Abstract
Artificial light at night can strongly alter organismal traits, but its role in shaping species interactions remains poorly understood, especially so in aquatic ecosystems. By capitalizing on a recently discovered antagonistic interaction between a brood-parasitic flatworm and Daphnia magna water fleas, we tested whether this interaction depends on exposure to artificial light at night. During a 19 day laboratory population growth experiment, we manipulated flatworm presence and night-time light conditions in a full-factorial design. We confirmed the negative effects of flatworm predation on Daphnia abundance at the population level. Importantly, we showed that the flatworm-caused reduction in the final population size of Daphnia under artificial light at night was twice as strong (81%) compared to under dark–night conditions (39%). Our findings are relevant when it comes to assessing the impact of artificial light at night on the development of Daphnia populations and thus top-down control of phytoplankton. Freshwater ecosystems in urbanized areas, where this parasitic interaction was first encountered, may be especially at risk, as these are typically exposed to high levels of stress factors, including light pollution.
Keywords: light at night, Strongylostoma simplex, parasite, microturbellarian, egg predation
1. Introduction
Human-induced environmental change shapes not only organismal traits but also species interactions [1]. Artificial light at night (ALAN) is a pervasive stressor for a wide range of taxa, influencing life history, behaviour and physiology, which in turn influence species interactions and ultimately community structure and ecosystem functions [2–4]. Whether and how species interactions are shaped by ALAN has recently gained attention among ecologists [5]. Aquatic habitats are suggested to be particularly threatened by ALAN, because aquatic organisms often have reduced available refuge to avoid light exposure, especially so in urban ponds with limited structural complexity [6] and are expected to be exposed to high levels of ALAN [7]. Despite this, the effects of ALAN on species interactions in aquatic ecosystems remain understudied [7].
Natural temporal light patterns produced by nocturnal celestial bodies are important environmental cues for many animals, including aquatic species [8–10]. For example, moonlight can induce the vertical migration of zooplankton down to a depth of 100 m [11]. The high sensitivity of aquatic organisms to low-intensity natural light makes them susceptible to disturbance even by low-intensity ALAN [7]. It is known that ALAN interrupts natural diel vertical migration patterns [12–14], where zooplankton species stay in deeper water layers during the day to minimize the risk of predation by visual planktivorous fish and only ascend to the surface water layers at night to feed on phytoplankton and microzooplankton [15]. Disruptions of diel activity and habitat selection patterns can result in altered temporal niche partitioning, which in turn can influence species interactions, for instance resulting in increased predation rates when interactors occupy the same temporal niche [5]. Aside from changed activity patterns, ALAN may also alter species interactions by imposing physiological stress on the interacting organisms (e.g. [10,16]).
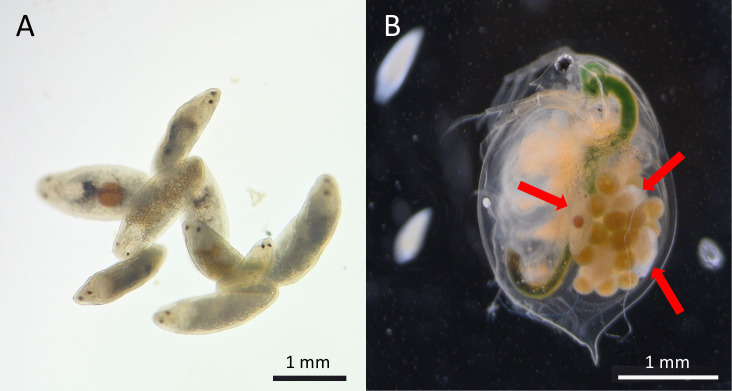
To test whether ALAN has an influence on species interactions in aquatic ecosystems, we capitalized on a recently discovered interaction between a flatworm and Daphnia magna water fleas [17]. The water flea Daphnia, a key ecological interactor in freshwater ecosystems, is well suited for this question. First, Daphnia show a changed pattern of diel vertical migration under ALAN, with the animals staying closer to the bottom during both day and night [12,14]. Second, Daphnia have numerous antagonistic interactions with a wide range of organisms. We have recently shown that the typhloplanid flatworm Strongylostoma simplex, an egg parasite of Daphnia (figure 1), reduced survival and offspring production in D. magna [17]. Here, we first aim to test the hypothesis that the effects of the flatworm on Daphnia individuals translate into changes at the population level. We also aim to test the hypothesis that ALAN significantly influences the effects of flatworms on Daphnia abundance. While little is known about the diel vertical migration patterns of flatworms (e.g. [18]), we predict the low-depth preference of Daphnia under ALAN to result in an increased encounter rate between Daphnia and flatworms (assuming the flatworm is benthic: [19]). We therefore expected a reduced Daphnia abundance under ALAN in the presence of flatworms.
