Abstract
In this report, we assessed the evolution of the cytotoxic T-lymphocyte (CTL) response induced by an epitope vaccine. In two macaques immunized with a mixture of lipopeptides derived from simian immunodeficiency virus (SIV) Nef and Gag proteins, CTL responses were directed against the same, single epitope of the Nef protein (amino acids 128 to 137) presenting an alanine at position 136 (Nef 128-137/136A). However, after 5 months of SIV infection, peripheral blood mononuclear cells from both macaques lost their ability to be stimulated by autologous SIV-infected cells while still retaining their capacity to generate cytotoxic responses after specific Nef 128-137/136A peptide stimulation. The sequences of the pathogenic viral isolate used for the challenge showed a mixture of several variants. Within the Nef epitopic sequence from amino acids 128 to 137, 82% of viral variants expressed the epitopic peptide Nef 128-137/136A; the remaining variants presented a threonine at position 136 (Nef 128-137/136T). In contrast, sequence analysis of cloned proviral DNA obtained from both macaques 5 months after SIV challenge showed a different pattern of quasi-species variants; 100% of clones presented a threonine at position 136 (Nef 128-137/136T), suggesting the disappearance of viral variants with an alanine at this position under antiviral pressure exerted by Nef 128-137/136A-specific CTLs. In addition, 12 months after SIV challenge, six of eight clones from one macaque presented a glutamic acid at position 131 (Nef 128-137/131E+136T), which was not found in the infecting isolate. Furthermore, CTLs generated very early after SIV challenge were able to lyse cells sensitized with the Nef 128-137/136A epitope. In contrast, lysis was significantly less effective or even did not occur when either the selected peptide Nef 128-137/136T or the escape variant peptide Nef 128-137/131E+136T was used in a target cell sensitization assay. Dose analysis of peptides used to sensitize target cells as well as a major histocompatibility complex (MHC)-peptide stability assay suggested that the selected peptide Nef 128-137/136T has an altered capacity to bind to the MHC. These data suggest that CTL pressure leads to the selection of viral variants and to the emergence of escape mutants and supports the fact that immunization should elicit broad CTL responses.
There is increasing evidence that cytotoxic T lymphocytes (CTL) play an essential role in the immune response against human immunodeficiency virus (HIV). A positive role for these effector cells has been strongly suggested by many studies. For example, CTL have been associated with the initial reduction of early viremia during primary infection (2, 20). In addition, CTL have been shown to play an important role in the maintenance of the asymptomatic phase of infection, and the reduction of HIV type 1-specific CTL precursors has been correlated with disease progression (5, 17, 34). Furthermore, CD8+ T lymphocytes can exert a very efficient inhibition of virus replication through nonlytic mechanisms (15, 23, 38, 40). Some individuals exposed to HIV but uninfected and thus apparently protected have HIV-specific CTL responses (6, 21, 35, 36). Likewise, recent studies with simian immunodeficiency virus (SIV) and feline immunodeficiency virus models have demonstrated the positive role of virus-specific CTL in vaccine-induced protection (11, 12). Nevertheless CTL responses appear not to be sufficient to completely eradicate the virus, and AIDS ultimately develops.
We reasoned that if vaccine-induced CTL are an important defense mechanism for controlling virus replication associated with the asymptomatic stage and for retarding disease development, the selection of viral escape mutants under the pressure of CTL may occur, thereby accelerating disease progression. Indeed, recent studies with HIV-infected humans have pointed out the importance of viral mutations in epitopic peptides under strong antiviral pressure exerted in vivo by antigen-specific CTL (7). Moreover, the selection of viral escape mutants in both primary (3, 33) and late-stage (13) HIV infections has been demonstrated.
The present study was designed to compare CTL responses induced by epitope vaccination before and after SIV challenge with the aim of characterizing the evolution of epitopic peptides recognized by CTL. In two macaques, lipopeptide-induced CTL recognized one peptide from the Nef protein. Stimulation with autologous SIV-infected cells obtained at different times postchallenge showed that these epitope vaccine-induced CTL were generated in the first months after SIV infection but not at a later stage. Viral sequencing performed after the SIV challenge revealed changes within the epitope vaccine which, in turn, led to an immunologic escape variant.
MATERIALS AND METHODS
Sequences and synthesis of lipopeptides.
Five peptides from the SIV Nef protein were synthesized: LP1 (amino acids [aa] 101 to 126, LP2 (aa 125 to 147), LP3 (aa 155 to 178), LP4 (aa 201 to 225), and LP5 (aa 221 to 247). Two peptides from the SIV Gag protein were also produced: LP6 (aa 165 to 195) and LP7 (aa 246 to 281). The selected sequences were identical to sequences that have been reported elsewhere (4) but were modified at the C-terminal positions with enantiomerically pure Nɛ-palmitoyl-lysylamide. The lipopeptides were synthesized by solid-phase synthesis as previously described (9). The lipopeptides were purified to >90% homogeneity by reverse-phase high-pressure liquid chromatography and characterized by amino acid composition and molecular mass determinations.
Short peptides.
Overlapping peptides spanning the entire sequences of the lipopeptides were synthesized by Neosystem, Strasbourg, France.
Animals and immunization protocol.
Two rhesus macaques (Macaca mulatta) (C16 and C18) were immunized by subcutaneous injection of a mixture of seven lipopeptides (500 μg each) in incomplete Freund adjuvant on days 0, 21, and 42. The two macaques were challenged 6 months later by intravenous injection of 10 50% animal infectious doses. One such dose is the amount of virus that infects 50% of macaques; this dose was previously determined in an in vivo titration assay (1). All animal experiments were performed in accordance with European Economic Community guidelines.
Generation of CTL cell lines.
Peripheral blood mononuclear cells (PBMC) were isolated by density gradient centrifugation through lymphocyte separation medium (Pharmacia, Uppsala, Sweden) and were used immediately or stored at −180°C in liquid nitrogen. Antipeptide CTL cell lines were obtained by culturing monkey PBMC (2 × 106 cells/ml) in 24-well microtiter plates (Costar, Cambridge, Mass.) containing RPMI 1640 medium supplemented with 100 U of penicillin per ml, 100 μg of streptomycin per ml, 2 mM l-glutamine, 1% nonessential amino acids, 1 mM sodium pyruvate, 10 mM HEPES buffer, 2 × 10−5 M 2-mercaptoethanol, and 10% heat-inactivated fetal calf serum. A mixture of the seven free peptides (5 μM each) was added to each well. The plates were incubated for 3 days at 37°C, and interleukin 2 (Boehringer GmbH, Mannheim, Germany) was then added to each well (10 IU/ml). On days 7 and 14, effector cells were stimulated by adding fresh autologous PBMC that had been pulsed for 2 h with the peptide pool (5 μM each); stimulating cells were then washed and irradiated (4,000 rads) (effector/stimulator ratio, 1:2). PBMC from macaques that had been challenged with SIV were collected and cultured (106 cells/ml) in the same culture medium. The production of SIV antigens on infected CD4+ cells was induced by stimulating PBMC with 10 μg of concanavalin A (Sigma, St. Louis, Mo.) per ml for 3 days. Interleukin 2 (10 IU/ml) was then added, and cells were diluted to 5 × 105/ml twice a week as previously described (25).
Phenotypic analysis of CTL cell lines.
Phenotypes of cell lines were determined on the day of the chromium release test (CRT) (see below) by incubating cells with fluorescein isothiocyanate-conjugated anti-CD4 (OKT4; Ortho Diagnostic Systems, Raritan, N.J.) and phycoerythrin-conjugated anti-CD8 (Leu-2a; Becton Dickinson, Mountain View, Calif.) monoclonal antibodies for 30 min at 4°C. The cells were washed with phosphate-buffered saline and examined for the percentage of positively staining cells in an Epics Elite flow cytometer (Coulter, Margency, France). Isotype-matched irrelevant antibodies (Coulter) were used as controls.
