Abstract
Background
Depression in peri- and post-menopausal women burdens families and health systems. Physical activity is recognized as a complementary therapy for menopausal depressive symptoms, but prior studies lack direct comparisons of intervention types. This network meta-analysis fills this gap by evaluating multiple physical activity modalities via direct and indirect comparisons, establishing an evidence-based hierarchy to guide clinical decisions and provide actionable guidance for managing depression in these women.
Methods
A systematic search across four databases (PubMed, Cochrane Library, Embase, Web of Science) identified randomized controlled trials (RCTs ) on physical activity for depression in peri- and post-menopausal women. Search timeframe: database inception to April 2025. Two independent researchers selected studies, extracted data, and assessed quality via the Cochrane Risk of Bias Tool. We conducted frequentist network meta-analyses (Stata/SE 15.1) integrated direct and indirect evidence. Surface Under the Cumulative Ranking Curve (SUCRA) ranked interventions by efficacy probabilistically, with higher values indicating superior outcomes.
Results
Twenty-three RCTs were ultimately included in the meta-analytical synthesis. The results demonstrated superior therapeutic efficacy of aerobic exercise in alleviating depressive symptoms among peri-and post-menopausal women (SUCRA = 78.7%), closely followed by multi mode motion (SUCRA = 78.1%). Stretching exercise and mind-body exercises also achieved clinically meaningful improvements, with respective SUCRA values of 72.6% and 45.4%.
Conclusion
Our findings show aerobic exercise, multimodal motion, and stretching best reduce depressive symptoms in peri- and post-menopausal women. Clinicians should prioritize aerobic exercise; multi modal motion programs (combining aerobic and stretching) may boost adherence for those seeking variety. Despite physical activity’s proven antidepressant effects, key challenges include developing theory-based strategies to support long-term adherence, especially amid menopausal physiological changes that hinder consistent exercise.
Trial registration
This study has been registered on PROSPERO (CRD420251026378).
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-025-24398-1.
Keywords: Physical activity, Postmenopausal women, Depressive, Perimenopause, Mental health, Network meta-analysis
Introduction
Depressive disorders in menopausal women refer to persistent mood disturbances arising from the interplay of neuroendocrine alterations (particularly estrogen fluctuations) and psychosocial stressors (e.g., familial role transitions, occupational pressures) during the menopausal transition to the postmenopausal period [1–3]. Perimenopause is a period of changing ovarian function that begins 2 to 8 years before the final menstrual period, occurring in stages. In the early stage, the neurohormonal systems governing ovulation start to become dysregulated without obvious changes in cycle length; in the middle to late stage, menstrual cycles are irregular, with fluctuating hormone levels, possible symptoms like hot flashes, and changes in chronic disease risk indicators [4]. Postmenopause starts with the final menstrual period, marking the permanent cessation of ovarian function. It is clinically diagnosed retrospectively after 12 months of amenorrhea, with an average age of approximately 51 years [4]. This condition manifests through varied clinical presentations, encompassing both psychological symptoms (depressed mood, anhedonia) and characteristic somatic manifestations including vasomotor dysfunction (hot flashes, night sweats), sleep disturbances (sleep-onset insomnia, early morning awakening), and genitourinary symptoms (vaginal dryness, urinary frequency) [5, 6]. Epidemiological studies reveal a global prevalence of depression of 35.6% among menopausal women, with perimenopausal and postmenopausal depression exhibiting rates of 33.9% and 34.9%, respectively [7]. Crucially, these emotional and somatic symptoms worsen each other through multiple pathways: sleep impairment amplifies affective dysregulation while depressive states heighten somatic symptom perception, with social withdrawal further potentiating loneliness [8, 9]. These combined symptoms significantly compromise occupational performance, familial relationships, and health-related quality of life [10]. Given these multifaceted impacts on women’s physical and mental health, developing targeted preventive and therapeutic strategies constitutes a critical clinical and public health priority.
