Abstract
One hallmark of prion diseases is the accumulation of the abnormal isoform PrPSc of a normal cellular glycoprotein, PrPc, which is characterized by a high content of β-sheet structures and by its partial resistance to proteinase K. It was hypothesized that the PrP region comprising amino acid residues 109 to 122 [PrP(109–122)], which spontaneously forms amyloid when it is synthesized as a peptide but which does not display significant secondary structure in the context of the full-length PrPc molecule, should play a role in promoting the conversion into PrPSc. By using persistently scrapie-infected mouse neuroblastoma (Sc+-MNB) cells as a model system for prion replication, we set out to design dominant-negative mutants of PrPc that are capable of blocking the conversion of endogenous, wild-type PrPc into PrPSc. We constructed a deletion mutant (PrPcΔ114–121) lacking eight codons that span most of the highly amyloidogenic part, AGAAAAGA, of PrP(109–122). Transient transfections of mammalian expression vectors encoding either wild-type PrPc or PrPcΔ114–121 into uninfected mouse neuroblastoma cells (Neuro2a) led to overexpression of the respective PrPc versions, which proved to be correctly localized on the extracellular face of the plasma membrane. Transfection of Sc+-MNB cells revealed that PrPcΔ114–121 was not a substrate for conversion into a proteinase K-resistant isoform. Furthermore, its presence led to a significant reduction in the steady-state levels of PrPSc derived from endogenous PrPc. Thus, we showed that the presence of amino acids 114 to 121 of mouse PrPc plays an important role in the conversion process of PrPc into PrPSc and that a deletion mutant lacking these codons indeed behaves as a dominant-negative mutant with respect to PrPSc accumulation. This mechanism could form a basis for a new gene therapy and/or a prevention concept for prion diseases.
The transmissible spongiform encephalopathies, also termed prion diseases, comprise Creutzfeldt-Jakob disease (CJD), Gerstmann-Sträussler-Scheinker syndrome, and fatal familial insomnia in humans, scrapie in sheep, and bovine spongiform encephalopathy (BSE) in cattle (35). Prion diseases are characterized by the accumulation of an abnormal, proteinase K-resistant isoform of the prion protein, PrPSc (also called PrP-res), which is absent in control brains (47). The normal isoform, PrPc (also called PrP-sen), is commonly expressed in neurons and several other cell types and is protease sensitive. Recently, a variant form of CJD, characterized by some unusual clinical histopathological features, in a series of young European patients was reported (48). Recent data from several laboratories which have used complementary approaches provide compelling evidence that variant CJD is in fact caused by transmission of the BSE agent to humans (5, 12, 19, 26).
Prion diseases are transmissible, but the exact nature of the infectious agent is controversial. However, the central role of PrP in the pathogenesis of the encephalopathy and in agent replication has previously been proven by experiments showing the complete resistance of PrP null mice and cells to infection with exogenous prions (3, 6). According to the prion hypothesis, the infectious agent consists of PrPSc itself (34). Two models have been proposed to explain the conversion of PrPc into PrPSc. On the one hand, the refolding model postulates that PrPc must be partially unfolded and refolded under the direction of PrPSc as a template (34). On the other hand, the nucleation model implies a partly flexible conformation of PrPc which adapts to the conformation of a PrPSc polymer after binding to the latter, with the polymer thus acting like a seed (7). Both models require close physical interaction between PrPc and PrPSc at some point in the conversion process. In the last few years, the cellular site of conversion has been assigned to the endocytic pathway (9, 43) that is normally used by PrPc molecules located on the cell surface and attached to the plasma membrane by a glycosyl phosphatidylinositol (GPI) anchor. Recently, it has been shown more specifically that both PrPc and PrPSc are present in caveola-like domains, supporting the hypothesis that PrPSc formation occurs within this subcellular compartment (46). However, at least in the case of conversion that occurs as a result of a heritable CJD-specific PrP mutation (in the absence of preexisting PrPSc), additional compartments have previously been implicated (13).
Consistent with the notion that precise interactions between homologous PrP molecules are important in PrPSc formation, several hamster-specific codons inserted into a background of mouse PrP have previously been observed to interfere with the conversion of endogenous, wild-type mouse PrPc into PrPSc, thereby identifying critical residues for the observed hamster/mouse species barrier (32, 33).
PrP is rich in secondary structure, which is predominantly α-helical in the case of PrPc, but displays a high β-sheet content after conversion into PrPSc (10, 30, 40). The recently published nuclear magnetic resonance structure of full-length recombinant murine PrPc revealed that the region spanning amino acids 121 to 231 contains a high level of secondary structure, including three α-helices and a two-stranded antiparallel β-sheet, whereas the N-terminal segment (amino acids 23 to 120) is flexibly disordered (21, 38, 39). On the other hand, it has previously been shown that a region comprising residues 90 to 120 in PrPSc is protected against proteinase K digestion (17), indicating that there must be a major change in the structural arrangement of this region in PrPc and PrPSc. When they are synthesized as peptides, the region comprising amino acid residues 109 to 122 [PrP(109–122)] (termed H1 by Gasset et al. [16]) and PrP(106–126) spontaneously form β-sheets (15, 16), with the internal, completely conserved sequence AGAAAAGA displaying the highest tendency to form amyloid (16). Additional evidence points to the important role of the region from residues 109 to 122 in the context of PrP (14, 22). Interestingly, PrP(109–122) is also represented in PrP peptides which have previously proven to be toxic to PrPc-expressing neurons in brain cell cultures (4, 15, 20).
We set out to design dominant-negative mutants of PrPc which would be capable of interfering with the conversion process of wild-type PrPc. Such mutants should themselves no longer be convertible into PrPSc but should be capable of binding to wild-type PrPc and/or PrPSc, thereby blocking the binding sites required for homologous interaction between wild-type PrPc and wild-type PrPSc. If the models for the formation of PrPSc were correct, this should prevent the de novo synthesis of PrPSc.
The high amyloidogenic potential of PrP(109–122) suggested that this region has a critical role to play in the conversion of PrPc into PrPSc. We therefore deleted codons 114 to 121, which span most part of the subregion AGAAAAGA, thus creating the mutant PrPcΔ114–121. By using persistently scrapie-infected mouse neuroblastoma (Sc+-MNB [37]) cells as a model system for scrapie agent replication, we showed that this deletion mutant is not converted into a proteinase K-resistant isoform. Indeed, its overexpression resulted in inhibition of endogenous PrPSc accumulation.
MATERIALS AND METHODS
Cloning of constructs coding for mouse PrP.
The mouse PrP open reading frame (ORF) was amplified by PCR with genomic DNA from mouse Neuro2a cells. The primers P1 (nucleotides −20 to +2) and P5 (nucleotides 780 to 760) were designed to introduce two new restriction sites suitable for further subcloning steps (AatII and SacI, noncut enzymes for the PrP ORF). The PCR product was ligated into pUC19 to yield pUC-PrP. Sequence analysis of this construct confirmed its perfect identity at the amino acid sequence level with the published PrP sequence (27). The complete ORF was subcloned into plasmid vector pL15TK (24), containing the human cytomegalovirus immediate-early promoter/enhancer and the herpes simplex virus type 1 thymidine kinase gene poly(A) signal, resulting in expression plasmid pCMV-PrP.