In vitro transformation of B-cell lines.
B-lymphoblastoid cell lines (B-LCLs) were generated by incubating serial dilutions of PBMC with supernatants of the S594 cell line. This line (kindly provided by N. Letvin) produces the immortalizing baboon herpesvirus (herpesvirus papio). B-LCLs were then cultured in culture medium supplemented with 10% fetal calf serum.
Recombinant vaccinia viruses.
The sequence encoding the Nef protein was inserted into vaccinia virus. Wild-type vaccinia virus (Copenhagen strain) was used as a control. All the constructions were prepared by Transgene, Strasbourg, France.
CRT.
Target cells were sensitized with peptides. B-LCLs (106) were incubated either overnight or for 1 h with long or short peptides (concentration, 10−5 to 10−8 M), respectively, at 37°C in a humidified 5% CO2 atmosphere. To obtain target cells presenting SIVmac251 gene products, B-LCLs were incubated at a concentration of 106 cells/ml with recombinant vaccinia virus (20 PFU/cell) for 18 h under the same conditions. The B-LCLs were then washed, labeled with 100 μCi of Na251CrO4 (NEN Life Science Products, Courtaboeuf Les Ullis, France) for 1 h, washed twice, and used as target cells. The CRT was performed with V-bottom 96-well microtiter plates. The cytolytic activity of anti-SIV cell lines was measured by mixing 5 × 103 51Cr-labeled target cells with effector cells at various effector cell/target cell (E/T) ratios in a final volume of 200 μl/well. Duplicate wells were seeded for each E/T ratio. Plates were incubated for 4 h at 37°C; 100 μl of supernatant was then removed from each well and analyzed with a gamma counter. Spontaneous release was determined by incubating target cells with medium alone; it never exceeded 20% of the total 51Cr incorporated. Results are expressed as specific Cr release: 100 × [(experimental counts per minute − spontaneous counts per minute)/(maximum counts per minute − spontaneous counts per minute)]. The within-sample variation never exceeded 5% of the absolute counts per minute.
Major histocompatibility complex (MHC)-peptide stability assay.
B-LCL autologous target cells were pulsed with the short peptide (10 μM Nef 128-137/136T) for 1 h, washed, and incubated for 1 to 14 h before the addition of effector cells for the CRT.
DNA preparation.
PBMC were isolated as described above and washed in RPMI 1640 medium. Aliquots (107 cells) were then incubated overnight at 52°C in 1 ml of lysis buffer (10 mM Tris-HCl [pH 8.3], 50 mM KCl, 2.5 mM MgCl2, 0.45% Tween 20, 400 μg of proteinase K per ml). The DNA was extracted with phenol-chloroform and precipitated with ethanol. The pellet was washed with 70% ethanol, dried, resuspended in 10 mM Tris (pH 7.5), and quantified by measuring the optical density at 260 nm.
PCR amplification.
Nested PCRs were performed with 100-μl reaction mixtures containing 200 μM each deoxynucleotide triphosphate (dNTP) (Pharmacia), 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 2.5 U of Taq polymerase (Gibco BRL, Life Technologies, Gaithersburg, Md.), and 20 pmol of each primer (Genset, Paris, France). The primers used in the first round of PCR were Nef1 (5′-AGGCTCTCTGCGACCCTACG-3′) and Nef2 (5′-AGAACCTCCCAGGGCTCAATCT-3′). VJ11 (5′-ATGGGTGGAGCTATTTCCATG-3′) and VJ12 (5′-TTAGCCTTCTTCTAACCTC-3′) were used in the second round (representing the entire nef gene). Each initial reaction mixture contained 1 μg of DNA, and 5 μl of the first-round PCR mixture was used in the second round. The reactions were carried out with a DNA Thermocycler 9600 (Perkin-Elmer, Branchburg, N.J.) for 40 cycles. The first step was 1 min at 96°C; the following steps were 30 s at 95°C, 30 s at 55°C, and 1 min at 72°C, with a final incubation at 72°C for 5 min. Amplified products were visualized on a 1.5% agarose gel after staining with ethidium bromide.
Reverse transcription-PCR.
Viral isolate RNA was extracted from 400 μl of the viral stock by use of 300 μl of phenol acid (Appligene Oncor, Illkirch, France) and 300 μl of extraction buffer (7 M urea, 0.35 M NaCl, 10 mM Tris-HCl [pH 7.5], 10 mM EDTA, 1% sodium dodecyl sulfate). After vortexing and centrifugation, the supernatant was extracted twice with phenol and twice with chloroform and ethanol precipitated with 2 μg of tRNA. After centrifugation, the RNA pellet was washed with 70% ethanol, dried, and resuspended in 50 μl of sterile water. Five microliters was subjected to reverse transcription for 1 h at 37°C with a 25-μl reaction mixture containing 50 mM Tris-HCl (pH 8.3), 75 mM KCl, 3 mM MgCl2, 8 mM dithiothreitol, 400 μM each dNTP, 50 pmol of primer Nef2, 30 U of RNasin (Promega, Madison, Wis.), and 200 U of Moloney murine leukemia virus reverse transcriptase (Gibco BRL). The PCR mixture was incubated for 5 min at 90°C, and 5 μl of the cDNA mixture was amplified under the same PCR conditions as those described above but with VJ11 and VJ12.
Cloning and sequencing.
After purification on a Qiaquick column (Qiagen, Courtaboeuf Les Ullis, France), 50 ng of the PCR product was ligated overnight at 15°C with 50 ng of pTAG vector (R&D Systems Europe, Abingdon, United Kingdom) in a 10-μl reaction mixture containing 50 mM Tris-HCl (pH 7.6), 10 mM MgCl2, 1 mM ATP, 1 mM dithiothreitol, 5% polyethylene glycol 8000, and 1 U of T4 DNA ligase (Gibco BRL). The ligation product (0.1 μl) was transformed in Escherichia coli TG1, and the few white colonies obtained on Luria-Bertani plates with ampicillin were selected. DNA was extracted with the Easy Prep Plasmid Prep kit (Pharmacia), and 500 ng was sequenced with dye terminator chemistry on a 373A sequencer (ABI/Perkin-Elmer). All the sequences obtained were aligned with the SeqEd program.