Figure 1.
The typhloplanid flatworm Strongylostoma simplex (A) and the water flea Daphnia magna (B). Flatworms inside the brood chamber of a water flea with eggs are indicated with arrows. Note also on the left side of the water flea the two free-swimming flatworms. Photo credit for panel A: Mareike Brehm-Benedix.
2. Material and methods
(a). Experimental settings
To explore the effects of flatworms on Daphnia abundance under ALAN, we conducted a population growth experiment over 19 days. The experimental design consisted of a flatworm treatment (flatworm absent/present) crossed with an ALAN treatment (dark at night/light at night), using four replicates per treatment combination (n = 16). Treatments were assigned to experimental units using a randomization procedure.
To produce the light environment during day and night, we used LED lamps (Sylvania L300, 3000K, warm-white LED; Feilo Sylvania International Group, Hungary) and used neutral density filter foil (Reinan, USA) wrapped around the lamps to dim the light intensity for the ALAN treatment. Illuminance was measured on top of the jars with a sensitive illuminance metre (ILT-1600; International Light Technologies, USA). Both spectral distribution and correlated colour temperature (CCT) were measured using a spectroradiometer with a measurement range of 380−780 nm and 4.5 nm wavelength resolution (JETI Specbos 1211 UV; Jena Technische Instrumente, Jena, Germany; see also electronic supplementary material, figures S1 and S2). Illuminance and CCT during day hours were ca 400 lx (approximately 6.4 µmol photons m−2 s−1) and 3963 K, respectively. For the ALAN treatment, illuminance and CCT during night hours were ca 35 lx (approximately 0.5 µmol photons m−2 s−1) and 2978 K, respectively, compared to <0.02 lx (approximately 0.0004 µmol photons m−2 s−1, CCT below detection limit) for the control treatment. Both illuminance and CCT were within the typical range of recorded ALAN in urban areas, albeit the illuminance was at the higher end of that range [20].
Jars assigned to the flatworm treatment received five individual flatworms of similar size (ca 0.5 mm) at the beginning of the experiment. Flatworms were collected from a small artificial water body in a cemetery in Berlin and were identified as Strongylostoma simplex simplex [17]. These predatory freshwater flatworms have been previously recorded from natural lakes (see references in [17]) where Daphnia also occurs (e.g. Lake Mývatn in Iceland [21]). We used 1 l cylindrical glass jars (84 mm diameter, 210 mm height, WECK, Germany) filled with 750 ml of dechlorinated tap water. Each experimental population was started with seven D. magna individuals of mixed age: one adult, one subadult and five newly born juveniles. This starting composition was chosen to represent a realistic Daphnia population structure, as well as to reduce the possibility that all eggs would be attacked from the very start of the experiment, preventing any population development in the flatworm treatments, in case we were to start with mature adults only [17]. The number of Daphnia versus flatworms in experimental jars was informed by their relative abundance in the source habitats of the flatworms (N. Tüzün 2023, unpublished data). We used a single D. magna clone for this experiment, collected in 2022 from a very small artificial water pond in a cemetery in Berlin (52°31′19.6″ N 13°30′55.6″ E). The clone was kept in culture under standardized conditions (20°C, 14 : 10 light : dark photoperiod) in the laboratory of IGB Berlin (clone code: ZEN-4). The habitat from which the Daphnia clone was isolated did not contain the flatworm at the moment of isolation; therefore, we assume these Daphnia to be naive to the presence of Strongylostoma simplex. The experimental populations were fed daily with the green algae Acutodesmus obliquus at 1 mg C l-1. We refreshed the medium (dechlorinated tap water) three times per week.