We used pUC-PrP to delete codons 114 to 121, yielding pUC-PrPΔ114–121. This was done by cutting with restriction enzymes AlwNI and EcoO109I and religating the large fragment in the presence of the single-stranded 8-mer oligonucleotide GCCACCCC. The complete ORF PrPcΔ114–121 was subcloned into pL15TK, giving rise to plasmid pCMV-PrPΔ114–121, and checked by sequencing.
Cells and antibodies.
Neuro2a cells (purchased from the American Type Culture Collection) and Sc+-MNB cells (kind gift of B. Caughey and B. Chesebro) (37) were maintained at 37°C in 5% CO2 in Dulbecco’s modified Eagle’s minimal essential medium (DMEM) supplemented with 10% heat-inactivated fetal calf serum and penicillin-streptomycin.
A polyclonal rabbit antiserum (Kan72) directed against a peptide derived from mouse PrP (codons 89 to 103) was prepared; it was used for immunodetection of transiently expressed prion proteins. The immunogenicity of the peptide used was first shown by Caughey et al. (8).
Transient transfections.
One day before transfection, 106 Neuro2a cells were plated onto 10-cm-diameter dishes. In transfections of Sc+-MNB cells, cells were plated at about 20% confluency onto 75-cm2 plates. Cells were transfected by the modified CaPO4 precipitation protocol of Chen and Okayama (11). The total amount of transfected DNA was adjusted to 20 μg by adding calf thymus DNA (Sigma) as a carrier. After incubation for 8 to 19 h at 35°C and 3% CO2, cells were washed twice with serum-free medium and then incubated with complete medium at 37°C and 5% CO2. This time point was defined as 0 h after transfection.
Western blot analysis.
Cytoplasmic lysates were prepared by using ice-cold buffer containing deoxycholate and Triton X-100 (2). Lysates were boiled in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (25). SDS-PAGE (12.5% acrylamide) was performed as previously described (25). Proteins were transferred electrophoretically to nitrocellulose membranes (Schleicher & Schuell) by using a semidry blotting system (transfer buffer of 25 mM Tris-HCl [pH 8.0], 192 mM glycine, 20% methanol, 0.02% SDS). Blots were blocked in phosphate-buffered saline (PBS)–0.05% Tween 20–5% dry milk and subsequently incubated overnight at 4°C with polyclonal rabbit antiserum Kan72 (diluted 1:1,000 in blocking solution). After being washed in PBS-Tween 20, blots were incubated at room temperature for 1 h with a peroxidase-conjugated secondary antibody (Dianova) diluted 1:2,000 in blocking solution. Detection was performed by enhanced chemiluminescence (ECL kit; Amersham) exactly as described by the supplier.
Cell ELISA.
The initial steps of the cell enzyme-linked immunosorbent assay (ELISA) were the same as those of Taraboulos et al. (44). Briefly, cells were fixed with formaldehyde and permeabilized with Triton X-100. PrPc immunostaining was done with Kan72 (diluted 1:2,000 in blocking solution, as described above for Western blotting, preceded by incubation in blocking solution). For selective immunodetection of PrPSc, proteinase K digestion (20 μg/ml) was performed for 15 min at 37°C; digestion was terminated by the addition of phenylmethylsulfonyl fluoride (2 mM; 15 min at room temperature), followed by denaturation with 6 M guanidine hydrochloride. Fixed cells were extensively washed with PBS and subsequently incubated in blocking solution, followed by incubation with Kan72 as described above for PrPc detection. It is possible to discriminate between PrPc and PrPSc because PrPc is proteinase K sensitive and in this assay PrPSc is detectable only after denaturation with guanidine hydrochloride. The original method was further modified by using a secondary antibody coupled with alkaline phosphatase rather than peroxidase (diluted 1:2,000; Sigma) and by employing substrates that yield insoluble reaction products, thus allowing in situ staining of cells. The substrate solution was 4 mM MgCl2, 100 μg of nitroblue tetrazolium per ml, and 50 μg of 5-bromo-4-chloro-3-indolyl-phosphate per ml in 50 mM glycine (pH 9.7). McKinley et al. (28) have previously used the peroxidase reaction for in situ immunostaining of cellular PrPSc, but in our hands, its rate is often difficult to control. In contrast, alkaline phosphatase reactions proceed rather slowly and thus can be controlled much better.
Metabolic labeling and phospholipase treatment of live cells.
After transfection, cells were preincubated for 1 h in methionine- and cysteine-free DMEM containing 1% dialyzed fetal bovine serum, followed by metabolic labeling with 35S-labeled methionine-cysteine (150 μCi per ml; ICN) for 4 h. Then cells were incubated with 1.6 U of phosphatidylinositol phospholipase C (PIPLC) (Boehringer Mannheim) per ml in MEM for 1 h at 37°C. The cell-free supernatant was immunoprecipitated in lysing buffer (36) with polyclonal rabbit antiserum Kan72.
RESULTS
Strong overexpression of wild-type PrP or the deleted version PrPcΔ114–121 in transfected Neuro2a and Sc+-MNB cells.
PrP expression plasmids pCMV-PrP and pCMV-PrPΔ114–121 were tested for the ability to direct the overexpression of recombinant proteins by transient transfections in Neuro2a cells, followed by Western blot or immunoprecipitation analysis. For transient transfections, we used the calcium-phosphate coprecipitation protocol of Chen and Okayama (11). Indirect immunofluorescence (not shown) and Western blot analyses revealed significant overexpression of wild-type PrPc and PrPcΔ114–121 after transient transfection. In addition, we demonstrated the expected difference in molecular mass of about 1 kDa between the mutant and wild-type proteins on Western blots of cell extracts derived from tunicamycin-treated Neuro2a cells (Fig. 1). Tunicamycin prevents N-linked glycosylation of proteins, thus allowing easy comparisons of the apparent molecular weights of protein backbones. To check for any spontaneous production of PrPSc after overexpression of prion proteins in normal Neuro2a cells, we digested cytoplasmic extracts of transiently transfected Neuro2a cells with proteinase K. As expected, neither pCMV-PrP nor pCMV-PrPΔ114–121 led to the production of protease-resistant PrP in uninfected cells (data not shown).
FIG. 1.