RESULTS AND DISCUSSION
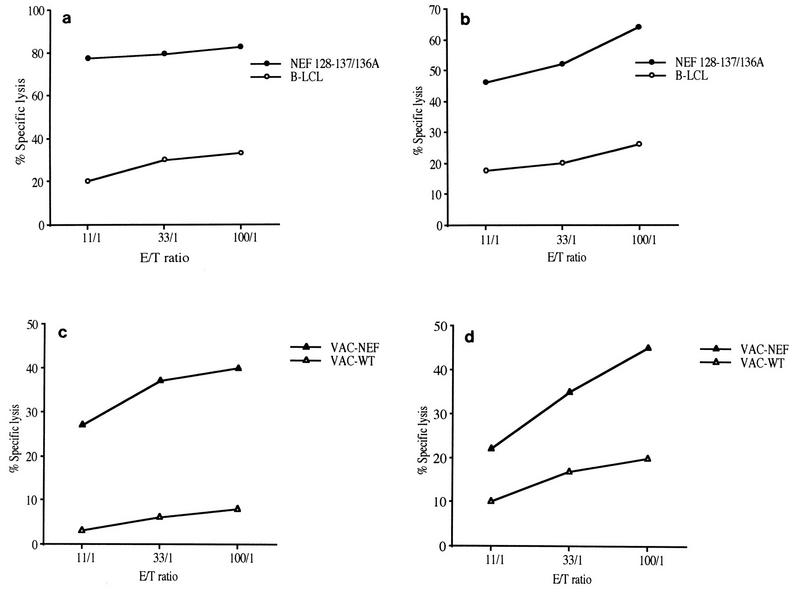
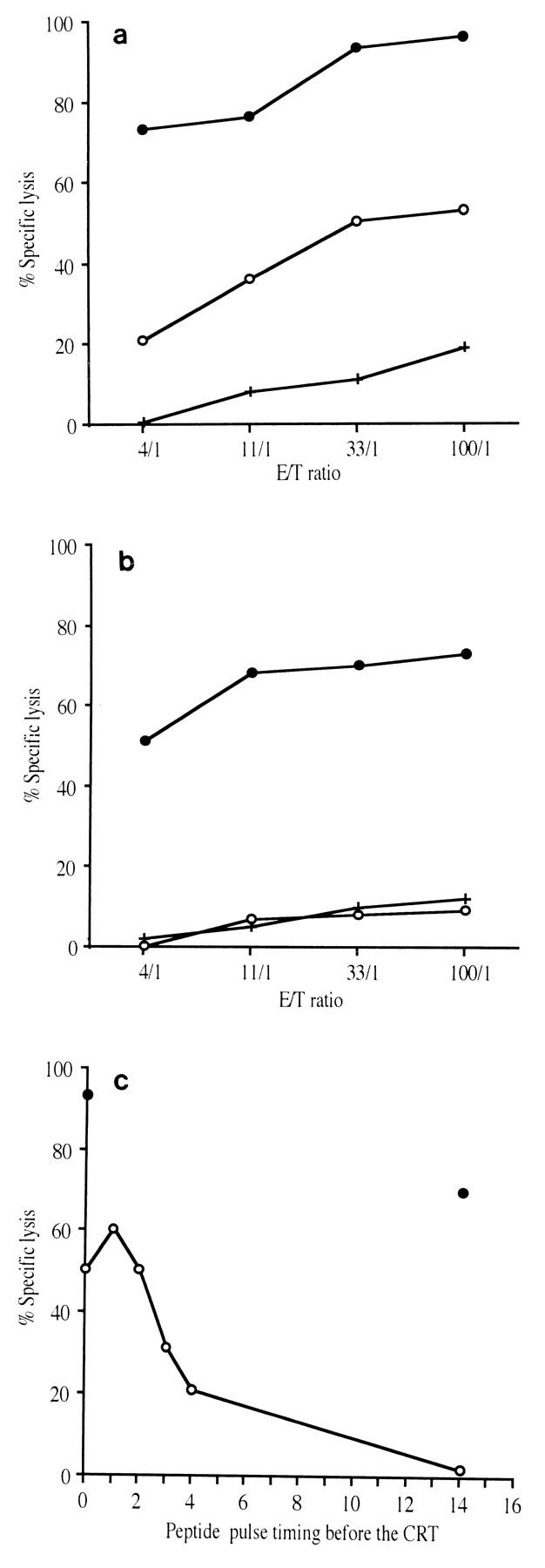
In order to obtain further insight into the development of an effective vaccine against HIV, we analyzed the precise characteristics of the CTL response and its evolution over time after epitope vaccination by using a macaque SIV model. Two macaques were immunized with seven lipopeptides derived from SIV Nef and Gag proteins. The vaccine-induced CTL responses were examined by stimulating macaque PBMC with a mixture of the corresponding seven free peptides. In both macaques the same single long peptide from the pool of immunizing peptides (Nef aa 125 to 147) was recognized. The minimal CTL epitope recognized by effector cells within this 23-mer sequence was identified by sensitizing target cells with short peptides in a CRT. The same single 10-mer peptide, Nef 128-137/136A (GLEGIYYSAR), was found to be the minimal epitope for CTL in both macaques (Table 1 and Fig. 1a and b). Additional experiments showed that Nef 128-137 peptide-sensitized B-LCLs from both C16 and C18 were lysed by peptide-specific effector CTL derived from both macaques in a cross-presentation assay (data not shown), strongly suggesting that the macaques share a common MHC molecule. On the day of the CRT, effector cells were >70% CD8+ T cells, as might be expected for class I-restricted antigen-specific CTL. It was important to determine whether these CTL also recognized and lysed virus-infected cells. We assessed the ability of antipeptide CTL to recognize naturally processed antigen by using autologous B-LCLs infected with a recombinant vaccinia virus encoding the SIV nef gene (Vac-Nef) as target cells. Antipeptide CTL from both macaques efficiently lysed these target cells, suggesting that peptide Nef 128-137/136A was endogenously processed (Fig. 1c and d).
TABLE 1.
Precise specificities of CTL in immunized macaques
| Peptide incubated with target cellsd | % Specific lysisa of effector cells from the following macaqueb at the indicated E/T ratioc:
|
|||||
|---|---|---|---|---|---|---|
| C16
|
C18
|
|||||
| 60:1 | 20:1 | 6:1 | 30:1 | 10:1 | 3:1 | |
| None | 5 | 2 | 1 | 1 | 1 | 0 |
| Nef 125-147 | 25 | 17 | 10 | 45 | 36 | 18 |
| Nef 125-138 | 32 | 17 | 11 | 26 | 10 | 9 |
| Nef 133-147 | 4 | 4 | 1 | 4 | 2 | 1 |
| Nef 128-137 | 43 | 30 | 12 | 21 | 13 | 8 |
The CRT was performed after three in vitro stimulations and was considered positive if the specific 51Cr release observed with target cells exceeded that observed with autologous cells alone by more than 10%.
Effector cells were obtained from macaque PBMC stimulated with the mixture of the seven peptides.
Target cells (5 × 103) were labeled with 51Cr and then incubated for 4 h with various numbers of effector cells.
Target cells were autologous B-LCLs immortalized by herpesvirus papio and sensitized with 10 μM each peptide.
FIG. 1.
Cytotoxic activities were tested with bulk cultured cells of macaques C16 (a and c) and C18 (b and d). These effector cells were CTL specific for immunodominant epitopic peptide Nef 128-137/136A (GLEGIYYSAR) as described in Materials and Methods. Target cells were autologous B-LCLs alone (○) or incubated with peptide Nef 128-137/136A (•) (a and b) and infected with wild-type vaccinia virus (▵) or with Vac-Nef recombinant vaccinia virus (▴) (c and d). Mean values for 51Cr release from target cells are expressed as percent specific lysis.
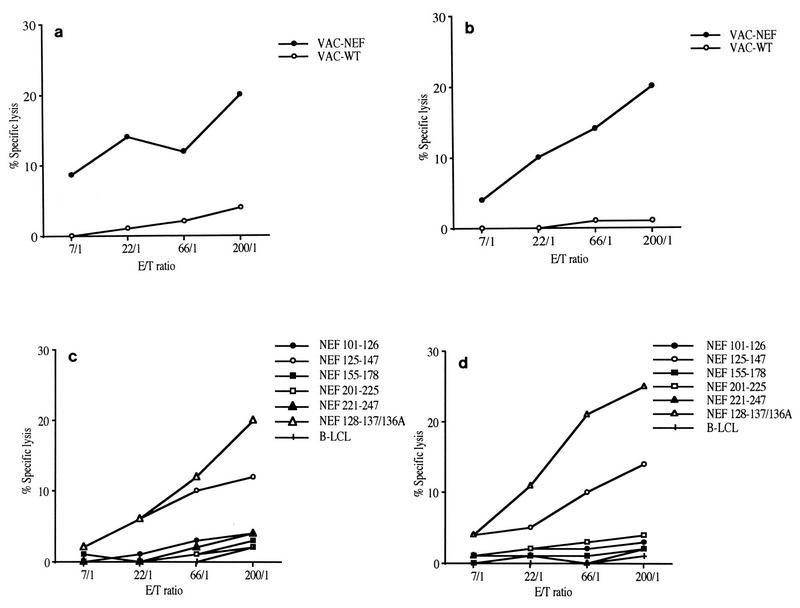
In order to examine the evolution of vaccination-induced anti-Nef CTL responses after SIV challenge, we assessed the ability of virus-infected cells to stimulate CTL responses as soon as day 30 after experimental infection. T-cell lines were then established by activating PBMC with autologous SIV-infected cells as described previously for the evaluation of these responses in infected macaques (25, 26, 39). CTL obtained from both macaques recognized Vac-Nef-infected B-LCL target cells (Fig. 2a and b). The lysing capacities of these cell lines were also evaluated with peptide-sensitized target cells. The five long Nef peptides (included in the immunogens) as well as overlapping peptides spanning the whole Nef sequence were tested. Only the CTL epitope of Nef 128-137/136A was recognized in both macaques (Fig. 2c and d), indicating that this peptide is immunodominant in these macaques.
