another residue of STING at the Lys 150 (K150) site. K107 is a composition of multiple amino acid residues, including THR-192, GLU-195, GLN-159, GLU-188, and ASN-195 [77]. Metformin binds with the GLU-195 in AXIN-1 via hydrogen bonds to induce the binding of AXIN-1 and STING at the Lys 150 site [77]. STING was found to be the target of E3 ubiquitin ligases TRIM32 and TRIM56, which are important for recruiting TBK1 for STING activation. Overall, the findings demonstrated that metformin decreases K48-linked STING ubiquitination by reducing its binding with E3 ligand RNF5 to overcome the resistance of PD-1 inhibitors in NSCLC harboring STK11 mutations (Fig. 2).
Metformin and atezolizumab
Lu et al. [78] demonstrated that the antitumor effect of metformin is mediated through the regulation of AMPK and CCAAT-enhancer-binding protein beta (CEBPB)- PD-L1 expressions. PD-L1 is a ligand of PD-1, and CEBPB is a transcription factor having three isoforms: liver-enriched transcriptional activator protein (LAP)*, LAP, and liver-enriched inhibitory protein (LIP), where LAP* plays an important role in the inhibition of CEBPB. Besides, the activation or inhibition of CEBPB transcription depends on the ratio of LIP/LAP. The data demonstrated that metformin treatment inhibits cell proliferation and PD-L1 expression in a dose-dependent manner in A549 and H1299 NSCLC cell lines [96]. Next studies showed that PD-L1 has a binding site for CEBPB and that metformin upregulates CEBPB expression, suggesting that CEBPB could regulate PD-L1 transcription directly by binding to its specific site. Importantly, the overexpression of LAP in A549 cells resulted in increased cell proliferation and migration as well as PD-L1 upregulation, whereas overexpression of LIP in A549 cells had opposite effects on cell proliferation and PD-L1 expression [78]. Importantly, metformin upregulates AMPKα1 activation, LAP* and LIP expression, and downregulates PD-L1 expression to exert anti-tumor effects. In addition, AMPKα1 activation significantly reduced the expression of three isoforms of CEBPB, confirming that AMPKα1 can also regulate the expression of PD-L1 and CEBPB [78]. In a mouse model, metformin treatment decreased the growth of Lewis lung carcinoma tumor growth, and an enhanced antitumor effect was observed with anti-PD-L1 (atezolizumab) immunotherapy. Overall, these results confirmed that the metformin-induced anti-tumor effect is mediated via its ability to regulate the AMPKα1/CEBPB/PD-L1 signaling pathway (Fig. 2).
Evidence from the clinical studies with metformin or its combination with EGFR-TKIs, chemotherapy, and chemoradiotherapy
Metformin
In addition to in vitro and in vivo studies, clinical studies have determined the efficacy of metformin with or without other therapeutic agents in NSCLC patients. To that end, Xiao et al. [97] presented a meta-analysis on the association between metformin treatment and the risk of survival in lung cancer patients with type 2 diabetes. Overall, the data demonstrated that metformin treatment was associated with significantly decreased lung cancer incidence/risk and increased survival in lung cancer (both NSCLC and SCLC) patients. Interestingly, the subgroup analysis by ethnicity indicated that metformin’s protective effect on lung cancer risk was noticed in Asian patients but not in European patients. However, the beneficial effect of metformin on lung cancer survival was documented in both Asian and non-Asian lung cancer patients.
Notably, a study by Lu et al. [78] performed a retrospective analysis on lung adenocarcinoma patients who underwent surgery, and their tumor tissues were screened for PD-L1 gene expression. Tumor cells or tumor stromal cells with a score of ≥ 1% were defined as PD-L1 positive. Similar results were also observed in clinical specimen analysis of adenocarcinoma patients, demonstrating increased expression of CEBPB and decreased PD-L1 expression in the metformin-treated group compared to the control group. The survival probability analysis demonstrated that patients with low expression of CEBPB-LAP and PD-L1 had significantly better prognoses compared to those patients with high expression of CEBPB-LAP and PD-L1 [78]. Overall, these studies indicated that CEBPB regulates PD-L1 gene expression that could be modulated by metformin to achieve a better prognosis. The summary of clinical studies is given in Table 2 [74, 78, 96, 98–101].
Table 2.
Summary of clinical studies with metformin, or its combination with EGFR-TKIs or chemotherapeutic agents
| Study design/Patient subgroup selection | Diabetic/ Non-diabetic status |
Treatment arm(s) | Metformin dosage variation | Targets | PFS and OS | References |
|---|---|---|---|---|---|---|
| Adenocarcinoma patients | Not mentioned | Metformin | Retrospective study, metformin dose not given | CEBPB and PD-L1 | Low expression of CEBPB and PD-L1 was associated with better survival probability | [78] |
| Advanced-stage NSCLC patients with EGFR mutation | Diabetic | Metformin + EGFR-TKI | Retrospective study, metformin dose not given | EGFR mutation | Increase PFS and OS | [74] |
| Stage IIIB/IV lung adenocarcinoma patients with EGFR mutation | Non-diabetic | Metformin + EGFR-TKI | Started with 500 mg Bid and then reduced to 500 mg daily in patients who experienced drug-related adverse events | EGFR mutation | Longer median PFS and median OS | [98] |
| Stage IIIB/IV NSCLC with EGFR-ALK wild-type | Both diabetic and non-diabetic | Metformin + Chemotherapy | Started with 500 mg Bid for 1 week, and then increased to 1000 mg Bid if patients tolerated | – | No survival benefit in unselected patients | [96] |
| Stage IV chemotherapy naïve non-squamous NSCLC patients | Non-diabetic | Metformin + Pemetrexed + Carboplatin | 1st cycle started with 500 mg Bid. After a week, increased to 1000 mg first daily dose and 500 mg second dose. From the 2nd cycle, metformin was given 1000 mg Bid | STK11 mutation | No benefit in improving the PFS rate | [99] |
| Unresected stage IIIA or IIIB NSCLC | Non-diabetic | Metformin + Chemoradiotherapy | Started with 1000 mg daily. In week 2, the metformin dose increased to 1500 mg daily and then escalated to 2000 mg daily in week 3 | – | Poor PFS and OS, and higher adverse events | [100] |
| Unresected stage III NSCLC | Non-diabetic | Metformin + Chemoradiotherapy | Started with 500 mg Bid. In week 2, the metformin dose increased to 500 mg thrice a day | – | No benefit in improving survival | [101] |
NSCLC non-small cell lung cancer, EGFR epidermal growth factor receptor, Bid twice a day, CEBPB CCAAT-enhancer-binding protein beta, PD-L1 programmed cell death ligand-1, STK11 serine/threonine kinase 11, PFS progression-free survival, OS overall survival, TKI tyrosine kinase inhibitor, ALK anaplastic lymphoma kinase
Metformin combination with EGFR-TKIs
Regarding the metformin combination, a retrospective clinical study explored advanced-stage NSCLC patients with T2DM harboring EGFR mutations such as L858R and 19del who received either concurrent metformin or other hypoglycemic drugs with EGFR-TKIs treatment [74]. The data demonstrated that patients who received metformin treatment with EGFR-TKIs exhibited better response, including higher stable disease conditions, progression-free survival (PFS), and overall survival (OS) compared to non-metformin users [74]. Along similar lines, a prospective phase 2 randomized clinical trial was conducted to evaluate and compare the efficacy of a combination of metformin and EGFR-TKIs with EGFR-TKIs alone in stage IIIB-IV lung adenocarcinoma patients harboring an activating EGFR mutation [98]. The EGFR-TKIs included erlotinib hydrochloride, afatinib dimaleate, or gefitinib. The primary outcome was to assess the PFS, and secondary outcomes included objective response rate (ORR), disease control rate, OS, and safety. The data demonstrated that metformin treatment improves the clinical response of first/second-line EGFR-TKIs (i.e., significantly longer median PFS and median OS) compared to the EGFR-TKIs alone group [98]. However, the frequency of grade 3 or 4 adverse events (i.e., diarrhea, rash, nausea, and mucositis) was similar in both treatment groups. The limitations of these findings included the lack of randomization stratification per smoking status, the EGFR mutation profile/EGFR-TKIs, and conducting a non-double-blind study. Overall, these findings indicated that metformin addition could be used as a promising option to overcome EGFR-TKI resistance in NSCLC patients.