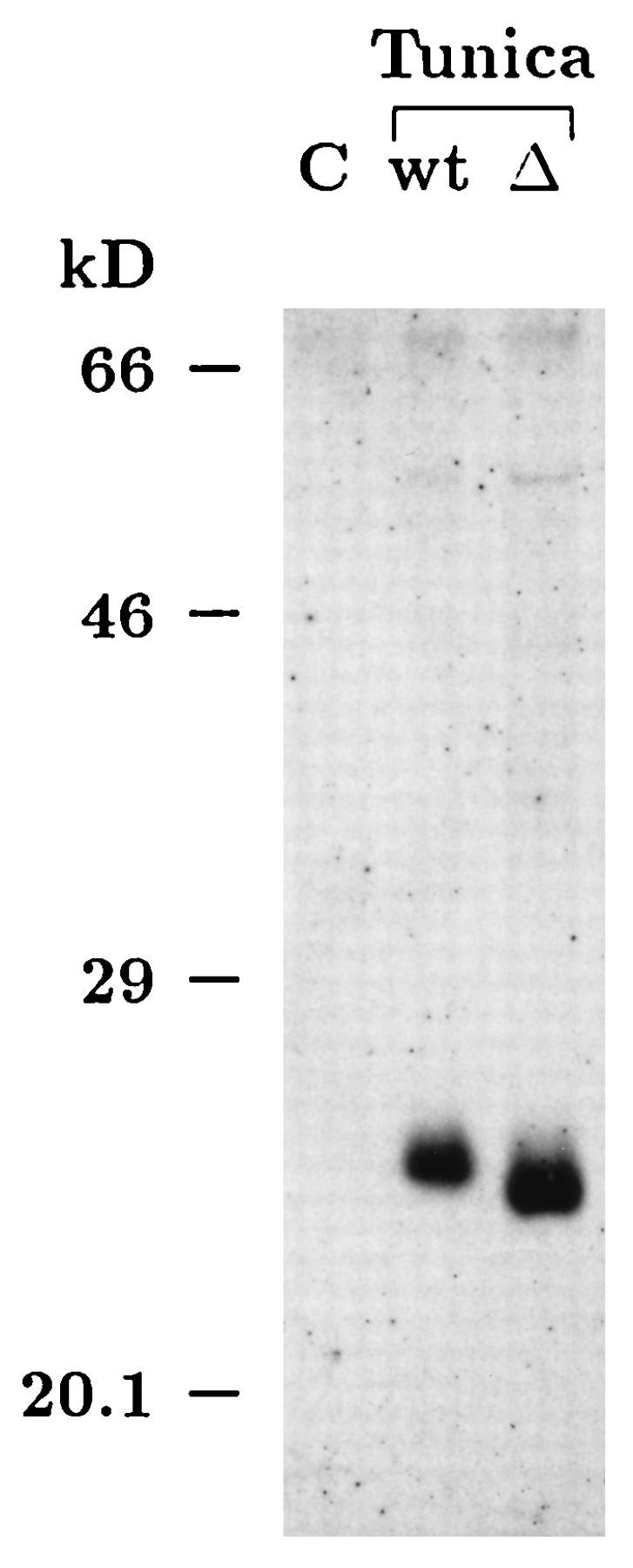
Reduced molecular weight of the deletion mutant PrPcΔ114–121. Plasmids pL15TK (empty vector) (C), pCMV-PrP (wild type [wt]), and pCMV-PrPΔ114–121 (Δ) were transfected into Neuro2a cells. Tunica indicates that cells were grown for 32 h in the presence of 10 μg of tunicamycin per ml to prevent N-linked glycosylation of proteins. Thirty-two hours after transfection, cytoplasmic extracts were prepared and methanol precipitated, and the resulting protein pellets were boiled in SDS-PAGE sample buffer. Samples were run on a 12.5% polyacrylamide gel and immunoblotted onto a nitrocellulose membrane. PrP immunodetection was carried out by using polyclonal rabbit antiserum Kan72 in conjunction with enhanced chemiluminescence detection. The blot clearly demonstrates that the mutant PrPcΔ114–121 has a reduced molecular mass. A comparison of the relative mobilities of the two proteins on a half-logarithmic calibration curve revealed that the observed difference in molecular mass is about 1 kDa (kD), as expected for a deletion of 8 amino acids. The exposure time for the Western blot was chosen to detect only overexpressed, exogenous prion proteins.
In situ detection of overexpressed recombinant PrPc or PrPSc by a modified cell ELISA.
To assess the percentage of cells overexpressing PrPc after transient transfections or the percentage of cells producing PrPSc in persistently infected cell cultures, we used a modified cell ELISA method. Taraboulos et al. (44) have described a cell ELISA procedure to identify PrPSc production in cell clones growing on microtiter plates. However, since a soluble product was formed in the peroxidase reaction used as an indicator, the status of individual cells could not be assessed. We modified the immunocytochemical protocol for convenient and reliable identification of individual cells overexpressing protease-sensitive PrPc or producing proteinase K-resistant PrPSc. Figure 2 shows the cell ELISA results for Sc+-MNB cells transiently transfected with the empty vector or pCMV-PrPΔ114–121. By performing a protocol designed to detect overexpressed PrPc, endogenous PrPSc was not detected due to the lack of denaturation with guanidine hydrochloride (Fig. 2A), whereas the levels of endogenous PrPc were too low to be detected. Overexpressed PrPcΔ114–121, on the other hand, was easily monitored by strong cytoplasmic staining of cells and revealed transfection efficiencies ranging from 30 to 80%. However, when proteinase K digestion and subsequent guanidine hydrochloride treatments were performed, PrPSc was stained (Fig. 2B). As expected, control Neuro2a cells did not stain after proteinase K digestion and subsequent guanidine hydrochloride treatment (data not shown). Interestingly, the staining patterns of PrPc and PrPSc in the cell ELISA were different. PrPSc seemed to be more concentrated in a smaller region in the cytoplasm, whereas overexpressed PrPc appeared to be diffusely dispersed in the cytoplasm.
FIG. 2.
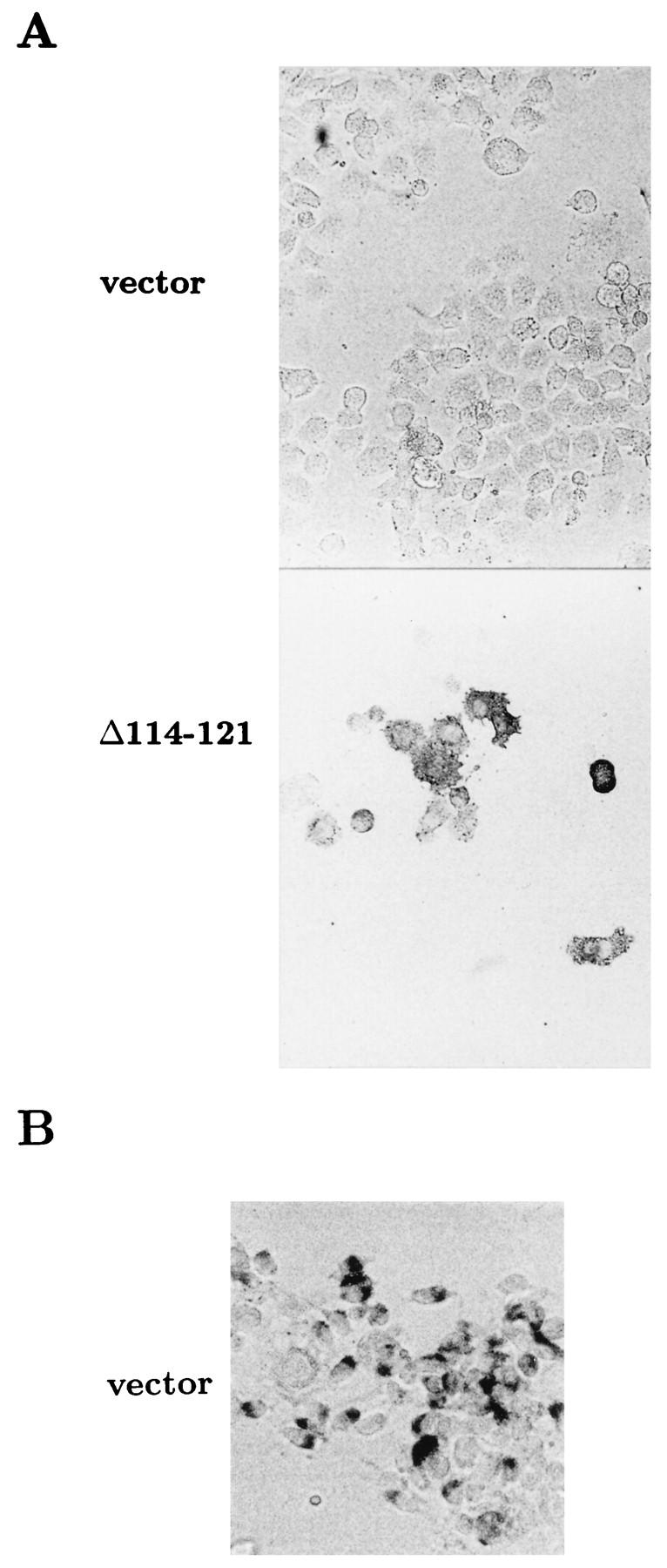
Cell ELISA for the detection of cells overexpressing PrPc or producing PrPSc. Sc+-MNB cells were transfected with plasmid pL15TK (vector) or pCMV-PrPΔ114–121 (Δ114–121) and subsequently incubated for 53 h. After fixation with formaldehyde and permeabilization, cells were either directly incubated with antiserum Kan72 to stain PrPc (A) or incubated with proteinase K and denatured with 6 M guanidine hydrochloride before incubation with antibody to stain PrPSc (B). Subsequently, incubation was done with alkaline phosphatase-coupled secondary antibody, followed by incubation with substrate. Note that in panel A, the exposure time for the vector-transfected control was longer to make negative cells clearly visible, whereas in the pCMV-PrPΔ114–121 transfection, negative cells are hardly visible.