FIG. 2.
Cytotoxic activities were assayed with CTL obtained by stimulating PBMC of both macaques (C16 [a and c] and C18 [b and d]) with autologous SIV-infected cells after infection, as described in Materials and Methods. Target cells were autologous B-LCLs infected with wild-type vaccinia virus (○) or with Vac-Nef recombinant vaccinia virus (•) (a and b) and incubated with various peptides or without peptide (+) (c and d).
Several studies have shown that a narrowed oligoclonal CTL response may disappear soon after HIV infection, and this pattern of response has been considered to be an indicator of a poor prognosis (3, 29, 30). Here we show that Nef-specific CTL which recognized the Nef 128-137/136A epitope were found regularly during the first few months of infection after stimulation of PBMC with autologous SIV-infected cells. However, no CTL could be detected by this procedure 5 months after infection, either against Vac-Nef-infected target cells (Fig. 3) or against peptide-sensitized target cells (data not shown). This loss of response could have been due to the disappearance of CTL precursors, as demonstrated in the lymphocytic choriomeningitis virus (LCMV)-docile murine model (27, 28). However, this hypothesis can be ruled out, since macaque PBMC could still be stimulated by the original peptide Nef 128-137/136A in vitro. The antipeptide cell lines were capable of lysing Vac-Nef-infected target cells and CTL precursors were still detectable 1 year postinfection (data not shown).
FIG. 3.
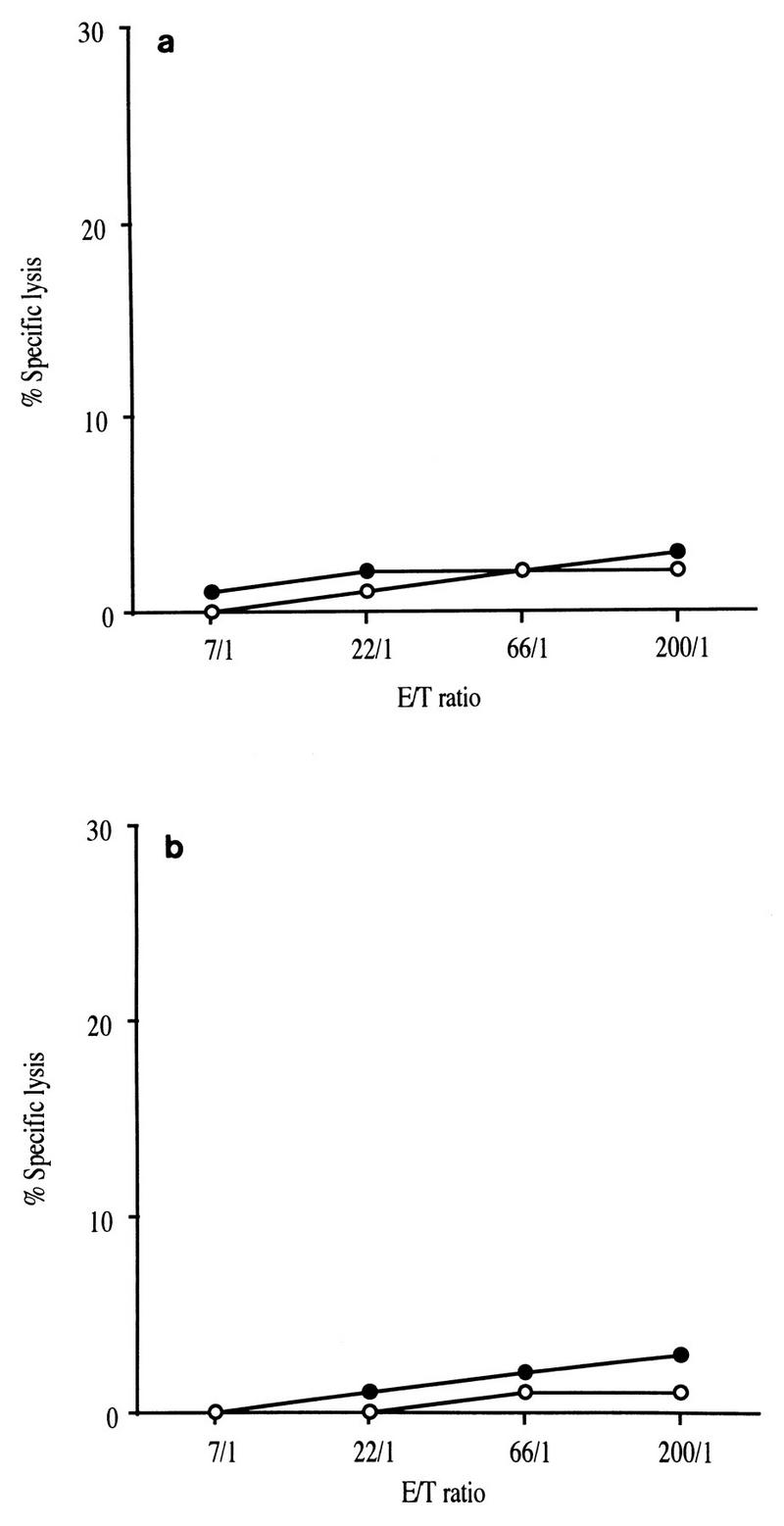
Cytotoxic activities were assayed with CTL stimulated by autologous SIV-infected cells after 5 months of experimental infection. Target cells were autologous B-LCLs infected with wild-type vaccinia virus (○) or with Vac-Nef recombinant vaccinia virus (•). (a) Macaque C16. (b) Macaque C18.
Another explanation for the loss of the CTL response is that a mutation occurred within the viral sequence, leading to viral escape from recognition by the CTL. Selection of a viral escape mutant was first demonstrated in vivo with LCMV in mice (32), and several reports have documented the occurrence of viral escape mutants within the Env, Gag and Nef proteins during HIV infection (3, 7, 13, 24, 31, 33). This phenomenon can take place at several stages during HIV infection, from the primary stages (3, 33) to the asymptomatic phase (7, 24, 31) and even in the late stages of infection, after the sequence has remained stable for several years (13). The relevance of such mutations to the development of increased viral burden or the onset of AIDS is still controversial, although some studies have described a link between these events (3, 13). The precise mechanisms determining the occurrence of escape mutations are not fully understood, but there is growing evidence that HIV-specific CTL responses may select viral escape mutants by exerting a strong controlling pressure on virus replication in vivo. Indeed, these mutations appear to be particularly deleterious when they occur within a unique and/or immunodominant epitopic sequence (29, 30). In this context, selection for mutant variants and subsequent disease progression were observed after an HIV-1-specific CTL clone was transferred to an AIDS patient (19).