Metformin combination with chemotherapy
Given that metformin in combination with EGFR-TKIs was found to improve the PFS and OS of NSCLC patients, its efficacy was evaluated with chemotherapy regimens. To that end, Lee et al. [96] conducted a randomized phase II clinical trial in chemo-native, EGFR-anaplastic lymphoma kinase (ALK) wild-type, stage IIIB/IV NSCLC patients. The treatment group included metformin combination with chemotherapy (i.e., gemcitabine + carboplatin), and the control group received chemotherapy alone. The patient outcomes included the risk of disease progression and survival. Metformin combination significantly decreased the risk of progression and improved the survival of those squamous cell carcinoma (SqCC) patients who exhibited high fluorodeoxyglucose uptake in SqCC in positron-emission tomography imaging compared to non-SqCC patients [96]. However, no survival benefit was seen in patients whose insulin level was reduced after the metformin combination, or who had hyperinsulinemia. While alterations in TP53, RAS, and PI3K-Akt-mTOR genes were not predictive of PFS and OS, significantly increased levels of high-density lipoprotein (HDL)-cholesterol in the metformin group were associated with better PFS and OS [96]. Overall, these findings suggested that while metformin combination exerted a synergistic antitumor effect in selected SqCC patients, whose tumors are highly dependent on glucose metabolism, it did not show any survival benefits in unselected non-SqCC NSCLC patients.
Along similar lines, Verma et al. [99] conducted a single-arm phase 2 clinical trial to determine the efficacy of metformin with pemetrexed and carboplatin in treatment-naïve advanced-stage non-squamous NSCL patients. The primary outcome is 6 month PFS, and secondary outcomes are the safety, OS, ORR, proportion, and effect of STK11 mutation (a subgroup who are inherently resistant to immune checkpoint inhibitors) on the rate of 6 month PFS [99]. Out of 26 patients, 25 patients had a median PFS of 4.5 months, and the 6 month PFS rate was 28%. Forty percent of patients experienced partial response, 32% had stable disease, and the ORR was 72%. The mutation analysis was done in only 9 patients, and 2 patients with STK11 mutation had relatively low PFS (< 12 weeks), indicating limitations to precisely draw any conclusions on the efficacy of combination therapy in STK11-mutated patients. Overall, these findings indicated that while the combination therapy was found to be safe and tolerable with common gastrointestinal toxicities such as nausea, vomiting, and diarrhea, no improvement in the 6 month PFS rate was documented [99].
Metformin combination with chemoradiotherapy
Given that locally-advanced NSCLC (LA-NSCLC) patients experience poor OS with concurrent chemoradiotherapy, Tsakiridis et al. [100] determined the efficacy of metformin in the same context in a multicenter phase 2 randomized clinical trial, termed The Ontario Clinical Oncology Advanced lung Cancer Treatment with Metformin and Chemoradiotherapy (OCOG-ALMERA) study. The metformin was given concurrently with chemotherapy regimens: cisplatin + etoposide, cisplatin + vinorelbine, carboplatin + etoposide, or paclitaxel combined with radiotherapy. The assessment of the proportion of patients who experienced a failure event was the primary outcome, which included disease progression, distant metastases, death, and not following treatment or evaluations within 12 months. [100]. The studies demonstrated treatment failure in 18 out of 26 patients (69.2%) in the metformin arm compared to 12 out of 28 (42.9%) in the control (without metformin) arm. Notably, in the metformin arm, the 1 year PFS and OS rates were 34.8% and 47.4% compared to 63.0% and 85.2% in the control arm. In addition, the adverse events were higher in the metformin (53.8%) compared to the control (25%) arm [100]. The limitations included a lack of double-blinding, placebo control, and limited accrual due to the exclusion of patients with diabetes. Overall, these findings indicated that metformin combination with chemoradiotherapy was associated with poor treatment efficacy and adverse effects. While the underlying mechanisms of treatment failure and associated side effects with metformin are not adequately explored in the manuscript, these findings indicate that metformin should not be used with chemoradiotherapy in LA-NSCLC patients.
Likewise, Skinner et al. [101] conducted an open-label phase 2, NRG-LU001 randomized clinical trial to examine the effects of metformin combination with concurrent chemoradiation and chemoradiation alone in non-diabetic unresected stage III NSCLC patients. The primary outcome was 1-year PFS, and the secondary outcomes included OS, time to local–regional recurrence and metastasis, and adverse events. The data demonstrated no significant differences in the recurrence, metastasis, and adverse events; however, the 1 year PFS and 1 year OS rates in the metformin combination were 51.3% and 80.8% compared to 60.4% and 80.2% in the control arm [101]. The limitations of these findings included: 1) while 63.2% of patients received metformin, only 39% of patients were able to maintain it at the given dose without modifications; and 2) the absence of a placebo group. Overall, these findings suggest that while metformin in combination with concurrent chemoradiation was well tolerated, it did not improve the PFS and OS in NSCLC patients.