Correct subcellular localization of recombinant prion proteins in Neuro2a cells.
PrPc is a membrane protein which is anchored in the plasma membrane via its GPI moiety. PIPLC specifically cleaves the GPI anchor and thereby releases GPI-anchored proteins into the extracellular space. To determine whether overexpressed wild-type PrPc and PrPcΔ114–121 are correctly localized and, in particular, whether they are present on the cell surface, we transfected Neuro2a cells with either pCMV-PrP or pCMV-PrPΔ114–121 and performed a PIPLC digestion of the live-cell monolayer. The cell-free supernatant was analyzed by immunoprecipitation with the PrP-specific rabbit antiserum Kan72. As shown in Fig. 3, transfection with either construct resulted in high-level expression of prion proteins which could be released by PIPLC into the cell culture medium. The pattern of PrP bands indicated the presence of unglycosylated, singly glycosylated, and doubly glycosylated PrP, as expected.
FIG. 3.
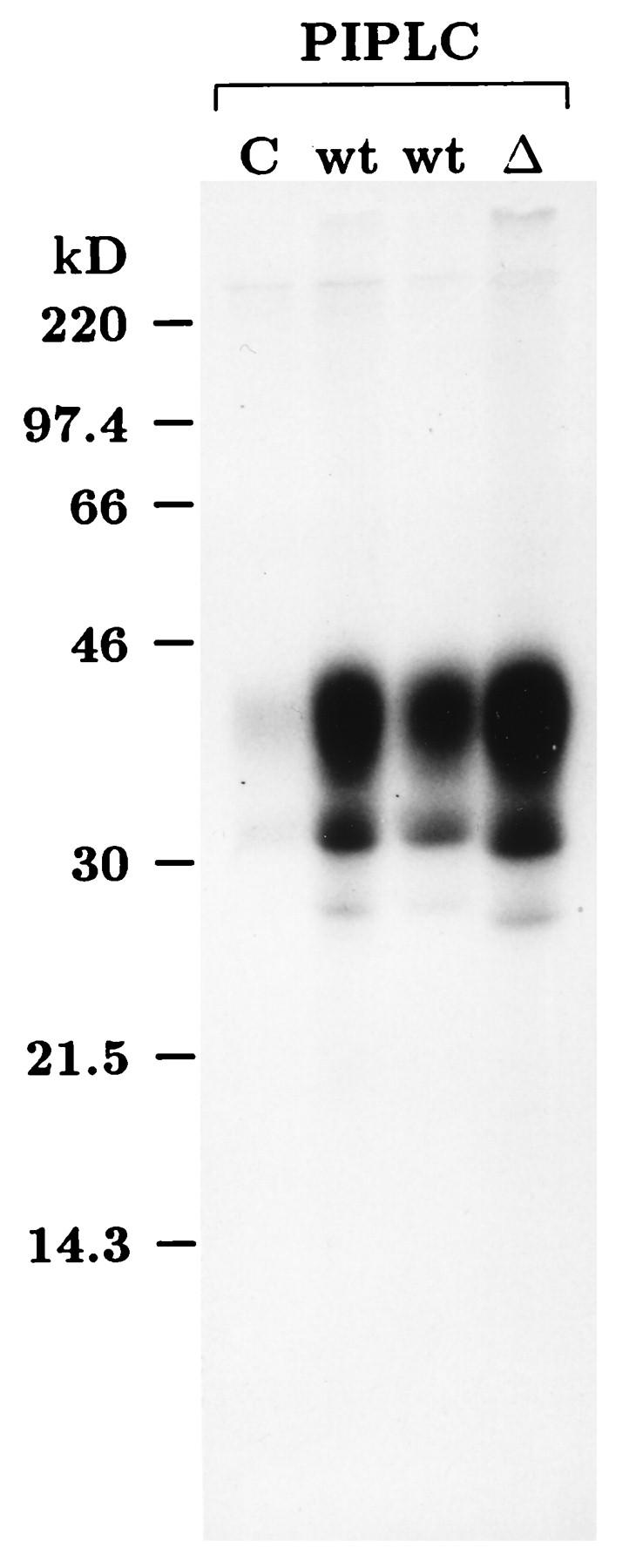
Localization on the plasma membrane and strong overexpression of recombinant prion proteins in transiently transfected Neuro2a cells. Neuro2a cells were transfected with the empty vector pL15TK (C), pCMV-PrP (wild type [wt]), or pCMV-PrPΔ114–121 (Δ). Twenty-four hours after transfection, cells were metabolically labeled with [35S]methionine-cysteine for 4 h and then incubated with MEM containing PIPLC for 1 h at 37°C. The cell-free supernatant was immunoprecipitated with rabbit antiserum Kan72 in lysing buffer (36). After SDS-PAGE on a 12.5% polyacrylamide gel, autoradiography was done for 5 days. The two lanes marked wt were derived from cultures transfected in parallel with equal amounts of pCMV-PrP. kD, kilodaltons.
Inability of PrPcΔ114–121 to acquire protease resistance and its dominant-negative phenotype with respect to PrPSc accumulation.