The experiment was run in a temperature-controlled incubator (Pol-Eko ST3 Smart, Poland) at 20°C with a photoperiod of 14 : 10 light : dark (typical for August in Berlin). The experiment ran for 19 days, during which we manually counted the number of Daphnia when we refreshed the medium, i.e. three times per week. Dead individuals were removed from experimental jars. When counting the Daphnia, we also checked the number of worms in each jar and added new flatworms when necessary. The average number of flatworms per experimental jar throughout the trial period was 4.64 ± 0.64 (mean ± s.d.) for the control and 4.72 ± 0.57 for the light pollution treatment and was not significantly different between these groups (Wilcoxon test: W = 610, p = 0.577).
(b). Statistical analyses
To test for the effects of flatworms on Daphnia abundance in the absence and presence of night-time light pollution, we constructed a linear model with time of the experiment (continuous), flatworm treatment (categorical: flatworms absent/present) and light pollution treatment (categorical: dark at night/light at night) as fixed effects. We included all interaction terms of these fixed effects in the model. Given the typical nonlinear shape of Daphnia abundance over time, we fitted a second-order polynomial function of time. In addition, we fitted logistic growth curves to test for differences in carrying capacity and growth rate during the exponential phase. These are reported as electronic supplementary material, table S1 and figure S3. Note that logistic growth models could only be estimated for the non-flatworm treatments, as the models would not converge for the flatworm treatment groups due to the fact that they did not reach a plateau. Pairwise comparisons of treatment effects and regression slopes using estimated marginal means were calculated using the R package emmeans [22]. Logistic growth models were fitted using the R package nlme [23], and the ‘reduced’ (no treatment differences in logistic curve parameters) versus ‘full’ (treatment-specific logistic curve parameters) models were compared using a likelihood ratio test. All analyses were performed in R v. 4.3.2 [24].
3. Results
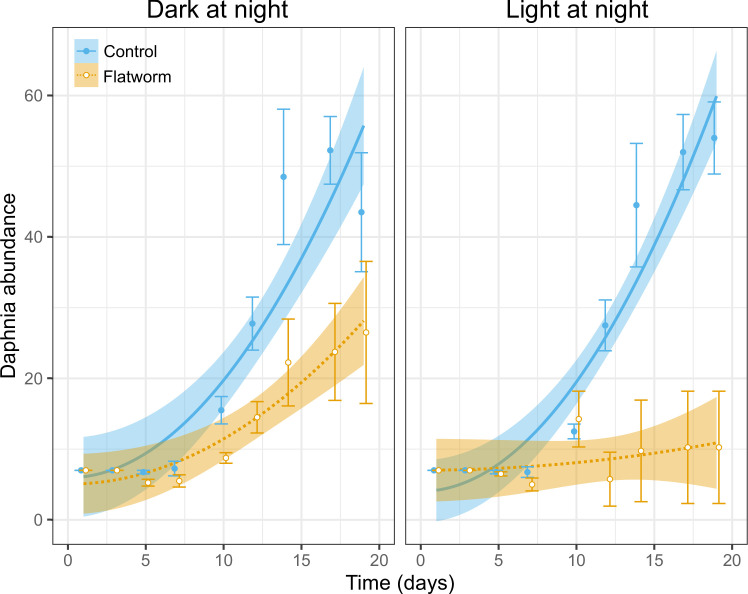
Daphnia abundance on average increased over time, with a steeper increase after the relatively stable first week (indicated by the significant quadratic term of time, table 1, figure 2). The increase in Daphnia abundance over time was on average less pronounced in the flatworm treatment compared to the no-flatworm treatment, and this pattern was more prominent after the first week of the experiment (time2 × flatworm treatment, table 1, figure 2). The significant three-way interaction further shows that in the light pollution treatment, the flatworm-induced negative effect on the increase in Daphnia abundance over time was more pronounced compared to the control light conditions, especially towards the end of the experiment (table 1, figure 2). This is further reflected as a significant difference between the linear growth rates (i.e. slopes) of flatworm-exposed Daphnia at control versus light pollution conditions (contrast test: t ratio = 2.59, p = 0.011). Considering only the no-flatworm treatments, the logistic growth curves did not differ between the control and light pollution treatments (likelihood ratio test = 1.71, p = 0.634, electronic supplementary material, figure S3); this is reflected in the similar values for the three logistic curve parameters across light pollution treatments (electronic supplementary material, table S1).