The possible reasons/underlying mechanisms of metformin failure in improving chemoradiation efficacy in OCOG-ALMERA and NRG-LU001 clinical trials may be as follows. (1) These trials did not include patients with preexisting diabetes, compared to other trials where improved EGFR-TKIs responses were noted with metformin in diabetic NSCLC patients [74, 98]. (2) Lack of evaluation of genetic and serum biomarker testing in these trials, as NSCLC patients harboring EGFR mutations and increased levels of serum HDL-cholesterol were associated with better PFS and OS in the metformin combination group [74, 96]. While it is questionable whether metformin should further be explored with chemoradiotherapy, emerging evidence from preclinical studies and phase 1b trial indicates that, in addition to EGFR-TKIs, metformin may augment immune-based therapy such as anti-PD1 and anti-PD-L1 immune checkpoint inhibitors [102–105].
Notably, the common inconsistencies between preclinical and clinical studies that affect the overall responses of metformin combination therapy are summarized in Table 3.
Table 3.
Disparities between preclinical studies and clinical trials with metformin combination regimens
| Parameters | Preclinical studies | Clinical trials | Translational inconsistencies |
|---|---|---|---|
| Metformin dose variation | Often 1 – 10 mmol/L in vitro and high doses in animal models | 500 – 2000 mg/d | Efficacy may not be achievable in human tumors |
| Treatment schedule | Started before, soon after tumor implantation, or during early tumor growth | Started in the advanced stage or metastatic disease | Early intervention in preclinical studies may enhance treatment response |
| Schedule for drug combination | Precisely timed at controlled intervals | Timing is based on patient tolerance and clinical logistics | Synergy is impacted if timing varies |
| Tumor model | Homogeneous cell lines, xenografts, or genetically-engineered mouse models | Heterogeneous tumors with several driver mutations | Higher genetic variability in human responses |
| Tumor microenvironment (TME) and immunity | Often lacks immune and stromal components | Consists of immune and stromal components | Human TME can hinder drug efficacy |
| State of health | Healthy, young, or non-diabetic | Often older with comorbid conditions (e.g., diabetes) | Comorbid conditions can affect drug tolerability |
| Study control | Tightly controlled environment and diet | Variable environment, diet, and medications | Variability in patients adds confounding factors |
|
Common endpoints/ outcome measures |
Suppression of tumor volume/biomarker changes in a short period of time | PFS and OS over months to years | Patient outcomes are often challenging to improve significantly |
PFS progression-free survival, OS overall survival
Conclusion, limitations, and future perspectives
Several in vitro and in vivo studies have confirmed the potential anti-cancer activity of metformin against NSCLC. Metformin targets various activities of tumor cells, including proliferation, migration, invasion, and angiogenesis, to induce cell cytotoxicity, cell cycle arrest, and apoptosis, resulting in decreased tumor growth and metastasis as documented in in vitro, in vivo, and ex vivo studies. The ability of metformin to target multiple cell signaling pathways provides a rationale for its exploration with other therapeutic agents for the treatment of NSCLC. Several classes of anti-cancer agents have been evaluated with metformin and found to exert synergistic antitumor effects. Notably, metformin has also been used to overcome drug resistance mechanisms. Importantly, metformin in combination with EGFR-TKIs has been shown to improve the clinical outcomes in NSCLC patients. Moreover, metformin can be used as a promising combination approach to target other potential signaling cascades involved in tumor development and/or impede the efficacy of therapeutic agents [106–108].
While metformin combination with chemotherapeutic agents resulted in synergistic antitumor effects in experimental in vitro and in vivo models, it did not show preferable outcomes in terms of PFS and OS in clinical settings, which remain the ongoing challenges and limitations to be explored in future research. Moreover, metformin’s side effects, such as gastrointestinal issues (e.g., nausea and diarrhea), B12 deficiency, and lactic acidosis, which are found to be associated with higher dosing regimens and longer treatment duration, also affect its clinical translation [109–111]. Notably, these deficiencies can cause anemia and peripheral neuropathy, which have significant overlap with the side effects of chemotherapy or radiotherapy that lung cancer patients experience. Furthermore, compared to other repurposed drugs such as statins and aspirin, which are associated with reduced lung cancer incidence/mortality, only a weak association was noticed between metformin and overall survival of lung cancer patients [100, 101, 112–116]. These studies suggest that greater attention should be given to dosing optimization and patient stratification (e.g., with characteristics similar to those of lung cancer patients who benefited from statin or aspirin) to explore metformin’s effect in combination with other therapeutic agents.
Along similar lines, biomarkers guide lung cancer treatment by predicting therapy outcomes or emerging resistance [117]. For example, EGFR mutations (exon 19 deletion or L858R) predict responses to osimertinib (i.e., 46% disease-free survival in stage IIA-IIIA NSCLC patients) [118]. Thus, the identification of potential biomarkers and their implications in selecting a subgroup of NSCLC patients who could benefit from metformin treatment is important to determine the best combination approaches, including strategies to avoid adverse events.
In addition, the emerging trends indicate that metformin analogs such as phenformin show promising and stronger anti-cancer effects than metformin, or exert synergistic efficacy with other therapeutic agents in experimental NSCLC models [119, 120]. Moreover, nanotechnology-based delivery of metformin via hyaluronan-coated mesoporous silica nanoparticles or PEGylated niosomal nanoparticles has not only shown an enhanced cellular uptake and cytotoxicity compared to free metformin, but also increased synergy with other drugs [121, 122]. Thus, these new strategies could also be explored in enhancing metformin-based combination approaches for NSCLC treatment. Taken together, metformin should be evaluated as a novel therapeutic strategy with careful consideration of other anti-cancer agents in clinical trials for the treatment of NSCLC.
Acknowledgements
Not applicable.