As a model system to study the influence of PrPcΔ114–121 on the accumulation of PrPSc, we used a persistently scrapie-infected cell clone, Sc+-MNB, which had been derived from the same mouse neuroblastoma cell line as Neuro2a (37). We transfected in parallel the empty expression vector, the mutant construct pCMV-PrPΔ114–121, and the wild-type construct pCMV-PrP into Sc+-MNB cells. After 42 h, cells were lysed for the preparation of cytoplasmic extracts, and we performed a limited proteinase K digestion, followed by denaturation with 3 M guanidine thiocyanate and methanol precipitation. Extracts were digested with peptide–N-glycosidase F (PNGase F) to reduce the heterogeneity of PrPSc due to the presence of asparagine-linked oligosaccharides. The results are shown in Fig. 4. To allow for the detection of PrPSc derived from PrPcΔ114–121, we used a long separating gel, the electrophoretic resolution of which was even higher than that of the gel shown in Fig. 1, where the difference in migration between the wild type and PrPcΔ114–121 is clearly evident. The Western blot (Fig. 4A) shows that the mutant protein PrPcΔ114–121 was not converted into a proteinase K-resistant isoform in Sc+-MNB cells, since no faster-migrating band appeared below wild-type PrPSc. In addition to not being converted into PrPSc, the presence of PrPcΔ114–121 led to a significant reduction in the steady-state level of protease-resistant, wild-type PrPc-derived PrPSc (Fig. 4A, lane 2). (Note that comparable amounts of cell extracts were loaded in all lanes, as is evident from a parallel gel stained with Coomassie fast stain [Fig. 4B].) To exclude the remote possibility that the observed inhibition was due to sensitization of PrPSc to proteinase K by PrPcΔ114–121 after cell lysis in the presence of detergents, we performed the following mixing experiment. Cleared cytoplasmic extracts from Sc+-MNB cells were used to lyse Neuro2a cells transiently transfected with pCMV-PrPΔ114–121 and, in parallel, cells transfected with the empty vector or pCMV-PrP. As shown by indirect immunofluorescence assay, about 50% of cells transiently transfected with pCMV-PrP or pCMV-PrPΔ114–121 did in fact overexpress exogenous prion proteins (data not shown). Lysates were then treated in exactly the same manner as described for the experiment shown in Fig. 4, including proteinase K digestion and incubation with PNGase F. However, after Western blotting, there was no visible difference in the intensity of the 19-kDa band representing PrPSc (data not shown). This experiment, therefore, revealed that the loss of signal intensity detected in lane 2 of Fig. 4A is indeed due to a reduction in the steady-state level of PrPSc, resulting from some process that occurs in living, pCMV-PrPΔ114–121-transfected cells before lysis.
FIG. 4.
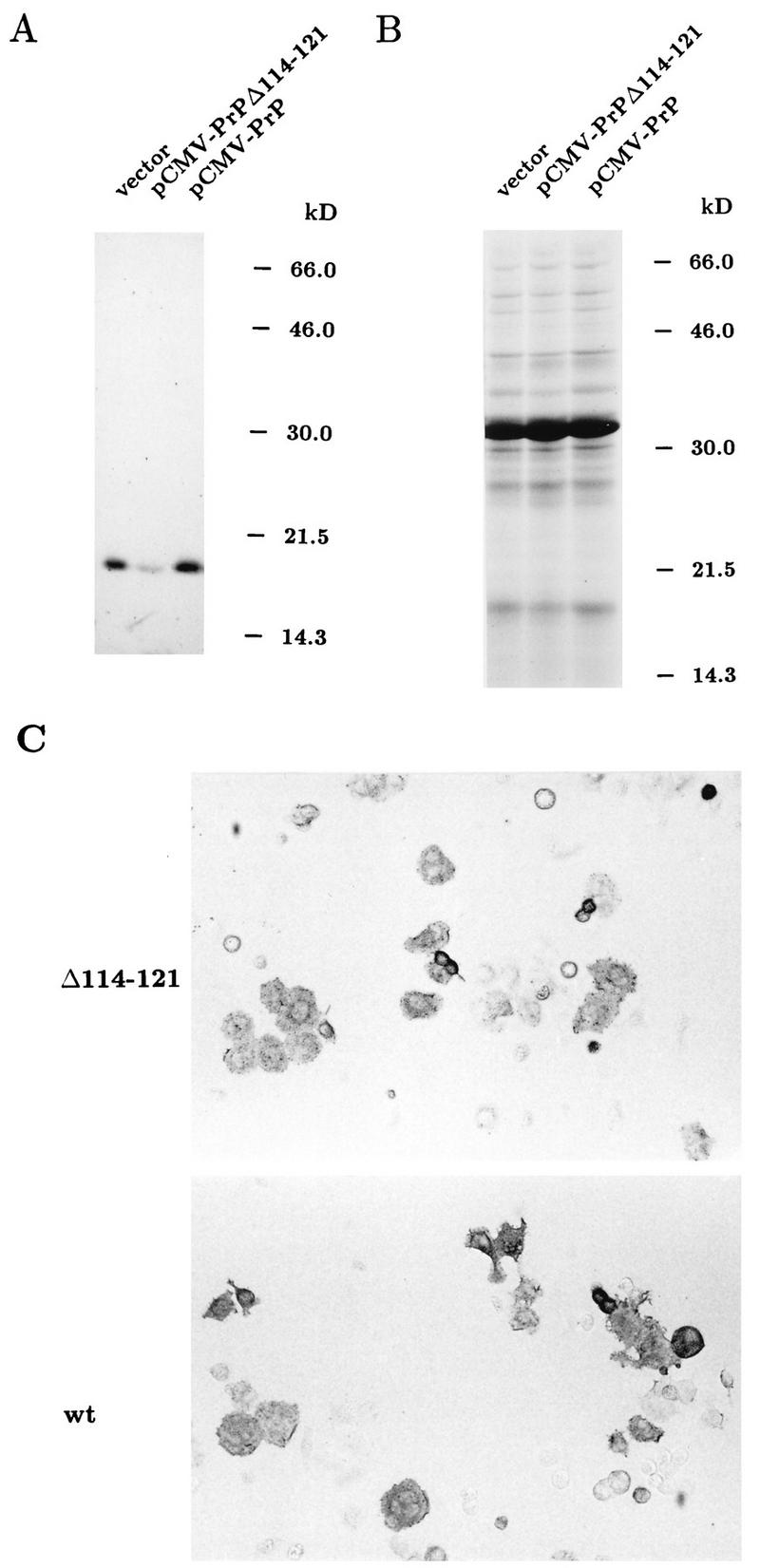
Western blot analysis of Sc+-MNB cells transfected with pCMV-PrPΔ114–121. Sc+-MNB cells were transfected with either the empty vector pL15TK, pCMV-PrPΔ114–121, or pCMV-PrP and were lysed 42 h later with deoxycholate and Triton X-100. Cytoplasmic extracts were digested with proteinase K (20 μg/ml) for 1 h at 37°C. After stopping the reaction with phenylmethylsulfonyl fluoride (2 mM for 15 min at room temperature), proteins were methanol precipitated, denatured in 3 M guanidine thiocyanate, and methanol precipitated again. The resulting protein pellet was resuspended in 1× denaturing buffer (NEB), digested with PNGase F for 5 h under conditions specified by the supplier, mixed with SDS-PAGE sample buffer, and run on a 12.5% polyacrylamide gel. (A) After blotting to nitrocellulose, the blot was reacted under standard conditions with polyclonal antibody Kan72; PrP-specific bands were visualized by enhanced chemiluminescence. As a result of digestion with PNGase F, the signal was reduced to a single band migrating to 19 kDa (kD). (B) A parallel gel loaded with precisely 50 times the volume of the same samples was incubated in Coomassie fast stain to visualize the total amount of protein per lane. The prominent band just above the 30-kDa marker represents proteinase K. (C) Sc+-MNB cells transfected in parallel with the same constructs used in panels A and B were analyzed for overexpressed PrPc by our modified cell ELISA method. Note that untransfected cells are hardly visible, whereas cells overexpressing PrP constructs are clearly stained. wt, wild type.