In order to test the hypothesis of a viral escape mutation in the Nef epitopic sequence of the vaccinated and infected macaques, we first sequenced the challenge viral isolate used in this study. The viral stock (originally donated by R. Desrosiers) came from the lymphocytes of an infected macaque; it had subsequently been passaged once on macaque PBMC and was therefore probably multiclonal. Sequence analysis confirmed some degree of heterogeneity in the sequence of Nef aa 128 to 137. Changes were observed at position 136, the majority of sequenced clones (9 of 11; 82%) having an alanine at this position but two clones (18%) having a threonine (Fig. 4). Proviral DNA obtained from the macaques PBMC 5 months postinfection was amplified, cloned, and sequenced; all the resulting viral sequences were identical (Fig. 4) and included a threonine at position 136. The clones obtained 1 year postinfection showed a similar pattern, and there was an additional change in samples from macaque C18: six of eight clones (75%) had a glutamic acid at position 131. All clones from the original isolate had a glycine at this position. These data suggest that the immune pressure induced by CTL influenced the selection of variants (Nef 128-137/136T) and then led to the emergence of viral escape mutants (Nef 128-137/131E+136T). However, it is possible that the viruses having an A at position 136 had a restricted replicative capacity in vivo, leading to preferential expansion of the Nef 128-137/136T variants. This is unlikely, since our viral stock had been passaged only once on macaque PBMC after ex vivo isolation from a macaque with AIDS. We checked this conclusion by analyzing the sequences of material collected from infected unrelated macaques at various times after infection. In all cases, the majority of clones had an A at position 136, suggesting that a virus with this characteristic can replicate in vivo.
FIG. 4.
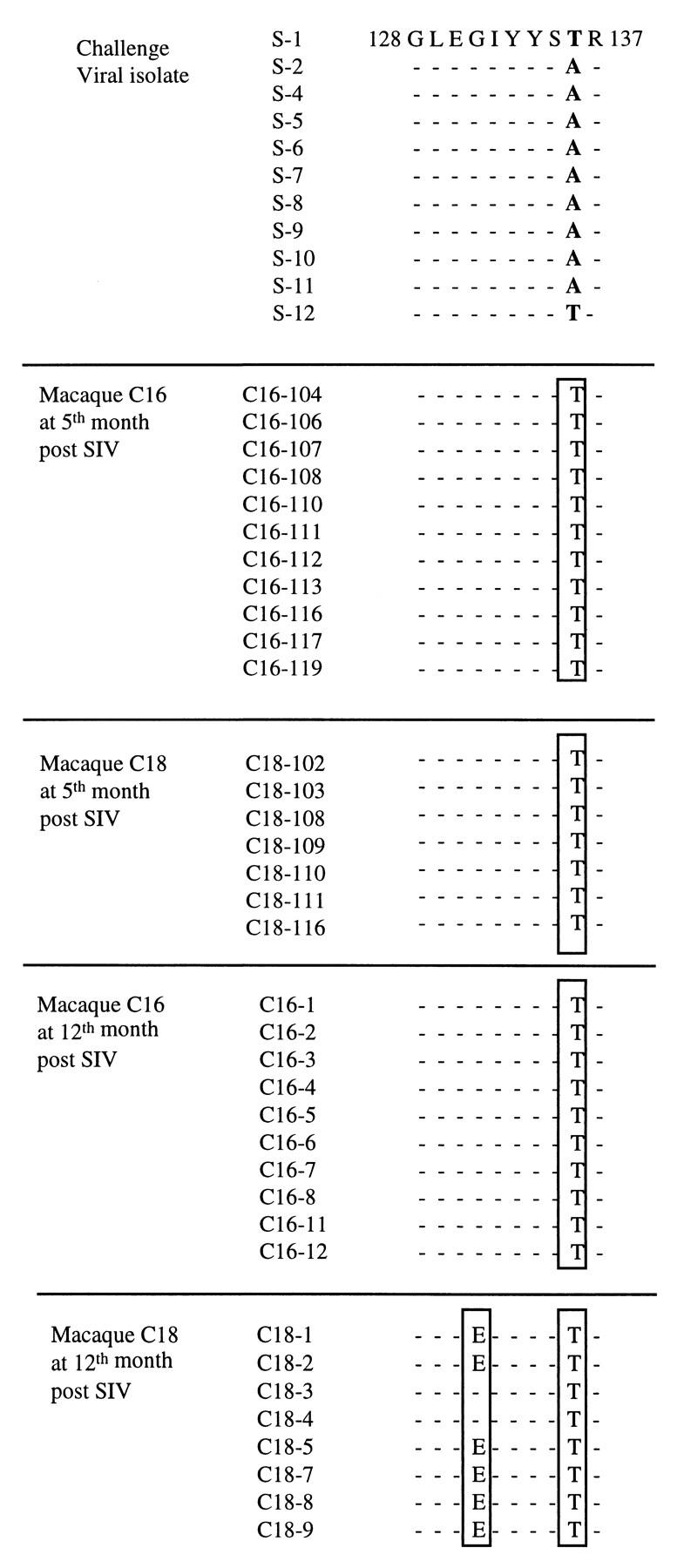
Comparison of different sequences of viral clones isolated from PBMC of infected macaques at 5 and 12 months after SIV challenge with pathogenic viral isolate SIVmac251. Analysis was performed within the epitopic sequence Nef 128-137.
The modified epitopes could escape CTL surveillance in several ways. Antigen processing and the transport of peptides may be affected by mutations within the flanking regions (7, 8, 10), peptide binding to the MHC may be altered by mutations in the anchor residues (7, 31, 32), and mutations may affect recognition by T-cell receptors (TCR). Mutations that affect recognition by TCR may lead to nonrecognition by CTL (22) or to an additional escape mechanism by antagonism and anergy of effector cells (1a, 14, 16, 18, 37).
The impact of these changes in the Nef epitopic sequence from aa 128 to 137 on CTL activity was then analyzed. Antipeptide CTL cell lines were obtained by stimulating PBMC with the peptide Nef 128-137/136A, since the immunizing peptides were based on the sequence of molecular clone BK28, which has an A at position 136. We tested the ability of these CTL cell lines to recognize and lyse autologous target cells sensitized with the various Nef peptides (Nef 128-137/136A, Nef 128-137/136T, and Nef 128-137/131E+136T). CTL cell lines from macaques C16 and C18 recognized autologous target cells sensitized with either peptide Nef 128-137/136A or peptide Nef 128-137/136T, although the activity against peptide Nef 128-137/136T was much lower (Fig. 5). In contrast, peptide Nef 128-137/131E+136T was not recognized at all by the CTL cell line from macaque C18 (Fig. 5b). This result suggests that one mutation in a CTL epitope can occur under CTL pressure and that multiple alterations within a particular epitope may allow the virus to escape CTL recognition.
FIG. 5.