Table 1.
Result of the linear model testing for effects of time, flatworm treatment, light pollution treatment, as well as their interactions, on Daphnia abundance. Note that time was included as a second-order polynomial term (implemented using the poly(x,2) function in R).
|
fixed effects |
d.f. |
F-value |
p-value |
|---|---|---|---|
|
time2 |
2 |
97.44 |
<0.001 |
|
flatworm treatment |
1 |
67.83 |
<0.001 |
|
light pollution treatment |
1 |
2.15 |
0.145 |
|
time2 × flatworm |
2 |
34.41 |
<0.001 |
|
time2 × light pollution |
2 |
1.04 |
0.357 |
|
flatworm × light pollution |
1 |
2.69 |
0.103 |
|
time2 × flatworm × light pollution |
2 |
3.97 |
0.021 |
Significant p-values (p < 0.05) are indicated in bold.
Figure 2.
Daphnia abundance over time as a function of the presence or absence of predatory flatworms and the presence or absence of light pollution at night. The left panel represents the no light pollution (i.e. dark at night) condition, and the right panel represents the light pollution condition. Shown are regression lines for the control (blue, full line) and flatworm treatments (yellow, dashed line). Shown are also raw means (±1 s.e.) per time point. Bands around lines represent 95% confidence intervals (derived from the linear model; see table 1). Logistic growth curves fitted for control treatments are shown in electronic supplementary material, figure S3.
4. Discussion
Our results show that ALAN can exacerbate the adverse effects of brood-parasitic flatworm on D. magna water fleas. The interaction between these Strongylostoma and Daphnia was recently described for the first time, with observations clearly suggesting an egg parasitism behaviour of the flatworm, with first indications that survival (possibly due to injury during infection) and offspring production of D. magna can be strongly reduced in the presence of flatworms [17]. In the current study, we indeed confirm that this impact has negative effects on population development. Importantly, we show that the reduction in Daphnia abundance caused by flatworm parasitism interacts synergistically with ALAN, i.e. it is twice as pronounced under ALAN when compared to control light conditions (final population size reduced by 81% versus 39%, respectively). Our findings have relevance for assessing the impact of ALAN on the development of Daphnia populations and thus top-down control of phytoplankton in standing freshwater ecosystems, especially in urban areas, as these are typically exposed to high levels of light pollution [7].
While organismal responses to ALAN are well documented [3,4], there have been far fewer studies studies on species interactions under ALAN [5,25]. Light pollution can shape species interactions among others by altering behaviour and physiology in a way that affects the encounter rate between the interactors, as illustrated for host–parasite interactions in aquatic systems [26]. Fish predation, which also induces zooplankton to reside at greater depths in the water column, has been linked to increased parasitic infection in Daphnia due to increased exposure to parasite spores, which are found in the sediment [27]. Most typhloplanid flatworms prefer benthic habitats [19], which may explain our observation that the flatworms have a stronger impact under ALAN. Nevertheless, as we have not explicitly tested for altered depth preference in Daphnia under ALAN in the current study, this idea remains to be experimentally verified. An alternative pathway is that ALAN-induced physiological impacts (e.g. in phytoplankton [28]; in Daphnia [29]; in fish [30]) may have an effect on resource allocation, potentially making Daphnia more vulnerable to flatworm parasitism.