A comparison of the amount of PrPSc formed after transfection of pCMV-PrP with the amount in vector-transfected cells did not show a substantial increase in wild-type-PrP-transfected cells (Fig. 4A). This may be explained by saturation of the pathway of PrPSc formation in the Sc+-MNB cell population we used and/or by increased toxicity and loss of cells with high-level PrPSc accumulation. The transfection efficiency in this experiment (Fig. 4) was checked by our modified cell ELISA method with cultures transfected in parallel with those analyzed in Fig. 4A and B. As shown in Fig. 4C, the transfection efficiencies for the wild-type expression construct, pCMV-PrP, and mutant pCMV-PrPΔ114–121 were quite similar, with about 50% of cells overexpressing. Interestingly, the observed inhibition after transfection of PrPcΔ114–121 seemed to be stronger than expected, assuming that the inhibition of de novo PrPSc synthesis in each transfected cell is 100% and that 50% of cells are reached by transfection. We hypothesize that the strong inhibition we observed may have been due to a bystander effect, i.e., inhibition of PrPSc synthesis in nontransfected cells also. Borchelt et al. (1) have previously shown that PrPc can be released into the culture medium to some extent. In addition, Hay et al. showed that in in vitro systems under certain conditions, PrPc can exist in a secretory form (18). Assuming that to be the case for PrPcΔ114–121, it is possible that neighboring, nontransfected cells were reached through the culture medium, thus resulting in the inhibition of PrPSc production in these cells also.
DISCUSSION
We have shown here that a deletion of eight amino acids (codons 114 to 121) in mouse PrPc abrogates the conversion of the mutant protein into PrPSc. In addition, we have shown that this mutant inhibits in a trans-dominant fashion the accumulation of endogenous PrPSc in persistently scrapie-infected Sc+-MNB cells.
Strong overexpression of this mutant and in parallel wild-type PrPc was achieved by transfection of human cytomegalovirus immediate-early promoter/enhancer-driven expression constructs carrying the PCR-cloned wild-type or mutagenized ORF. We followed a strategy to amplify the PrP ORF with as little 5′ and 3′ untranslated sequence as possible so as to avoid the presence of any putative cis-acting negative regulatory elements and thus to achieve efficient overexpression. We always observed very similar expression levels of recombinant prion proteins after transfection of pCMV-PrP and pCMV-PrPΔ114–121 into uninfected or persistently infected mouse neuroblastoma cells. Furthermore, we demonstrated the expected reduction in the molecular mass of the mutant protein by Western blotting of extracts of tunicamycin-treated cells. In addition, in situ analysis of transiently transfected cells by a modified cell ELISA technique demonstrated a high percentage (50% on average) of cells strongly overexpressing the transfected transgene in both noninfected mouse Neuro2a cells and Sc+-MNB cells. The modified cell ELISA technique was also used to detect PrPSc accumulation in individual cells. In this case, PrPSc was rendered accessible to immunodetection by denaturation with guanidine hydrochloride. To discriminate between PrPc and PrPSc, cells were pretreated with proteinase K. Thus, this method allows us to alternatively visualize the overexpression of PrPc or the accumulation of PrPSc in situ.
The correct localization of the recombinant prion proteins on the cell membrane mediated through GPI anchors was verified by incubation of a live-cell monolayer with PIPLC and subsequent immunoprecipitation of the supernatant.
The inability of our deletion mutant to convert into PrPSc is consistent with many findings pointing to the region from codons 109 to 122 as being extremely critical for the conversion process. Gasset et al. demonstrated that PrP(109–122), especially the sequence AGAAAAGA (which is perfectly conserved among all of the species analyzed), showed the strongest tendency to form amyloid (16). Additional peptide studies have always come to the conclusion that the region from codons 109 to 122 is essential for PrPSc formation. For example, starting with a peptide encompassing codons 90 to 145, Zhang et al. narrowed down the region that forms a proteinase K-resistant core to codons 109 to 141 (49). Furthermore, a sequence comparison of a number of mammalian PrP genes indicated that amino acid exchanges in the region from codons 90 to 130 have a pronounced influence on the transmissibility of prions across species (41). Support for this view has also come from in vivo studies. It has previously been possible to reconstitute PrP knockout mice with PrP versions N-terminally truncated up to residue 80 (14). The C terminus of the critical region was narrowed down to as far as residue 145 because of the existence of an amber mutation in the PrP gene of a patient presenting with a variant of Gerstmann-Sträussler-Scheinker syndrome (22).
Interestingly, although the data mentioned above demonstrate the high fibrillogenic potential of PrP(109–122), the same region does not possess any significant secondary structure in the context of PrPc(23–231) (21, 39).
By partial unfolding and refolding studies of PrPSc, Kocisko et al. identified a 16-kDa resistant core region which seems to be essential for the conversion process (23). Mapping was performed by using antibodies directed against different epitopes in PrP. The result was that the N-terminal border of the resistant core may well overlap with the N terminus of the region from codons 109 to 122, which is compatible with our findings.
Muramoto et al. performed transfection experiments with PrP versions carrying deletions in various regions, including the complete H1 region (29). Their H1 deletion mutant was unable to be converted into a protease-resistant form. However, the data for our PrPcΔ114–121 mutant provide new information in this regard, since our deletion is much more subtle than the one described by Muramoto et al., thus allowing us to considerably narrow down the critical codons. Finally, they reported no information about a possible dominant-negative phenotype for their H1 deletion mutant.
In this work, when Sc+-MNB cells were transiently transfected with pCMV-PrPΔ114–121 and harvested 42 h later for Western blot analysis of PrPSc, the steady-state level was clearly reduced in comparison to that of the vector-transfected control, which is indicative of trans-dominant inhibition of PrPSc accumulation. The extent of the observed inhibition was even greater than expected if only successfully transfected cells had been completely blocked for PrPSc accumulation. The fact that PrPc molecules can be secreted to some extent (1, 18) leads us to hypothesize that even in nontransfected, neighboring cells, there may have been some inhibition of PrPSc accumulation due to shedding of PrPcΔ114–121, thus causing a so-called bystander effect. The theoretical possibility that PrPcΔ114–121 exerted its inhibiting effect by sensitization of PrPSc to proteinase K only after cells had been lysed in the presence of detergents was excluded (data not shown). Most probably, the underlying mechanism of the effect we have observed is the prevention of new PrPSc formation. However, the possibility that PrPcΔ114–121 destabilizes preexisting PrPSc in the context of living cells cannot be excluded at this moment.
Several previous reports have discussed strategies for inhibiting PrPSc accumulation. One major problem with drugs shown to exert antiscrapie effects is their intrinsic property to induce a wide variety of side effects. This is also reflected in the recently tested anthracycline IDX (42). Although this compound resulted in a significant reduction in scrapie-associated symptoms in hamsters, IDX could be given only intracerebrally at the same time the scrapie agent was inoculated, due to its intrinsic cytotoxicity and its limited ability to pass the blood-brain barrier. The same problems may be encountered when using the amyloid-binding dye Congo red or certain sulfated glycans, for which inhibitory effects on PrPSc accumulation in cell cultures and in animal experiments have previously been demonstrated (31). Another recent paper has described the use of so-called chemical chaperones (e.g., glycerol or dimethyl sulfoxide) for the reduction of PrPSc accumulation in cell culture experiments (45). In this case, however, many side effects are also to be expected.