Abbreviations
- 2-dDG
2-deoxy D-glucose
- Akt
Protein kinase B
- AMP
Adenosine monophosphate
- AMPK
AMP activated protein kinase
- ATP
Adenosine triphosphate
- ASS
Acetyl-coA carboxylase
- CDKN1B
Cyclin-dependent kinase inhibitor 1B
- CEBPB
CCAAT-enhancer-binding protein beta
- Deptor
DEP domain-containing mTOR-interacting protein
- 4E-BP1
Eukaryotic translation initiation factor 4E binding protein 1
- EGFR
Epidermal growth factor receptor
- ERK
Extracellular signal-regulated kinase
- FADD
Fas-associated death domain
- GLP-1
Glucagon-like peptide-1
- HER2
Human epidermal growth factor receptor 2
- HIF-1α
Hypoxia-inducible factor-1α
- HO-1
Heme oxygenase-1
- IGF
Insulin growth factor
- IGF-1R
IGF-1 receptor
- KRAS
Kirsten rat sarcoma viral oncogene homolog
- LAP
Liver-enriched transcriptional activator protein
- LDH
Lactose dehydrogenase
- LKB1
Liver kinase B1
- LIP
Liver-enriched inhibitory protein
- MAPK
Mitogen-activated protein kinase
- MEK
Mitogen-activated protein kinase kinase
- mTORC1
Mammalian target of rapamycin complex 1
- NF-κB
Nuclear factor-kappa B
- Nrf2
Nuclear factor erythroid-2 related factor 2
- NSCLC
Non-small cell lung cancer
- OCT1
Organic cation transporter 1
- OS
Overall survival
- PARP
Poly(ADP-ribose) polymerase
- PD-1
Programmed cell death protein-1
- PD-L1
Programmed cell death-ligand 1
- PDX
Patient-derived xenograft
- PFS
Progression-free survival
- PI3K
Phosphatidylinositol 3-kinase
- PRAS40
Proline-rich protein kinase B substrate of 40 kD
- RAF
Rapidly accelerated fibrosarcoma
- RHEB
Ras homolog enriched in brain
- RAS
Rat sarcoma viral oncogene homolog
- ROS
Reactive oxygen species
- RTKs
Receptor tyrosine kinases
- SCLC
Small cell lung cancer
- S6K1
Ribosomal protein S6 kinase B1
- SqCC
Squamous cell carcinoma
- SOS
Son of sevenless
- STK11
Serine/threonine kinase 11
- STING
Stimulator of interferon genes
- TBK1
TANK-binding kinase 1
- TKIs
Tyrosine kinase inhibitors
- TRAIL
Tumor necrosis factor-related apoptosis-inducing ligand
- TRF3
TATA-box-binding protein-related factor 3
- TSC
Tuberous sclerosis protein
Authors' contributions
AT, VG, and RPS designed the study. AT generated the figures. AT, VG, and RPS contributed to writing and revising the manuscript. All authors read, edited, and approved the final manuscript.
Funding
This work was supported by the National Institutes of Health (RPS, R21 ES033806).
Data availability
Not applicable.
Declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Contributor Information
Anita Thyagarajan, Email: anita.thyagarajan@wright.edu.
Ravi P. Sahu, Email: ravi.sahu@wright.edu
References
- 1.Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74:12–49. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization (WHO). Global Health Estimates 2020: deaths by cause, age, sex, by country and by region, 2000-2019. Geneva: WHO, 2020. https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death.
- 3.Ashrafizadeh M, Mirzaei S, Hushmandi K, Rahmanian V, Zabolian A, Raei M, et al. Therapeutic potential of AMPK signaling targeting in lung cancer: Advances, challenges and future prospects. Life Sci. 2021;278:119649. [DOI] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2023;73(1):17–48. [DOI] [PubMed] [Google Scholar]
- 5.American Cancer Society. Facts & figures 2024. American Cancer Society. Atlanta, Ga. 2024. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/2024-cancer-facts-figures.html.
- 6.Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. Global cancer observatory: Cancer today. Lyon: International agency for research on cancer; 2020. https://gco.iarc.fr/today, Accessed Feb 2022.
- 7.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. [DOI] [PubMed] [Google Scholar]
- 8.Araujo LH, Horn L, Merritt RE, Shilo K, Xu-Welliver M, Carbone DP. cancer of the lung: non-small cell lung cancer and small cell lung cancer. In: Niederhuber JE, Armitage JO, Doroshow JH, Kastan MB, Tepper JE, editors. Abeloff’s Clinical Oncology. 6th ed. Philadelphia: Elsevier; 2020. p. 1108–58. [Google Scholar]
- 9.Chiang A, Detterbeck FC, Stewart T, Decker RH, Tanoue L. non-small cell lung cancer. In: DeVita VT, Lawrence TS, Rosenberg SA, editors. DeVita, Hellman, and Rosenberg’s Cancer: Principles and Practice of Oncology. 11th ed. Philadelphia: Lippincott Williams & Wilkins; 2019. [Google Scholar]
- 10.Hann CL, Wu A, Rekhtman N, Rudin CM. small cell and neuroendocrine tumors of the lung. In: DeVita VT, Lawrence TS, Rosenberg SA, editors. DeVita, Hellman, and Rosenberg’s Cancer: Principles and Practice of Oncology. 11th ed. Philadelphia: Lippincott Williams & Wilkins; 2019. p. 671–700. [Google Scholar]
- 11.Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Annals of Oncology. 2018;29(4):192–237. [DOI] [PubMed] [Google Scholar]
- 12.Wolf J, Seto T, Han JY, Reguart N, Garon EB, Groen HJM, et al. Capmatinib in MET exon 14-mutated or MET-amplified non-small-cell lung cancer. N Engl J Med. 2020;383(10):944–57. [DOI] [PubMed] [Google Scholar]
- 13.Travis WD. Update on small cell carcinoma and its differentiation from squamous cell carcinoma and other non-small cell carcinomas. Mod Pathol. 2012;25(Suppl 1):S18-30. [DOI] [PubMed] [Google Scholar]
- 14.Siddiqui F, Siddiqui AH. Lung Cancer. 2021 Sep 3. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021.
- 15.Giaquinto AN, Miller KD, Tossas KY, Winn RA, Jemal A, Siegel RL. Cancer statistics for African American/black people 2022. CA Cancer J Clin. 2022;72(3):202–29. [DOI] [PubMed] [Google Scholar]
- 16.Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, et al. SEER cancer statistics review, 1975–2017, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2017/, based on November 2019 SEER data submission, posted to the SEER web site, April 2020.