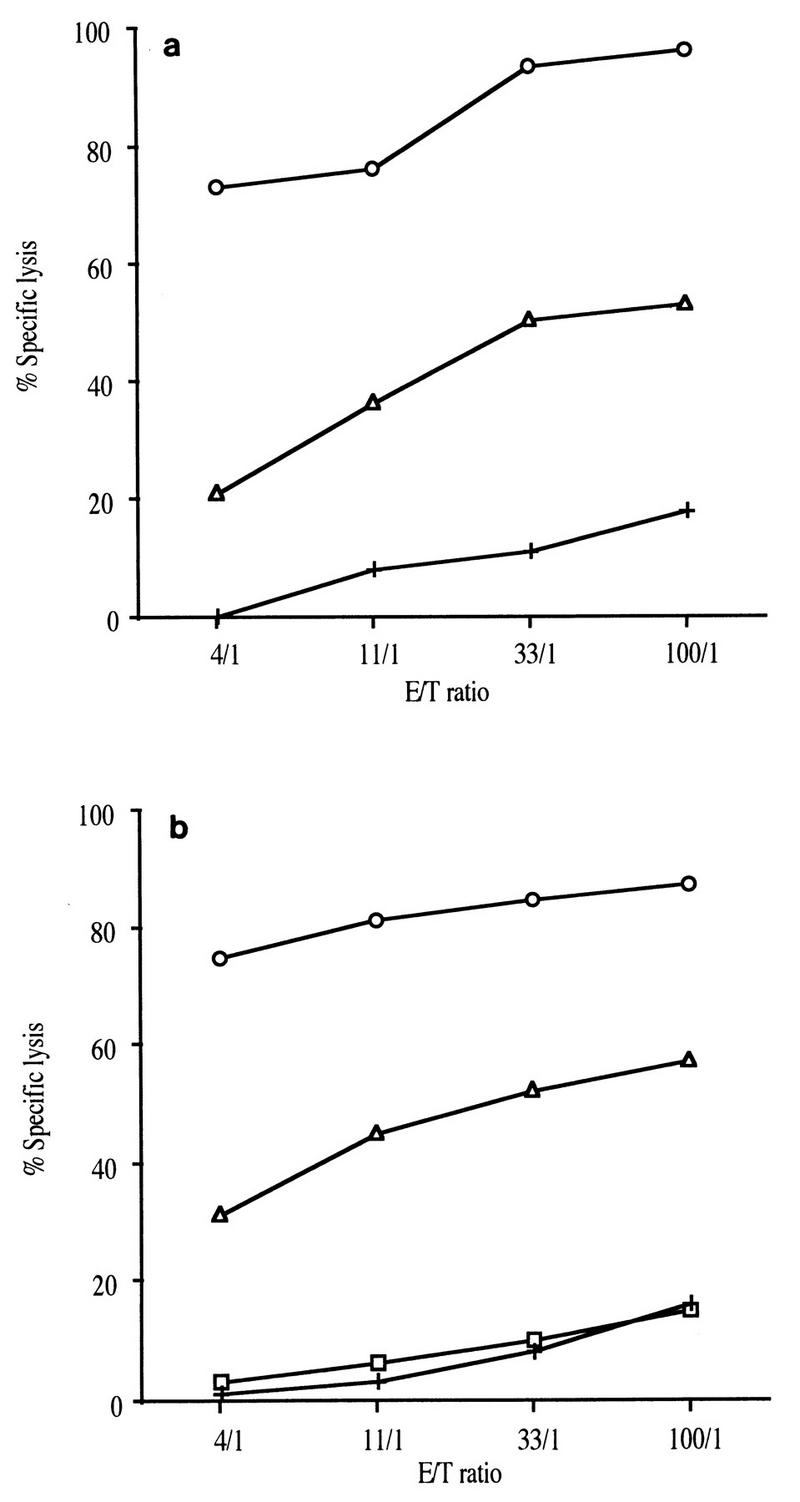
(a) Anti-Nef 128-137/136A CTL cell lines from macaque C16 were tested against autologous target cells pulsed with the original peptide Nef 128-137/136A (○), with the selected peptide Nef 128-137/136T (▵), or without peptide (+). (b) Identical data for macaque C18 with autologous target cells pulsed with the original peptide Nef 128-137/136A (○), with the selected peptide Nef 128-137/136T (▵), with the mutant peptide Nef 128-137/131E+136T (□), or without peptide (+).
In order to gain further insight into the molecular mechanisms of CTL-peptide interactions, we tested the ability of the anti–peptide Nef 128-137/136A CTL cell line to recognize and lyse B-LCL target cells sensitized by peptide Nef 128-137/136A or Nef 128-137/136T in a peptide dilution assay. CTL cell lines were established by stimulating PBMC with peptide Nef 128-137/136A and were tested on autologous target cells sensitized with serial dilutions of peptides ranging from 10−5 to 10−8 M. The impairment of lysis by recognition by CTL from infected macaques was 1 log10 unit for CTL from C16 and 0.5 log10 unit for CTL from C18 (Fig. 6). This result suggests that the changes in the amino acid sequence altered the interactions among the MHC, the peptide, and TCR within this trimolecular complex of antigen recognition. The nature of this impairment was further examined in an MHC-peptide stability assay with CTL from macaque C16. Class I surface proteins loaded with peptide Nef 128-137/136T were not stable, since autologous target cells were not recognized when sensitized with this peptide 14 h before the CRT (Fig. 7). Incubation for only 4 h prior to the CRT led to a 60% reduction in CTL activity (Fig. 7c). In contrast, the MHC–peptide Nef 128-137/136A complex appeared to be very stable, since target cells were very efficiently lysed after incubation with peptide Nef 128-137/136A overnight (Fig. 7a and b).
FIG. 6.
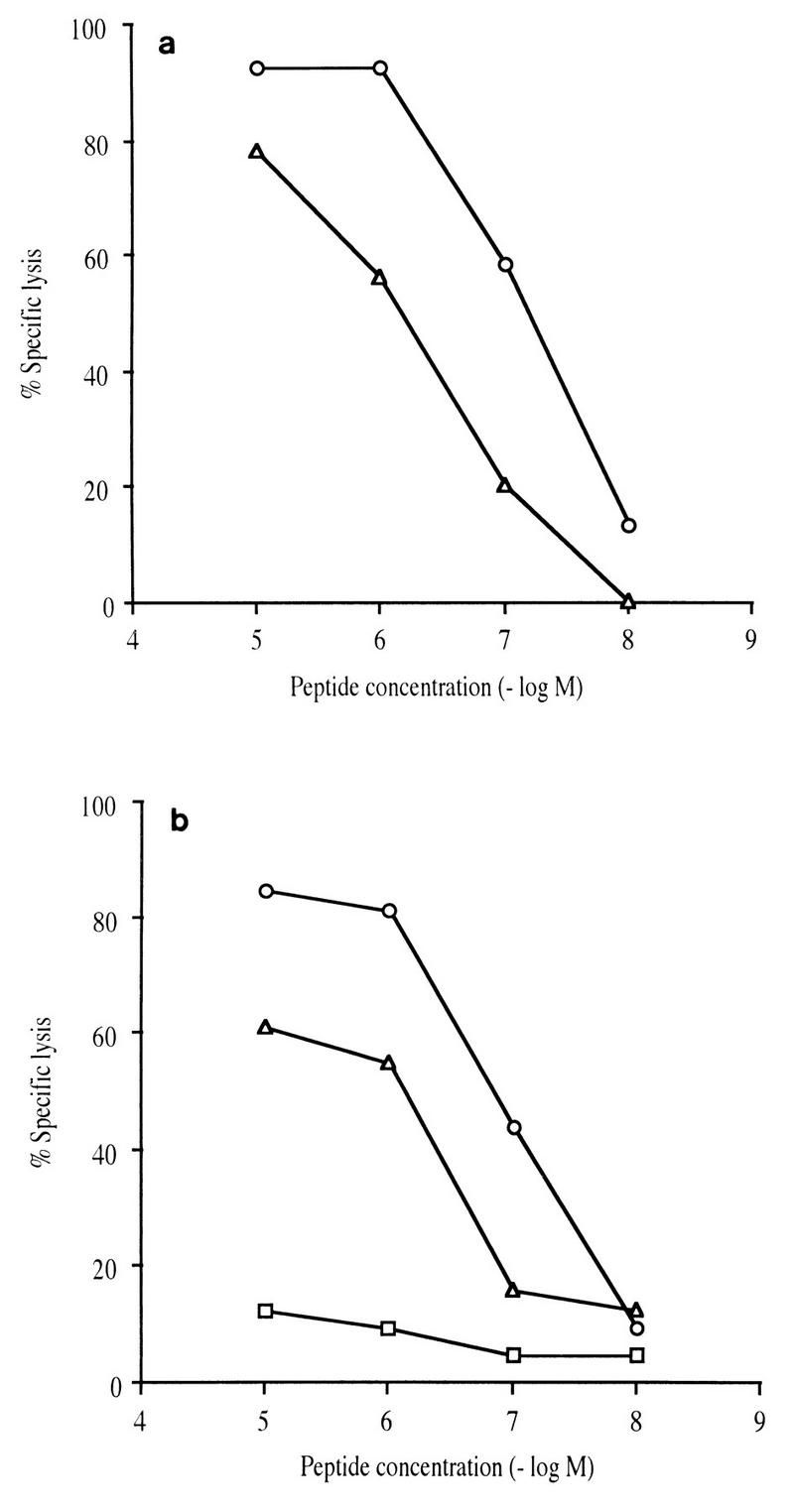
Specific lysis observed on target cells sensitized with different peptides: the original peptide Nef 128-137/136A (○), the selected peptide Nef 128-137/136T (▵), and the mutant peptide Nef 128-137/131E+136T (□). Target cells were autologous B-LCLs preincubated for 1 h with 10-fold serial dilutions of the different peptides. The CTL cell lines were obtained by stimulation of PBMC with the original Nef 128-137/136A peptide from macaque C16 (a) and macaque C18 (b). The E/T cell ratio was 10:1.