Increased (egg) predation pressure by flatworms under ALAN can potentially strongly impact population dynamics of Daphnia, given that typhoplanid flatworms can be important predators of Daphnia [19]. This may not only affect Daphnia densities and top-down control of phytoplankton but may also lead to profound changes in zooplankton community composition (e.g. [31]). This is because S. simplex is a parasite of eggs that are in the brood chamber of Daphnia, which likely makes large-bodied individuals and species more vulnerable than smaller ones (as shown for copepods predating on Daphnia eggs [32]). For example, increased fish predation on zooplankton under ALAN reduced the mean body size of zooplankton and changed the zooplankton community structure [33]. It remains to be tested whether ALAN has similar effects on the flatworm–Daphnia interaction, potentially shaping zooplankton population structure, community composition, and top-down control of phytoplankton.
The effects of ALAN on species interactions and ecosystem functions may be further exacerbated by other stressors, a key one in an urbanization and climate change context being warmer night temperatures [34]. A recent study revealed that higher temperatures increased the top-down control of predatory Mesostoma flatworms on Daphnia, changing the zooplankton community structure and affecting algal biomass [31]. Therefore, we suggest further exploration of the interaction between warming, ALAN and flatworm parasitism on Daphnia population dynamics covering time periods that exceed the one in the present study, in urban ponds that are typically exposed to both the heat island effect and the light pollution.
Contributor Information
Nedim Tüzün, Email: nedim.tuezuen@igb-berlin.de.
Franz Hölker, Email: franz.hoelker@igb-berlin.de.
Luc De Meester, Email: luc.demeester@igb-berlin.de.
Ethics
This work did not require ethical approval from a human subject or animal welfare committee.
Data accessibility
Data from the experimental trial are available from the Figshare repository [35].
Supplementary material is available online [36].
Declaration of AI use
We have not used AI-assisted technologies in creating this article.
Authors’ contributions
N.T.: conceptualization, formal analysis, investigation, writing—original draft, writing—review and editing; F.H.: conceptualization, methodology, writing—original draft, writing—review and editing; L.D.M.: conceptualization, methodology, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
We greatly appreciate the assistance of Johannes Reichenbach during the experiment. N.T. was supported by the Alexander von Humboldt Research Fellowship and Marie Skłodowska-Curie Actions Fellowship. F.H. was supported by the European Union under the Horizon Europe Programme, grant agreement no. 101135471 (AquaPLAN).
References
- 1. Guiden PW, Bartel SL, Byer NW, Shipley AA, Orrock JL. 2019. Predator–prey interactions in the Anthropocene: reconciling multiple aspects of novelty. Trends Ecol. Evol. 34, 616–627. ( 10.1016/j.tree.2019.02.017) [DOI] [PubMed] [Google Scholar]
- 2. Gaston KJ, Visser ME, Hölker F. 2015. The biological impacts of artificial light at night: the research challenge. Phil. Trans. R. Soc. B 370, 20140133. ( 10.1098/rstb.2014.0133) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sanders D, Frago E, Kehoe R, Patterson C, Gaston KJ. 2021. A meta-analysis of biological impacts of artificial light at night. Nat. Ecol. Evol. 5, 74–81. ( 10.1038/s41559-020-01322-x) [DOI] [PubMed] [Google Scholar]
- 4. Hölker F, et al. 2021. 11 Pressing research questions on how light pollution affects biodiversity. Front. Ecol. Evol. 9, 896. ( 10.3389/fevo.2021.767177) [DOI] [Google Scholar]