- 17.Berg CD, Schiller JH, Boffetta P, Cai J, Connolly C, Kerpel-Fronius A, et al. Air pollution and lung cancer: a review by International Association for the Study of Lung Cancer early detection and screening committee. J Thorac Oncol. 2023;18(10):1277–89. [DOI] [PubMed] [Google Scholar]
- 18.Henley SJ, Thun MJ, Chao A, Calle EE. Association between exclusive pipe smoking and mortality from cancer and other diseases. J Natl Cancer Inst. 2004;96(11):853–61. [DOI] [PubMed] [Google Scholar]
- 19.Siegel R. Surveillance Research, American Cancer Society, 250 Williams Street, NW, Atlanta, GA 30303–1002. American Cancer Society. Cancer Facts and Figures 2021. Atlanta; American Cancer Society: 2020.
- 20.Zhang T, Joubert P, Ansari-Pour N, Zhao W, Hoang PH, Lokanga R, et al. Genomic and evolutionary classification of lung cancer in never smokers. Nat Genet. 2021;53(9):1348–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cannon-Albright LA, Carr SR, Akerley W. Population-based relative risks for lung cancer based on complete family history of lung cancer. J Thorac Oncol. 2019;14(7):1184–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kneuertz PJ, Ferrari-Light D, Altorki NK. Sublobar resection vs lobectomy for stage IA non-small cell lung carcinoma-takeaways from modern randomized trials. Ann Thorac Surg. 2024;117(5):897–903. [DOI] [PubMed] [Google Scholar]
- 23.Salvicchi A, Tombelli S, Mugnaini G, Gonfiotti A. Lung segmentectomy in NSCLC surgery. Life. 2023;13(6):1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang C, Leighl NB, Wu YL, Zhong WZ. Emerging therapies for non-small cell lung cancer. J Hematol Oncol. 2019;12(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsuda A, Yamaoka K, Kunitoh H, Seto T, Tsuboi M, Ohira T, et al. Quality of life with docetaxel plus cisplatin versus paclitaxel plus carboplatin in patients with completely resected non-small cell lung cancer: quality of life analysis of TORG 0503. Qual Life Res. 2023;32(9):2629–37. [DOI] [PubMed] [Google Scholar]
- 26.Schneider BJ, Daly ME, Kennedy EB, Antonoff MB, Broderick S, Feldman J, et al. Stereotactic body radiotherapy for early-stage non-small-cell lung cancer: American society of clinical oncology endorsement of the American society for radiation oncology evidence-based guideline. J Clin Oncol. 2018;36(7):710–9. [DOI] [PubMed] [Google Scholar]
- 27.Huang YJ, Huang TW, Lin FH, Chung CH, Tsao CH, Chien WC. Radiation therapy for invasive breast cancer increases the risk of second primary lung cancer: a nationwide population-based cohort analysis. J Thorac Oncol. 2017;12(5):782–90. [DOI] [PubMed] [Google Scholar]
- 28.Brozos-Vázquez EM, Díaz-Peña R, García-González J, León-Mateos L, Mondelo-Macía P, Peña-Chilet M, et al. Immunotherapy in nonsmall-cell lung cancer: current status and future prospects for liquid biopsy. Cancer Immunol Immunother. 2021;70(5):1177–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uprety D, Mandrekar SJ, Wigle D, Roden AC, Adjei AA. Neoadjuvant immunotherapy for NSCLC: current concepts and future approaches. J Thorac Oncol. 2020;15(8):1281–97. [DOI] [PubMed] [Google Scholar]
- 30.Hsu PC, Jablons DM, Yang CT, You L. Epidermal growth factor receptor (EGFR) pathway, Yes-associated protein (YAP) and the regulation of programmed death-ligand 1 (PD-L1) in non-small cell lung cancer (NSCLC). Int J Mol Sci. 2019;20(15):3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herbst RS, Wu YL, John T, Grohe C, Majem M, Wang J, et al. Adjuvant osimertinib for resected EGFR-mutated stage IB-IIIA non-small-cell lung cancer: updated results from the phase III randomized ADAURA trial. J Clin Oncol. 2023;41(10):1830–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saw SPL, Ong BH, Chua KLM, Takano A, Tan DSW. Revisiting neoadjuvant therapy in non-small-cell lung cancer. Lancet Oncol. 2021;22(11):e501–16. [DOI] [PubMed] [Google Scholar]
- 33.Liao WY, Chen JH, Wu M, Shih JY, Chen KY, Ho CC, et al. Neoadjuvant chemotherapy with docetaxel-cisplatin in patients with stage III N2 non-small-cell lung cancer. Clin Lung Cancer. 2013;14(4):418–24. [DOI] [PubMed] [Google Scholar]
- 34.Rudrapal M, Khairnar SJ, Jadhav AG. Drug repurposing (DR): an emerging approach in drug discovery. Drug repurposing - hypothesis, molecular aspects and therapeutic applications. London: IntechOpen. 2020.