FIG. 7.
Time dependence of the peptide sensitization of targets. The CTL cell line was obtained by stimulating PBMC from macaque C16 with the original peptide Nef 128-137/136A. Autologous target cells were sensitized with the original peptide Nef 128-137/136A (•) or with the selected peptide Nef 128-137/136T (○) for 1 h, washed, and incubated for a further 1 h (a) or for 14 h (b) prior to the addition of effector cells. +, no peptide. (c) Data obtained at an E/T cell ratio of 100:1 with different incubation times for peptides with target cells (range, 1 to 14 h).
A recent study showed that vaccine-induced monospecific cytotoxic CD4+ cells may be irrelevant for protection if the host becomes contaminated with a viral isolate whose sequence differs from the CTL epitopic sequence (16). Our study demonstrates that induction by a vaccine of CD8+ CTL recognizing only one epitope might not be sufficient to protect against virus and may also favor the selection of viral variants and the emergence of viral escape mutants. In conclusion, these data support the fact that immunization should induce multispecific CTL responses.
ACKNOWLEDGMENTS
This work was supported by the Agence Nationale de Recherche sur le SIDA and by European Union Programme EVA. Lorenzo Mortara holds a Sidaction/Ensemble Contre le SIDA fellowship.
We thank Anne Marie Aubertin for the gift of the Nef1 and Nef2 primers and Claire Bony and Corinne Rommens for excellent technical assistance.
REFERENCES
- 1.Aubertin, A. M. Personal communication.
- 1a.Bertoletti A, Sette A, Chisari F V, Penna A, Levrero M, De Carli M, Fiaccadori F, Ferrari C. Natural variants of cytotoxic epitopes are T-cell receptor antagonists for antiviral cytotoxic T cells. Nature (London) 1994;369:407–410. doi: 10.1038/369407a0. [DOI] [PubMed] [Google Scholar]
- 2.Borrow P, Lewicki H, Hahn B H, Shaw G M, Oldstone M B A. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borrow P, Lewicki H, Wei X, Horwitz M S, Peffer N, Meyers H, Nelson J A, Gairin J E, Hahn B H, Oldstone M B A, Shaw G M. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat Med. 1997;3:205–211. doi: 10.1038/nm0297-205. [DOI] [PubMed] [Google Scholar]
- 4.Bourgault I, Chirat F, Tartar A, Lévy J P, Guillet J G, Venet A. Simian immunodeficiency virus as model for vaccination against HIV. Induction in rhesus macaques of GAG- or NEF-specific cytotoxic T lymphocytes by lipopeptides. J Immunol. 1994;152:2530–2537. [PubMed] [Google Scholar]
- 5.Carmichael A, Jin X, Sissons P, Borysiewicz L. Quantitative analysis of the human immunodeficiency virus type 1 (HIV-1)-specific cytotoxic T lymphocyte (CTL) response at different stages of HIV-1 infection: differential CTL responses to HIV-1 and Epstein-Barr virus in late disease. J Exp Med. 1993;177:249–256. doi: 10.1084/jem.177.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheynier R, Langlade-Demoyen P, Marescot M R, Blanche S, Blondin G, Wain-Hobson S, Griscelli C, Vilmer E, Plata F. Cytotoxic T lymphocyte responses in the peripheral blood of children born to human immunodeficiency virus-1-infected mothers. Eur J Immunol. 1992;22:2211–2217. doi: 10.1002/eji.1830220905. [DOI] [PubMed] [Google Scholar]
- 7.Couillin I, Culmann-Penciolelli B, Gomard E, Choppin J, Lévy J P, Guillet J G, Saragosti S. Impaired cytotoxic T lymphocyte recognition due to genetic variations in the main immunogenic region of the human immunodeficiency virus 1 NEF protein. J Exp Med. 1994;180:1129–1134. doi: 10.1084/jem.180.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Couillin I, Connan F, Culmann-Penciolelli B, Gomard E, Guillet J G, Choppin J. HLA-dependent variations in human immunodeficiency virus Nef protein alter peptide/HLA binding. Eur J Immunol. 1995;25:728–732. doi: 10.1002/eji.1830250315. [DOI] [PubMed] [Google Scholar]
- 9.Deprez B, Sauzet J P, Boutillon C, Martinon F, Tartar A, Sergheraert C, Guillet J G, Gomard E, Gras-Masse H. Comparative efficiency of simple lipopeptide constructs for in vivo induction of virus-specific CTL. Vaccine. 1996;14:375–382. doi: 10.1016/0264-410x(95)00220-u. [DOI] [PubMed] [Google Scholar]
- 10.Eisenlohr L C, Yewdell J W, Bennink J R. Flanking sequences influence the presentation of an endogenously synthesized peptide to cytotoxic T lymphocytes. J Exp Med. 1992;175:481–487. doi: 10.1084/jem.175.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flynn J N, Keating P, Hosie M J, Mackett M, Stephens E B, Beatty J A, Neil J C, Jarrett O. Env-specific CTL predominate in cats protected from feline immunodeficiency virus infection by vaccination. J Immunol. 1996;157:3658–3665. [PubMed] [Google Scholar]
- 12.Gallimore A, Cranage M, Cook N, Almond N, Bootman J, Rud E, Silvera P, Dennis M, Corcoran T, Stott J, McMichael A, Gotch F. Early suppression of SIV replication by CD8+ nef-specific cytotoxic T cells in vaccinated macaques. Nat Med. 1995;1:1167–1173. doi: 10.1038/nm1195-1167. [DOI] [PubMed] [Google Scholar]
- 13.Goulder P J R, Phillips R E, Colbert R A, McAdam S, Ogg G, Nowak M A, Giangrande P, Luzzi G, Morgan B, Edwards A, McMichael A J, Rowland-Jones S. Late escape from an immunodominant cytotoxic T-lymphocyte response associated with progression to AIDS. Nat Med. 1997;3:212–217. doi: 10.1038/nm0297-212. [DOI] [PubMed] [Google Scholar]
- 14.Jameson S C, Carbone F R, Bevan M J. Clone-specific T cell receptor antagonists of major histocompatibility complex class I-restricted cytotoxic T cells. J Exp Med. 1993;177:1541–1550. doi: 10.1084/jem.177.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kannagi M, Chalifoux L V, Lord C I, Letvin N L. Suppression of simian immunodeficiency virus replication in vitro by CD8+ lymphocytes. J Immunol. 1988;140:2237–2242. [PubMed] [Google Scholar]
- 16.Kent S J, Greenberg P D, Hoffman M C, Akridge R E, McElrath M J. Antagonism of vaccine-induced HIV-1-specific CD4+ T cells by primary HIV-1 infection. Potential mechanism of vaccine failure. J Immunol. 1997;158:807–815. [PubMed] [Google Scholar]
- 17.Klein M R, van Baalen C A, Holwerda A M, Kerkhof Garde S R, Bende R J, Keet I P M, Eeftinck-Schattenkerk J K M, Osterhaus A D M E, Schuitemaker H, Miedema F. Kinetics of gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long-term asymptomatics. J Exp Med. 1995;181:1365–1372. doi: 10.1084/jem.181.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klenerman P, Rowland-Jones S, McAdam S, Edwards J, Daenke S, Lalloo D, Köppe B, Rosenberg W, Boyd D, Edwards A, Giangrande P, Phillips R E, McMichael A J. Cytotoxic T-cell activity antagonized by naturally occurring HIV-1 Gag variants. Nature (London) 1994;369:403–407. doi: 10.1038/369403a0. [DOI] [PubMed] [Google Scholar]