- 5. Seymoure B, Dell A, Hölker F, Kalinkat G. 2023. A framework for untangling the consequences of artificial light at night on species interactions. Phil. Trans. R. Soc. B 378, 20220356. ( 10.1098/rstb.2022.0356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oertli B, Parris KM. 2019. Review: toward management of urban ponds for freshwater biodiversity. Ecosphere 10, e02810. ( 10.1002/ecs2.2810) [DOI] [Google Scholar]
- 7. Hölker F, Jechow A, Schroer S, Tockner K, Gessner MO. 2023. Light pollution of freshwater ecosystems: principles, ecological impacts and remedies. Phil. Trans. R. Soc. B 378, 20220360. ( 10.1098/rstb.2022.0360) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kühne JL, van Grunsven RHA, Jechow A, Hölker F. 2021. Impact of different wavelengths of artificial light at night on phototaxis in aquatic insects. Integr. Comp. Biol. 61, 1182–1190. ( 10.1093/icb/icab149) [DOI] [PubMed] [Google Scholar]
- 9. Marangoni LFB, et al. 2022. Impacts of artificial light at night in marine ecosystems—a review. Glob. Chang. Biol. 28, 5346–5367. ( 10.1111/gcb.16264) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ganguly A, Candolin U. 2023. Impact of light pollution on aquatic invertebrates: behavioral responses and ecological consequences. Behav. Ecol. Sociobiol. 77 1–15. ( 10.1007/s00265-023-03381-z) [DOI] [Google Scholar]
- 11. Last KS, Hobbs L, Berge J, Brierley AS, Cottier F. 2016. Moonlight drives ocean-scale mass vertical migration of zooplankton during the arctic winter. Curr. Biol. 26, 244–251. ( 10.1016/j.cub.2015.11.038) [DOI] [PubMed] [Google Scholar]
- 12. Moore MV, Pierce SM, Walsh HM, Kvalvik SK, Lim JD. 2000. Urban light pollution alters the diel vertical migration of Daphnia. Verh. Int. Verein. Limnol. 27, 779–782. ( 10.1080/03680770.1998.11901341) [DOI] [Google Scholar]
- 13. Ludvigsen M, et al. 2018. Use of an autonomous surface vehicle reveals small-scale diel vertical migrations of zooplankton and susceptibility to light pollution under low solar irradiance. Sci. Adv. 4, eaap9887. ( 10.1126/sciadv.aap9887) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maszczyk P, Tałanda J, Babkiewicz E, Leniowski K, Urban P. 2021. Daphnia depth selection in gradients of light intensity from different artificial sources: an evolutionary trap? Limnol. Oceanogr. 66, 1367–1380. ( 10.1002/lno.11691) [DOI] [Google Scholar]
- 15. De Meester L, Mehner T, Scofield A. 2022. Diel vertical migration. In Encyclopedia of inland waters (eds Mehner T, Tockner K), pp. 281–291, 2nd edn. Oxford, UK: Elsevier. ( 10.1016/B978-0-12-819166-8.00166-3) [DOI] [Google Scholar]
- 16. Grubisic M, et al. 2019. Light pollution, circadian photoreception, and melatonin in vertebrates. Sustainability 11, 6400. ( 10.3390/su11226400) [DOI] [Google Scholar]
- 17. Tüzün N, Lemke N, Diez YL, Artois T, Monnens M. 2025. Tiny killers: first record of rhabdocoel flatworms feeding on water flea embryos. Ecol. Evol. 15, e71277. ( 10.1002/ece3.71277) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. De Meester L, Dumont HJ. 1990. Laboratory observations on the vertical distribution of a tropical pelagic flatworm (Mesostoma sp.) in relation to satiation. Hydrobiologia 198, 103–106. ( 10.1007/BF00048626) [DOI] [Google Scholar]
- 19. Dumont HJ, Rietzler AC, Han BP. 2014. A review of typhloplanid flatworm ecology, with emphasis on pelagic species. Inland Waters 4, 257–270. ( 10.5268/IW-4.3.558) [DOI] [Google Scholar]
- 20. Hänel A, et al. 2018. Measuring night sky brightness: methods and challenges. J. Quant. Spectrosc. Radiat. Transf. 205, 278–290. ( 10.1016/j.jqsrt.2017.09.008) [DOI] [Google Scholar]
- 21. Jónasson PM. 1979. The Lake Mývatn ecosystem, Iceland. Oikos 32, 289. [Google Scholar]
- 22. Lenth RV, et al. 2023. emmeans: Estimated Marginal Means, aka Least-Squares Means. See https://CRAN.R-project.org/package=emmeans.