- 35.Parvathaneni V, Kulkarni NS, Muth A, Gupta V. Drug repurposing: a promising tool to accelerate the drug discovery process. Drug Discov Today. 2019;24(10):2076–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li B, Dai C, Wang L, Deng H, Li Y, Guan Z, et al. A novel drug repurposing approach for non-small cell lung cancer using deep learning. PLoS ONE. 2020;15(6):e0233112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pushpakom S, Iorio F, Eyers PA, Escott KJ, Hopper S, Wells A, et al. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov. 2019;18(1):41–58. [DOI] [PubMed] [Google Scholar]
- 38.Heckman-Stoddard BM, DeCensi A, Sahasrabuddhe VV, Ford LG. Repurposing metformin for the prevention of cancer and cancer recurrence. Diabetologia. 2017;60(9):1639–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care. 2009;32(9):1620–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanchez-Rangel E, Inzucchi SE. Metformin: clinical use in type 2 diabetes. Diabetologia. 2017;60(9):1586–93. [DOI] [PubMed] [Google Scholar]
- 41.Baker C, Retzik-Stahr C, Singh V, Plomondon R, Anderson V, Rasouli N. Should metformin remain the first-line therapy for treatment of type 2 diabetes? Ther Adv Endocrinol Metab. 2021;12:2042018820980225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bailey CJ. Metformin: historical overview. Diabetologia. 2017;60(9):1566–76. [DOI] [PubMed] [Google Scholar]
- 43.Andrews M, Soto N, Arredondo M. Effect of metformin on the expression of tumor necrosis factor-α, Toll like receptors 2/4 and C reactive protein in obese type-2 diabetic patients. Rev Med Chil. 2012;140(11):1377–82. [DOI] [PubMed] [Google Scholar]
- 44.Arai M, Uchiba M, Komura H, Mizuochi Y, Harada N, Okajima K. Metformin, an antidiabetic agent, suppresses the production of tumor necrosis factor and tissue factor by inhibiting early growth response factor-1 expression in human monocytes in vitro. J Pharmacol Exp Ther. 2010;334(1):206–13. [DOI] [PubMed] [Google Scholar]
- 45.Li C, Xue Y, Xi YR, Xie K. Progress in the application and mechanism of metformin in treating non-small cell lung cancer. Oncology Letter. 2017;13:2873–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pernicova I, Korbonits M. Metformin–mode of action and clinical implications for diabetes and cancer. Nat Rev Endocrinol. 2014;10:143–56. [DOI] [PubMed] [Google Scholar]
- 47.Madiraju AK, Erion DM, Rahimi Y, Zhang XM, Braddock DT, Albright RA, et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature. 2014;510(7506):542–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dowling RJO, Niraula S, Stambolic V, Goodwin PJ. Metformin in cancer: translational challenges. J Mol Endocrinol. 2012;48(3):R31-43. [DOI] [PubMed] [Google Scholar]
- 49.Lenhard JM, Croom DK, Minnick DT. Reduced serum dipeptidyl peptidase-IV after metformin and pioglitazone treatments. Biochem Biophys Res Commun. 2004;324(1):92–7. [DOI] [PubMed] [Google Scholar]
- 50.Malin SK, Kashyap SR. Effects of metformin on weight loss: potential mechanisms. Curr Opin Endocrinol Diabetes Obes. 2014;21(5):323–9. [DOI] [PubMed] [Google Scholar]
- 51.Chen K, Li Y, Guo Z, Zeng Y, Zhang W, Wang H. Metformin: current clinical applications in nondiabetic patients with cancer. Aging (Albany NY). 2020;12(4):3993–4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saxena A, Becker D, Preeshagul I, Lee K, Katz E, Levy B. Therapeutic effects of repurposed therapies in non-small cell lung cancer: what is old is new again. Oncologist. 2015;20(8):934–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guo Q, Liu Z, Jiang L, Liu M, Ma J, Yang C, et al. Metformin inhibits growth of human non-small cell lung cancer cells via liver kinase B-1-independent activation of adenosine monophosphate-activated protein kinase. Mol Med Rep. 2016;13(3):2590–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kalender A, Selvaraj A, Kim SY, Gulati P, Brûlé S, Viollet B, et al. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab. 2010;11(5):390–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cha JH, Yang WH, Xia W, Wei Y, Chan LC, Lim SO, et al. Metformin promotes antitumor immunity via endoplasmic reticulum-associated degradation of PD-L1. Mol Cell. 2018;71(4):606-20.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Levy A, Doyen J. Metformin for non-small cell lung cancer patients: opportunities and pitfalls. Crit Rev Oncol Hematol. 2018;125:41–7. [DOI] [PubMed] [Google Scholar]
- 57.Yenmis G, Yaprak Sarac E, Besli N, Soydas T, Tastan C, Dilek Kancagi D, et al. Anti-cancer effect of metformin on the metastasis and invasion of primary breast cancer cells through mediating NF-kB activity. Acta Histochem. 2021;123(4):151709. [DOI] [PubMed] [Google Scholar]
- 58.Nurwidya F, Andarini S, Takahashi F, Syahruddin E, Takahashi K. Implications of insulin-like growth factor 1 receptor activation in lung cancer. Malays J Med Sci. 2016;23(3):9–21. [PMC free article] [PubMed] [Google Scholar]
- 59.Liu S, Polsdofer EV, Zhou L, Ruan S, Lyu H, Hou D, et al. Upregulation of endogenous TRAIL-elicited apoptosis is essential for metformin-mediated antitumor activity against TNBC and NSCLC. Mol Ther. 2021;21:303–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang J, Gao Q, Wang D, Wang Z, Hu C. Metformin inhibits growth of lung adenocarcinoma cells by inducing apoptosis via the mitochondria-mediated pathway. Oncol Lett. 2015;10(3):1343–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hua Y, Zheng Y, Yao Y, Jia R, Ge S, Zhuang A. Metformin and cancer hallmarks: shedding new lights on therapeutic repurposing. J Transl Med. 2023;21(1):403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Han P, Zhou J, Xiang J, Liu Q, Sun K. Research progress on the therapeutic effect and mechanism of metformin for lung cancer. Oncol Rep. 2023;49(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Galal MA, Al-Rimawi M, Hajeer A, Dahman H, Alouch S, Aljada A. Metformin: a dual-role player in cancer treatment and prevention. Int J Mol Sci. 2024;25(7):4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thyagarajan A, Shaban A, Sahu RP. Microrna-directed cancer therapies: implications in melanoma intervention. J Pharmacol Exp Ther. 2018;364(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thyagarajan A, Tsai KY, Sahu RP. Microrna heterogeneity in melanoma progression. Semin Cancer Biol. 2019;59:208–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wahdan-Alaswad RS, Cochrane DR, Spoelstra NS, Howe EN, Edgerton SM, Anderson SM, et al. Metformin-induced killing of triple-negative breast cancer cells is mediated by reduction in fatty acid synthase via miRNA-193b. Horm Cancer. 2014;5(6):374–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kalogirou C, Schäfer D, Krebs M, Kurz F, Schneider A, Riedmiller H, et al. Metformin-derived growth inhibition in renal cell carcinoma depends on miR-21-mediated PTEN expression. Urol Int. 2016;96(1):106–15. [DOI] [PubMed] [Google Scholar]