The nonconvertibility and the efficacy of the trans-dominant inhibitory effect of PrPcΔ114–121 when it is expressed in a living organism remain to be established. Nevertheless, our work presented here may form a basis for a novel therapeutic or prophylactic strategy against prion diseases, namely, the use of deleted PrP molecules acting in a very specific manner to trans dominantly inhibit the accumulation of PrPSc. Any side effects of PrPcΔ114–121 or similar mutants, which in principle could be delivered by somatic gene therapy, or peptides mimicking PrPcΔ114–121 should be minimal compared to those of the chemical compounds mentioned above.
ACKNOWLEDGMENTS
We thank H. zur Hausen for continuous support and interest in this work. Furthermore, we thank B. Caughey and B. Chesebro for providing the persistently infected cell line Sc+-MNB and U. Ackermann for excellent photographic reproductions.
This work was supported by the Federal Ministry for Education, Science, Research and Technology (BMBF) in the framework of a national research network on transmissible spongiform encephalopathies (grant 01KI9457 to A.B.).
REFERENCES
- 1.Borchelt D R, Rogers M, Stahl N, Telling G, Prusiner S B. Release of the cellular prion protein from cultured cells after loss of its glycoinositol phospholipid anchor. Glycobiology. 1993;3:319–329. doi: 10.1093/glycob/3.4.319. [DOI] [PubMed] [Google Scholar]
- 2.Borchelt D R, Scott M, Taraboulos A, Stahl N, Prusiner S B. Scrapie and cellular prion proteins differ in their kinetics of synthesis and topology in cultured cells. J Cell Biol. 1990;110:743–752. doi: 10.1083/jcb.110.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brandner S, Isenmann S, Raeber A, Fischer M, Sailer A, Kobayashi Y, Marino S, Weissmann C, Aguzzi A. Normal host prion protein necessary for scrapie-induced neurotoxicity. Nature. 1996;379:339–343. doi: 10.1038/379339a0. [DOI] [PubMed] [Google Scholar]
- 4.Brown D R, Schmidt B, Kretzschmar H A. Role of microglia and host prion protein in neurotoxicity of a prion protein fragment. Nature. 1996;380:345–347. doi: 10.1038/380345a0. [DOI] [PubMed] [Google Scholar]
- 5.Bruce M E, Will R G, Ironside J W, McConnell I, Drummond D, Suttie A, McCardle L, Chree A, Hope J, Birkett C, Cousens S, Fraser H, Bostock C J. Transmissions to mice indicate that “new variant” CJD is caused by the BSE agent. Nature. 1997;389:498–501. doi: 10.1038/39057. [DOI] [PubMed] [Google Scholar]
- 6.Büeler H, Aguzzi A, Sailer A, Greiner R-A, Autenried P, Aguet M, Weissmann C. Mice devoid of PrP are resistant to scrapie. Cell. 1993;73:1339–1347. doi: 10.1016/0092-8674(93)90360-3. [DOI] [PubMed] [Google Scholar]
- 7.Caughey B, Chesebro B. Prion protein and the transmissible spongiform encephalopathies. Trends Cell Biol. 1997;7:56–62. doi: 10.1016/S0962-8924(96)10054-4. [DOI] [PubMed] [Google Scholar]
- 8.Caughey B, Race R E, Vogel M, Buchmeier M J, Chesebro B. In vitro expression in eukaryotic cells of a prion protein gene cloned from scrapie-infected mouse brain. Proc Natl Acad Sci USA. 1988;85:4657–4661. doi: 10.1073/pnas.85.13.4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caughey B, Raymond G J. The scrapie-associated form of PrP is made from a cell surface precursor that is both protease- and phospholipase-sensitive. J Biol Chem. 1991;266:18217–18223. [PubMed] [Google Scholar]
- 10.Caughey B W, Dong A, Bhat K S, Ernst D, Hayes S F, Caughey W S. Secondary structure analysis of the scrapie-associated protein PrP27-30 in water by infrared spectroscopy. Biochemistry. 1991;30:7672–7680. doi: 10.1021/bi00245a003. [DOI] [PubMed] [Google Scholar]
- 11.Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collinge J, Sidle K C L, Meads J, Ironside J, Hill A F. Molecular analysis of prion strain variation and the aetiology of “new variant” CJD. Nature. 1996;383:685–690. doi: 10.1038/383685a0. [DOI] [PubMed] [Google Scholar]
- 13.Daude N, Lehmann S, Harris D A. Identification of intermediate steps in the conversion of a mutant prion protein to a scrapie-like form in cultured cells. J Biol Chem. 1997;272:11604–11612. doi: 10.1074/jbc.272.17.11604. [DOI] [PubMed] [Google Scholar]
- 14.Fischer M, Rülicke T, Raeber A, Sailer A, Moser M, Oesch B, Brandner S, Aguzzi A, Weissmann C. Prion protein (PrP) with amino-proximal deletions restoring susceptibility of PrP knockout mice to scrapie. EMBO J. 1996;15:1255–1264. [PMC free article] [PubMed] [Google Scholar]
- 15.Forloni G, Angeretti N, Chiesa R, Monzani E, Salmona M, Bugiani O, Tagliavini F. Neurotoxicity of a prion protein fragment. Nature. 1993;362:543–546. doi: 10.1038/362543a0. [DOI] [PubMed] [Google Scholar]
- 16.Gasset M, Baldwin M A, Lloyd D H, Gabriel J-M, Holtzman D M, Cohen F, Fletterick R, Prusiner S B. Predicted α-helical regions of the prion protein when synthesized as peptides from amyloid. Proc Natl Acad Sci USA. 1992;89:10940–10944. doi: 10.1073/pnas.89.22.10940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrison P M, Bamborough P, Daggett V, Prusiner S B, Cohen F E. The prion folding problem. Curr Opin Struct Biol. 1997;7:53–59. doi: 10.1016/s0959-440x(97)80007-3. [DOI] [PubMed] [Google Scholar]
- 18.Hay B, Prusiner S B, Lingappa V R. Evidence for a secretory form of the cellular prion protein. Biochemistry. 1987;26:8110–8115. doi: 10.1021/bi00399a014. [DOI] [PubMed] [Google Scholar]
- 19.Hill A F, Desbruslais M, Joiner S, Sidle K C L, Gowland I, Collinge J, Doey L J, Lantos P. The same prion strain causes vCJD and BSE. Nature. 1997;389:448–450. doi: 10.1038/38925. [DOI] [PubMed] [Google Scholar]
- 20.Hope J, Shearman M S, Baxter H C, Chong A, Kelly S M, Price N C. Cytotoxicity of prion protein peptide (PrP106–126) differs in mechanism from the cytotoxic activity of the Alzheimer’s disease amyloid peptide, A beta 25–35. Neurodegeneration. 1996;5:1–11. doi: 10.1006/neur.1996.0001. [DOI] [PubMed] [Google Scholar]
- 21.Hornemann S, Korth C, Oesch B, Riek R, Wider G, Wüthrich K, Glockshuber R. Recombinant full-length murine prion protein, mPrP(23–231): purification and spectroscopic characterization. FEBS Lett. 1997;413:277–281. doi: 10.1016/s0014-5793(97)00921-6. [DOI] [PubMed] [Google Scholar]
- 22.Kitamoto T, Iizuka R, Tateishi J. An amber mutation of prion protein in Gerstmann-Sträussler syndrome with mutant PrP plaques. Biochem Biophys Res Commun. 1993;192:525–531. doi: 10.1006/bbrc.1993.1447. [DOI] [PubMed] [Google Scholar]