- 23. Pinheiro J, Bates D, R.Core Team . 2023. nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1-163. See https://CRAN.R-project.org/package=nlme.
- 24. R Core Team . 2023. R: a language and environment for statistical computing. R foundation for statistical computing. Vienna, Austria. See https://ropensci.org/blog/2021/11/16/how-to-cite-r-and-r-packages/. [Google Scholar]
- 25. Hirt MR, Evans DM, Miller CR, Ryser R. 2023. Light pollution in complex ecological systems. Phil. Trans. R. Soc. B 378, 20220351. ( 10.1098/rstb.2022.0351) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Poulin R. 2023. Light pollution may alter host–parasite interactions in aquatic ecosystems. Trends Parasitol. 39, 1050–1059. ( 10.1016/j.pt.2023.08.013) [DOI] [PubMed] [Google Scholar]
- 27. Decaestecker E, De Meester L, Ebert D. 2002. In deep trouble: habitat selection constrained by multiple enemies in zooplankton. Proc. Natl Acad. Sci. USA 99, 5481–5485. ( 10.1073/pnas.082543099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Diamantopoulou C, Christoforou E, Dominoni DM, Kaiserli E, Czyzewski J, Mirzai N, Spatharis S. 2021. Wavelength-dependent effects of artificial light at night on phytoplankton growth and community structure. Proc. R. Soc. B 288, 20210525. ( 10.1098/rspb.2021.0525) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li D, Huang J, Zhou Q, Gu L, Sun Y, Zhang L, Yang Z. 2022. Artificial light pollution with different wavelengths at night interferes with development, reproduction, and antipredator defenses of Daphnia magna. Environ. Sci. Technol. 56, 1702–1712. ( 10.1021/acs.est.1c06286) [DOI] [PubMed] [Google Scholar]
- 30. Kupprat F, Hölker F, Kloas W. 2020. Can skyglow reduce nocturnal melatonin concentrations in Eurasian perch? Environ. Pollut. 262, 114324. ( 10.1016/j.envpol.2020.114324) [DOI] [PubMed] [Google Scholar]
- 31. Devkota N, Salis RK, Hansson L. 2023. Warming reshapes the invertebrate predation pressure on the plankton community. Freshw. Biol. 68, 365–377. ( 10.1111/fwb.14031) [DOI] [Google Scholar]
- 32. Gliwicz ZM, Lampert W. 1994. Clutch-size variability in Daphnia: body-size related effects of egg predation by cyclopoid copepods. Limnol. Oceanogr. 39, 479–485. ( 10.4319/lo.1994.39.3.0479) [DOI] [Google Scholar]
- 33. Tałanda J, Maszczyk P, Babkiewicz E, Rutkowska K, Ślusarczyk M. 2022. The short-term effects of planktivorous fish foraging in the presence of artificial light at night on lake zooplankton. J. Plankton Res. (ed. Koski M), 44, 942–946. ( 10.1093/plankt/fbac046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tougeron K, Sanders D. 2023. Combined light pollution and night warming as a novel threat to ecosystems. Trends Ecol. Evol. 38, 701–704. ( 10.1016/j.tree.2023.05.012) [DOI] [PubMed] [Google Scholar]
- 35. Tüzün N, Hölker F, De Meester L. 2025. Data for flatworm–Daphnia trial under light pollution. Figshare. ( 10.6084/m9.figshare.28457171) [DOI]
- 36. Tüzün N, Hölker F, De Meester L. 2025. Supplementary material from: Artificial light at night intensifies effects of a parasitic flatworm on the water flea Daphnia magna. Figshare. ( 10.6084/m9.figshare.c.8007250) [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data from the experimental trial are available from the Figshare repository [35].
Supplementary material is available online [36].