- 68.Dong J, Peng H, Yang X, Wu W, Zhao Y, Chen D, et al. Metformin mediated microrna-7 upregulation inhibits growth, migration, and invasion of non-small cell lung cancer A549 cells. Anticancer Drugs. 2020;31(4):345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hou XB, Li TH, Ren ZP, Liu Y. Combination of 2-deoxy d-glucose and metformin for synergistic inhibition of non-small cell lung cancer: a reactive oxygen species and P-p38 mediated mechanism. Biomed Pharmacother. 2016;84:1575–84. [DOI] [PubMed] [Google Scholar]
- 70.Moro M, Caiola E, Ganzinelli M, Zulato E, Rulli E, Marabese M, et al. Metformin enhances cisplatin-induced apoptosis and prevents resistance to cisplatin in co-mutated KRAS/LKB1 NSCLC. J Thorac Oncol. 2018;13(11):1692–704. [DOI] [PubMed] [Google Scholar]
- 71.Huang S, He T, Yang S, Sheng H, Tang X, Bao F, et al. Metformin reverses chemoresistance in non-small cell lung cancer via accelerating ubiquitination-mediated degradation of Nrf2. Transl Lung Cancer Res. 2020;9(6):2337–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cao N, Lu Y, Liu J, Cai F, Xu H, Chen J, et al. Metformin synergistically enhanced the antitumor activity of celecoxib in human non-small cell lung cancer cells. Front Pharmacol. 2020;11:1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ko E, Baek S, Kim J, Park D, Lee Y. Antitumor activity of combination therapy with metformin and trametinib in non-small cell lung cancer cells. Dev Reprod. 2020;24(2):113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Han R, Jia Y, Li X, Zhao C, Zhao S, Liu S, et al. Concurrent use of metformin enhances the efficacy of EGFR-TKIs in patients with advanced EGFR-mutant non-small cell lung cancer-an option for overcoming EGFR-TKI resistance. Transl Lung Cancer Res. 2021;10(3):1277–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhu Z, Jiang T, Suo H, Xu S, Zhang C, Ying G, et al. Metformin potentiates the effects of anlotinib in NSCLC via AMPK/mTOR and ROS-mediated signaling pathways. Front Pharmacol. 2021;12:712181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang JL, Lan YW, Tsai YT, Chen YC, Staniczek T, Tsou YA, et al. Additive antiproliferative and antiangiogenic effects of metformin and pemetrexed in a non-small-cell lung cancer xenograft model. Front Cell Dev Biol. 2021;9:688062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang Z, Lu C, Zhang K, Lin C, Wu F, Tang X, et al. Metformin combining PD-1 inhibitor enhanced anti-tumor efficacy in STK11 mutant lung cancer through AXIN-1-dependent inhibition of STING ubiquitination. Front Mol Biosci. 2022;9:780200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lu T, Li M, Zhao M, Huang Y, Bi G, Liang J, et al. Metformin inhibits human non-small cell lung cancer by regulating AMPK-CEBPB-PDL1 signaling pathway. Cancer Immunol Immunother. 2022;71(7):1733–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.de Miguel J, Lemke A, Anel H, Walczak L, Martinez-Lostao. Onto better TRAILs for cancer treatment. Cell Death Differ. 2016;23(5):733–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yuan X, Gajan A, Chu Q, Xiong H, Wu K, Wu GS. Developing TRAIL/TRAIL death receptor-based cancer therapies. Cancer Metastasis Rev. 2018;37(4):733–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Skoulidis F, Byers LA, Diao L, Papadimitrakopoulou VA, Tong P, et al. Co-occurring genomic alterations define major subsets of KRAS-mutant lung adenocarcinoma with distinct biology, immune profiles, and therapeutic vulnerabilities. Cancer Discov. 2015;5(8):860–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sanchez-Cespedes M, Parrella P, Esteller M, Nomoto S, Trink B, Engles JM, et al. Inactivation of LKB1/STK11 is a common event in adenocarcinomas of the lung. Cancer Res. 2002;62(13):3659–62. [PubMed] [Google Scholar]
- 83.Hardie DG, Alessi DR. LKB1 and AMPK and the cancer-metabolism link - ten years after. BMC Biol. 2013;11:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Galluzzi L, Senovilla L, Vitale I, Michels J, Martins I, Kepp O, et al. Molecular mechanisms of cisplatin resistance. Oncogene. 2012;31(15):1869–83. [DOI] [PubMed] [Google Scholar]
- 86.Liang J, Lu T, Chen Z, Zhan C, Wang Q. Mechanisms of resistance to pemetrexed in non-small cell lung cancer. Transl Lung Cancer Res. 2019;8(6):1107–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hammad A, Namani A, Elshaer M, Wang XJ, Tang X. “NRF2 addiction” in lung cancer cells and its impact on cancer therapy. Cancer Lett. 2019;467:40–9. [DOI] [PubMed] [Google Scholar]
- 88.Furfaro AL, Traverso N, Domenicotti C, Piras S, Marinari UM, Pronzato MA, et al. The Nrf2/HO-1 axis in cancer cell growth and chemoresistance. Oxid Med Cell Longev. 2016;2016:1958174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shen G, Zheng F, Ren D, Du F, Dong Q, Wang Z, et al. Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol. 2018;11(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wu D, Nie J, Hu W, Dai L, Zhang J, Chen X, et al. A phase II study of anlotinib in 45 patients with relapsed small cell lung cancer. Int J Cancer. 2020;147(12):3453–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gao Y, Liu P, Shi R. Anlotinib as a molecular targeted therapy for tumors. Oncol Lett. 2020;20(2):1001–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26(21):3543–51. [DOI] [PubMed] [Google Scholar]
- 93.Belani CP, Brodowicz T, Ciuleanu TE, Krzakowski M, Yang SH, Franke F, et al. Quality of life in patients with advanced non-small-cell lung cancer given maintenance treatment with pemetrexed versus placebo (H3E-MC-JMEN): results from a randomised, double-blind, phase 3 study. Lancet Oncol. 2012;13(3):292–9. [DOI] [PubMed] [Google Scholar]
- 94.Jure-Kunkel M, Wu S, Xiao F, Abdullah SE, Gao G, Englert JM, et al. Somatic STK11/LKB1 mutations to confer resistance to immune checkpoint inhibitors as monotherapy or in combination in advanced NSCLC. J Clin Oncol. 2018;36(15_Suppl. l):3028. [Google Scholar]
- 95.Skoulidis F, Goldberg ME, Greenawalt DM, Hellmann MD, Awad MM, Gainor JF, et al. STK11/LKB1 mutations and PD-1 inhibitor resistance in KRAS-mutant lung adenocarcinoma. Cancer Discov. 2018;8(7):822–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee Y, Joo J, Lee YJ, Lee EK, Park S, Kim TS, et al. Randomized phase II study of platinum-based chemotherapy plus controlled diet with or without metformin in patients with advanced non-small cell lung cancer. Lung Cancer. 2021;151:8–15. [DOI] [PubMed] [Google Scholar]