- 23.Kocisko D A, Lansbury P T, Caughey B. Partial unfolding and refolding of scrapie-associated prion protein: evidence for a critical 16-kDa C-terminal domain. Biochemistry. 1996;35:13434–13442. doi: 10.1021/bi9610562. [DOI] [PubMed] [Google Scholar]
- 24.Küpper J H, DeMurcia G, Bürkle A. Inhibition of poly(ADP-ribosyl)ation by overexpressing the poly(ADP-ribose)polymerase DNA-binding domain in mammalian cells. J Biol Chem. 1990;265:18721–18724. [PubMed] [Google Scholar]
- 25.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 26.Lasmezas C I, Deslys J-P, Demaimay R, Adjou K T, Lamoury F, Dormont D, Robain O, Ironside J, Hauw J-J. BSE transmission to macaques. Nature. 1996;381:743–744. doi: 10.1038/381743a0. [DOI] [PubMed] [Google Scholar]
- 27.Locht C, Chesebro B, Race R, Keith J M. Molecular cloning and complete sequence of prion protein cDNA from mouse brain infected with the scrapie agent. Proc Natl Acad Sci USA. 1986;83:6372–6376. doi: 10.1073/pnas.83.17.6372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKinley M P, Taraboulos A, Kenaga L, Serban D, Stieber A, DeArmond S J, Prusiner S B, Gonatas N. Ultrastructural localization of scrapie prion proteins in cytoplasmic vesicles of infected cultured cells. Lab Invest. 1991;65:622–630. [PubMed] [Google Scholar]
- 29.Muramoto T, Scott M, Cohen F E, Prusiner S B. Recombinant scrapie-like prion protein of 106 amino acids in soluble. Proc Natl Acad Sci USA. 1996;93:15457–15462. doi: 10.1073/pnas.93.26.15457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan K-M, Baldwin M, Nguyen J, Gasset M, Serban A, Groth D, Mehlhorn I, Huang Z, Fletterick R J, Cohen F E, Prusiner S B. Conversion of α-helices into β-sheets features in the formation of the scrapie prion proteins. Proc Natl Acad Sci USA. 1993;90:10962–10966. doi: 10.1073/pnas.90.23.10962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Priola S A, Caughey B. Inhibition of scrapie-associated PrP accumulation. Mol Neurobiol. 1994;8:113–120. doi: 10.1007/BF02780661. [DOI] [PubMed] [Google Scholar]
- 32.Priola S A, Caughey B, Race R E, Chesebro B. Heterologous PrP molecules interfere with accumulation of protease-resistant PrP in scrapie-infected murine neuroblastoma cells. J Virol. 1994;68:4873–4878. doi: 10.1128/jvi.68.8.4873-4878.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Priola S A, Chesebro B. A single hamster PrP amino acid blocks conversion to protease-resistant PrP in scrapie-infected mouse neuroblastoma cells. J Virol. 1995;69:7754–7758. doi: 10.1128/jvi.69.12.7754-7758.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prusiner S B. Molecular biology of prion diseases. Science. 1991;252:1515–1522. doi: 10.1126/science.1675487. [DOI] [PubMed] [Google Scholar]
- 35.Prusiner S B, Telling G, Cohen F E, DeArmond S J. Prion diseases of humans and animals. Semin Virol. 1996;7:159–173. [Google Scholar]
- 36.Race R E, Caughey B, Graham K, Ernst D, Chesebro B. Analyses of frequency of infection, specific infectivity, and prion protein biosynthesis in scrapie-infected neuroblastoma cell clones. J Virol. 1988;62:2845–2849. doi: 10.1128/jvi.62.8.2845-2849.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Race R E, Fadness L H, Chesebro B. Characterization of scrapie infection in mouse neuroblastoma cells. J Gen Virol. 1987;68:1391–1399. doi: 10.1099/0022-1317-68-5-1391. [DOI] [PubMed] [Google Scholar]
- 38.Riek R, Hornemann S, Wider G, Billeter M, Glockshuber R, Wüthrich K. NMR structure of the mouse prion protein domain PrP(121–231) Nature. 1996;382:180–182. doi: 10.1038/382180a0. [DOI] [PubMed] [Google Scholar]
- 39.Riek R, Hornemann S, Wider G, Glockshuber R, Wüthrich K. NMR characterization of the full-length recombinant murine prion protein, mPrP(23–231) FEBS Lett. 1997;413:282–288. doi: 10.1016/s0014-5793(97)00920-4. [DOI] [PubMed] [Google Scholar]
- 40.Safar J, Roller P P, Gajdusek D C, Gibbs C J. Conformational transitions, dissociation, and unfolding of scrapie amyloid (prion) protein. J Biol Chem. 1993;268:20276–20284. [PubMed] [Google Scholar]
- 41.Schätzl H M, Da Costa M, Taylor L, Cohen F E, Prusiner S B. Prion protein gene variation among primates. J Mol Biol. 1995;245:362–374. doi: 10.1006/jmbi.1994.0030. [DOI] [PubMed] [Google Scholar]
- 42.Tagliavini F, McArthur R A, Canciani B, Giaccone G, Porro M, Bugiani M, Lievens P M-J, Bugiani O, Peri E, Dall’Ara P, Rocchi M, Poli G, Forloni G, Bandiera T, Varasi M, Suarato A, Cassutti P, Cervini M A, Lansen J, Salmona M, Post C. Effectiveness of anthracycline against experimental prion disease in syrian hamsters. Science. 1997;276:1119–1122. doi: 10.1126/science.276.5315.1119. [DOI] [PubMed] [Google Scholar]
- 43.Taraboulos A, Raeber A J, Borchelt D R, Serban D, Prusiner S B. Synthesis and trafficking of prion proteins in cultured cells. Mol Biol Cell. 1992;3:851–863. doi: 10.1091/mbc.3.8.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taraboulos A, Serban D, Prusiner S B. Scrapie prion proteins accumulate in the cytoplasm of persistently infected cultured cells. J Cell Biol. 1990;110:2117–2132. doi: 10.1083/jcb.110.6.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tatzelt J, Prusiner S B, Welch W J. Chemical chaperones interfere with the formation of scrapie prion protein. EMBO J. 1996;15:6363–6373. [PMC free article] [PubMed] [Google Scholar]
- 46.Vey M, Pilkuhn S, Wille H, Nixon R, DeArmond S J, Smart E J, Anderson R G, Taraboulos A, Prusiner S B. Subcellular colocalization of the cellular and scrapie prion proteins in caveolae-like membraneous domains. Proc Natl Acad Sci USA. 1996;93:14945–14949. doi: 10.1073/pnas.93.25.14945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weissmann C. Molecular biology of prion diseases. Trends Cell Biol. 1994;4:10–14. doi: 10.1016/0962-8924(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 48.Will R G, Ironside J W, Zeidler M, Cousens S N, Estibeiro K, Alperovitch A, Poser S, Pocchiari M, Hofman A, Smith P G. A new variant of Creutzfeldt-Jakob disease in the UK. Lancet. 1996;347:921–925. doi: 10.1016/s0140-6736(96)91412-9. [DOI] [PubMed] [Google Scholar]
- 49.Zhang H, Kaneko K, Nguyen J T, Livshits T L, Baldwin M A, Cohen F E, James T L, Prusiner S B. Conformational transitions in peptides containing two putative α-helices of the prion protein. J Mol Biol. 1995;250:514–526. doi: 10.1006/jmbi.1995.0395. [DOI] [PubMed] [Google Scholar]