- 19.Koenig S, Conley A J, Brewah Y A, Jones G M, Leath S, Boots L J, Davey V, Pantaleo G, Demarest J F, Carter C, Wannebo C, Yannelli J R, Rosenberg S A, Lane H C. Transfer of HIV-1-specific cytotoxic T lymphocytes to an AIDS patient leads to selection for mutant HIV variants and subsequent disease progression. Nat Med. 1995;1:330–336. doi: 10.1038/nm0495-330. [DOI] [PubMed] [Google Scholar]
- 20.Koup R A, Safrit J T, Cao Y, Andrews C A, McLeod G, Borkowsky W, Farthing C, Ho D D. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langlade-Demoyen P, Ngo-Giang-Huong N, Ferchal F, Oksenhendler E. Human immunodeficiency virus (HIV) nef-specific cytotoxic T lymphocytes in noninfected heterosexual contact of HIV-infected patients. J Clin Invest. 1994;93:1293–1297. doi: 10.1172/JCI117085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lill N L, Tevethia M J, Hendrickson W G, Tevethia S S. Cytotoxic T lymphocytes (CTL) against a transforming gene product select for transformed cells with point mutations within sequences encoding CTL recognition epitopes. J Exp Med. 1992;176:449–457. doi: 10.1084/jem.176.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mackewicz C E, Ortega H W, Levy J A. CD8+ cell anti-HIV activity correlates with the clinical state of the infected individual. J Clin Invest. 1991;87:1462–1466. doi: 10.1172/JCI115153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyerhans A, Dadaglio G, Vartanian J P, Langlade-Demoyen P, Frank R, Asjö B, Plata F, Wain-Hobson S. In vivo persistence of a HIV-1-encoded HLA-B27-restricted cytotoxic T lymphocyte epitope despite specific in vitro reactivity. Eur J Immunol. 1991;21:2637–2640. doi: 10.1002/eji.1830211051. [DOI] [PubMed] [Google Scholar]
- 25.Miller M D, Lord C I, Stallard V, Mazzara G P, Letvin N L. The GAG-specific cytotoxic T lymphocytes in rhesus monkeys infected with the simian immunodeficiency virus of macaques. J Immunol. 1990;144:122–128. [PubMed] [Google Scholar]
- 26.Miller M D, Yamamoto H, Hughes A L, Watkins D I, Letvin N L. Definition of an epitope and an MHC class I molecule recognized by GAG-specific cytotoxic T lymphocytes (CTL) in SIV infected rhesus monkeys. J Immunol. 1991;147:320–329. [PubMed] [Google Scholar]
- 27.Moskophidis D, Lechner F, Pircher H, Zinkernagel R M. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature (London) 1993;362:758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- 28.Moskophidis D, Laine E, Zinkernagel R M. Peripheral clonal deletion of antiviral memory CD8+ T cells. Eur J Immunol. 1993;23:3306–3311. doi: 10.1002/eji.1830231237. [DOI] [PubMed] [Google Scholar]
- 29.Pantaleo G, Demarest J F, Soudeyns H, Graziosi C, Denis F, Adelsberger J W, Borrow P, Saag M S, Shaw G M, Sekaly R P, Fauci A S. Major expansion of CD8+ T cells with a predominant Vβ usage during the primary immune response to HIV. Nature (London) 1994;370:463–467. doi: 10.1038/370463a0. [DOI] [PubMed] [Google Scholar]
- 30.Pantaleo G, Demarest J F, Schacker T, Vaccarezza M, Cohen O J, Daucher M, Graziosi C, Schnittman S S, Quinn T C, Shaw G M, Perrin L, Tambussi G, Lazzarin A, Sekaly R P, Soudeyns H, Corey L, Fauci A S. The qualitative nature of the primary immune response to HIV infection is a prognosticator of disease progression independent of the initial level of plasma viremia. Proc Natl Acad Sci USA. 1997;94:254–258. doi: 10.1073/pnas.94.1.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phillips R E, Rowland-Jones S, Nixon D F, Gotch F M, Edwards J P, Ogunlesi A O, Elvin J G, Rothbard J A, Bangham C R M, Rizza C R, McMichael A J. Human immunodeficiency virus genetic variation that can escape cytotoxic T cell recognition. Nature (London) 1991;354:453–459. doi: 10.1038/354453a0. [DOI] [PubMed] [Google Scholar]
- 32.Pircher H, Moskophidis D, Rohrer U, Bürki K, Hengartner H, Zinkernagel R M. Viral escape by selection of cytotoxic T cell-resistant virus variants in vivo. Nature (London) 1990;346:629–633. doi: 10.1038/346629a0. [DOI] [PubMed] [Google Scholar]
- 33.Price D A, Goulder P J R, Klenerman P, Sewell A K, Easterbrook P J, Troop M, Bangham C R M, Phillips R E. Positive selection of HIV-1 cytotoxic T lymphocyte escape variants during primary infection. Proc Natl Acad Sci USA. 1997;94:1890–1895. doi: 10.1073/pnas.94.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rinaldo C, Huang X L, Fan Z, Ding M, Beltz L, Logar A, Panicali D, Mazzara G, Liebmann J, Cottrill M, Gupta P. High levels of anti-human immunodeficiency virus type 1 (HIV-1) memory cytotoxic T-lymphocyte activity and low viral load are associated with lack of disease in HIV-1-infected long-term nonprogressors. J Virol. 1995;69:5838–5842. doi: 10.1128/jvi.69.9.5838-5842.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rowland-Jones S, Sutton J, Ariyoshi K, Dong T, Gotch F, McAdam S, Whitby D, Sabally S, Gallimore A, Corrah T, Takiguchi M, Schultz T, McMichael A, Whittle H. HIV-specific cytotoxic T-cells in HIV-exposed but uninfected Gambian women. Nat Med. 1995;1:59–64. doi: 10.1038/nm0195-59. [DOI] [PubMed] [Google Scholar]
- 36.Rowland-Jones S L, Nixon D F, Aldhous M C, Gotch F, Ariyoshi K, Hallam N, Simon Kroll J, Froebel K, McMichael A. HIV-specific cytotoxic T-cell activity in an HIV-exposed but uninfected infant. Lancet. 1993;341:860–861. doi: 10.1016/0140-6736(93)93063-7. [DOI] [PubMed] [Google Scholar]
- 37.Sloan-Lancaster J, Evavold B D, Allen P M. Induction of T-cell anergy by altered T-cell-receptor ligand on live antigen-presenting cells. Nature (London) 1993;363:156–159. doi: 10.1038/363156a0. [DOI] [PubMed] [Google Scholar]
- 38.Tsubota H, Lord C I, Watkins D I, Morimoto C, Letvin N L. A cytotoxic T lymphocyte inhibits acquired immunodeficiency syndrome virus replication in peripheral blood lymphocytes. J Exp Med. 1989;169:1421–1434. doi: 10.1084/jem.169.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Venet A, Bourgault I, Aubertin A M, Kiény M P, Lévy J P. Cytotoxic T lymphocyte response against multiple simian immunodeficiency virus (SIV) proteins in SIV-infected macaques. J Immunol. 1992;148:2899–2908. [PubMed] [Google Scholar]
- 40.Walker C M, Moody D J, Stites D P, Levy J A. CD8+ lymphocytes can control HIV infection in vitro by suppressing virus replication. Science (Washington, DC) 1986;234:1563–1566. doi: 10.1126/science.2431484. [DOI] [PubMed] [Google Scholar]