- 97.Xiao K, Liu F, Liu J, Xu J, Wu Q, Li X. The effect of metformin on lung cancer risk and survival in patients with type 2 diabetes mellitus: a meta-analysis. J Clin Pharm Ther. 2020;45(4):783–92. [DOI] [PubMed] [Google Scholar]
- 98.Arrieta O, Barrón F, Padilla MS, Avilés-Salas A, Ramírez-Tirado LA, Arguelles Jiménez MJ, et al. Effect of metformin plus tyrosine kinase inhibitors compared with tyrosine kinase inhibitors alone in patients with epidermal growth factor receptor-mutated lung adenocarcinoma: a phase 2 randomized clinical trial. JAMA Oncol. 2019;5(11):e192553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Verma S, Chitikela S, Singh V, Khurana S, Pushpam D, Jain D, et al. A phase II study of metformin plus pemetrexed and carboplatin in patients with non-squamous non-small cell lung cancer (METALUNG). Med Oncol. 2023;40(7):192. [DOI] [PubMed] [Google Scholar]
- 100.Tsakiridis T, Pond GR, Wright J, Ellis PM, Ahmed N, Abdulkarim B, et al. Metformin in combination with chemoradiotherapy in locally advanced non-small cell lung cancer: the OCOG-ALMERA randomized clinical trial. JAMA Oncol. 2021;7(9):1333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Skinner H, Hu C, Tsakiridis T, Santana-Davila R, Lu B, Erasmus JJ, et al. Addition of metformin to concurrent chemoradiation in patients with locally advanced non-small cell lung cancer: the NRG-LU001 phase 2 randomized clinical trial. JAMA Oncol. 2021;7(9):1324–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Eikawa S, Nishida M, Mizukami S, Yamazaki C, Nakayama E, Udono H. Immune-mediated antitumor effect by type 2 diabetes drug, metformin. Proc Natl Acad Sci USA. 2015;112(6):1809–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Scharping NE, Menk AV, Whetstone RD, Zeng X, Delgoffe GM. Efficacy of PD-1 blockade is potentiated by metformin-induced reduction of tumor hypoxia. Cancer Immunol Res. 2017;5(1):9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kubo T, Ninomiya T, Hotta K, Kozuki T, Toyooka S, Okada H, et al. Study protocol: phase-Ib trial of nivolumab combined with metformin for refractory/recurrent solid tumors. Clin Lung Cancer. 2018;19(6):e861–4. [DOI] [PubMed] [Google Scholar]
- 105.Amato L, De Rosa C, Di Guida G, Sepe F, Ariano A, Capaldo S, et al. Addition of metformin to anti-PD-1/PD-L1 drugs activates anti-tumor immune response in peripheral immune cells of NSCLC patients. Cell Death Dis. 2025;16(1):286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Thyagarajan A, Kadam SM, Liu L, Kelly LE, Rapp CM, Chen Y, et al. Gemcitabine induces microvesicle particle release in a platelet-activating factor-receptor-dependent manner via modulation of the MAPK pathway in pancreatic cancer cells. Int J Mol Sci. 2018. 10.3390/ijms20010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Khader S, Thyagarajan A, Sahu RP. Exploring signaling pathways and pancreatic cancer treatment approaches using genetic models. Mini Rev Med Chem. 2019;19(14):1112–25. [DOI] [PubMed] [Google Scholar]
- 108.Thyagarajan A, Alshehri MSA, Miller KLR, Sherwin CM, Travers JB, Sahu RP. Myeloid-derived suppressor cells and pancreatic cancer: implications in novel therapeutic approaches. Cancers. 2019;11(11):1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Du Y, Zhu YZ, Zhou YX, Ding J, Liu JY. Metformin in therapeutic applications in human diseases: its mechanism of action and clinical study. Mol Biomed. 2022;3(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dyatlova N, Tobarran NV, Kannan L, North R, Wills BK. Metformin-associated lactic acidosis (mala). In StatPearls [Internet], 2023. https://www.ncbi.nlm.nih.gov/books/NBK580485/. [PubMed]
- 111.See KC. Metformin-associated lactic acidosis: a mini review of pathophysiology, diagnosis and management in critically ill patients. World J Diabetes. 2024;15(6):1178–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen Y, Li X, Zhang R, Xia Y, Shao Z, Mei Z. Effects of statin exposure and lung cancer survival: a meta-analysis of observational studies. Pharmacol Res. 2019;141:357–65. [DOI] [PubMed] [Google Scholar]
- 113.Ung MH, MacKenzie TA, Onega TL, Amos CI, Cheng C. Statins associate with improved mortality among patients with certain histological subtypes of lung cancer. Lung Cancer. 2018;126:89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hochmuth F, Jochem M, Schlattmann P. Meta-analysis of aspirin use and risk of lung cancer shows notable results. Eur J Cancer Prev. 2016;25(4):259–68. [DOI] [PubMed] [Google Scholar]
- 115.Xin WX, Fang L, Fang QL, Zheng XW, Ding HY, Huang P. Effect of hypoglycemic agents on survival outcomes of lung cancer patients with diabetes mellitus: a meta-analysis. Medicine. 2018. 10.1097/MD.0000000000010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Parikh AB, Kozuch P, Rohs N, Becker DJ, Levy BP. Metformin as a repurposed therapy in advanced non-small cell lung cancer (NSCLC): results of a phase II trial. Invest New Drugs. 2017;35(6):813–9. [DOI] [PubMed] [Google Scholar]
- 117.Moore DC, Guinigundo AS. The role of biomarkers in guiding clinical decision-making in oncology. J Adv Pract Oncol. 2023;14(Suppl 1):15–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Godoy LA, Chen J, Ma W, Lally J, Toomey KA, Rajappa P, et al. Emerging precision neoadjuvant systemic therapy for patients with resectable non-small cell lung cancer: current status and perspectives. Biomark Res. 2023;11(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang J, Xia SA, Zhu ZZ. Synergistic effect of phenformin in non-small cell lung cancer (NSCLC) ionizing radiation treatment. Cell Biochem Biophys. 2015;71(2):513–8. [DOI] [PubMed] [Google Scholar]
- 120.Zhang J, Nannapaneni S, Wang D, Liu F, Wang X, Jin R, et al. Phenformin enhances the therapeutic effect of selumetinib in KRAS-mutant non-small cell lung cancer irrespective of LKB1 status. Oncotarget. 2017;8(35):59008–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang F, Liu W, Long Y, Peng H. Targeted delivery of metformin against lung cancer cells via hyaluronan-modified mesoporous silica nanoparticles. Appl Biochem Biotechnol. 2023;195(7):4067–83. [DOI] [PubMed] [Google Scholar]
- 122.Salmani-Javan E, Jafari-Gharabaghlou D, Bonabi E, Zarghami N. Fabricating niosomal-PEG nanoparticles co-loaded with metformin and silibinin for effective treatment of human lung cancer cells. Front Oncol. 2023;13:1193708. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.