Pharmacotherapy for menopausal depression demonstrates efficacy in symptom management through neurotransmitter regulation (e.g., SSRIs/SNRIs) or hormone replacement (HRT) [11], yet faces constraints from interindividual variability and safety concerns—HRT elevates breast cancer risks [12], while certain antidepressants exhibit limited effectiveness against hormone fluctuation-associated depression alongside adverse effect profiles [13]. Nonpharmacological interventions offer targeted advantages addressing the disorder’s unique hormone-psychosocial pathogenic interactions [14]: Psychological modalities resolve role transition-induced psychological conflicts [15, 16]; exercise-based therapies modulate estrogen metabolism to ameliorate mood disturbances [17–19]. These approaches circumvent pharmacological risks, align with patient preferences for natural therapeutics, and demonstrate particular suitability for menopausal depression populations.
Physical activity interventions demonstrate significant therapeutic efficacy as nonpharmacological approaches in the clinical management of menopausal depression. Empirical studies indicate that structured exercise modalities, including aerobic exercise, yoga, and tai chi, effectively alleviate depressive symptoms while regulating hormonal profiles and enhancing sleep quality and psychological resilience [20–22]. Exercise therapy exhibits minimal adverse effects [23, 24], cost-effectiveness, and high patient adherence [25], characteristics particularly advantageous in contemporary healthcare contexts. Personalized exercise programs tailored to individual preferences and physical conditions, including appropriate exercise types and intensities, can further optimize therapeutic outcomes [17, 20]. These interventions may serve as standalone treatments or synergistically complement pharmacotherapy and psychological therapies to amplify clinical benefits. Collectively, exercise-based interventions represent a safe, effective, and easily implementable nonpharmacological strategy, particularly suitable for menopausal depression patients seeking alternatives to medication or prioritizing quality-of-life preservation.
Previous meta-analyses have predominantly examined the association between physical activity and menopausal depression [23, 26]. However, a critical gap persists in high-quality evidence supporting optimal intervention selection among diverse physical activity modalities. To address this gap and establish evidence-based guidance for precision therapeutic strategies in menopausal depression, this investigation conducts a systematic review with network meta-analysis of rigorously selected, high-quality RCTs. The dual objectives encompass comparative effectiveness evaluation and hierarchical ranking of physical activity interventions to identify optimal therapeutic modalities for menopausal depression management. Therefore, this network meta-analysis aims to directly address the following research question: What are the comparative effectiveness and hierarchy of different physical activity modalities in alleviating depressive symptoms among peri-and postmenopausal women?
Methods
Study design
This systematic review and network meta-analysis of RCTs evaluated physical activity interventions for depressive symptom management in peri- and postmenopausal women. Conducted in accordance with PRISMA 2020 guidelines and prospectively registered on PROSPERO, the study employed network meta-analysis to comparatively assess physical activity modalities and establish their efficacy hierarchy.
Protocol and registration
This systematic review was conducted in strict adherence to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines, following all guidelines for study inclusion criteria, data organization procedures, statistical analytical approaches, and results reporting standards. The study protocol underwent prospective registration with the International Prospective Register of Systematic Reviews (PROSPERO) under the unique identifier CRD 420,251,026,378.
Data sources and search strategy
A comprehensive systematic search was executed across four electronic databases (PubMed, Cochrane Library, Embase, Web of Science) to identify literature examining physical activity interventions for peri-and post-menopausal. To ensure complete coverage, the reference lists of eligible studies were screened for additional publications.The search timeframe encompassed records from database inception through 4 April 2025. Search strategies were developed by PICOS criteria (Population: perimenopausal women; Intervention: physical activity; Comparator: any control; Outcomes: depression metrics; Study design: RCTs). Key search terms included:“Exercises” or “Physical Activity” or “Activities, Physical” or “Physical Activities” or “Exercise, Physical” or “Physical Exercise” or “Depression” or “Depressive Symptom” or “Emotional Depression” or “Negative Emotion” or “Affective Disorder” or “perimenopause” or “menopause” or “postmenopause” or “climacteric” or “Hot Flashes” or “perimenopausal” or “Hot Flash” For detailed search strategies, please consult Onliine Appendix B and B1.
Study selection
Following implementation of the aforementioned search strategy, two researchers independently screened was conducted by two investigators (HYW and DL) following PRISMA guidelines. The initial screening phase involved title/abstract review to identify potentially eligible studies using predefined inclusion criteria. Articles meeting preliminary thresholds underwent full-text retrieval and critical appraisal. Final inclusion decisions for quantitative synthesis were made through agreement by discussion, with discrepancies resolved through iterative team discussions until unanimous agreement was achieved.
Inclusion and exclusion criteria
Inclusion criteria
The systematic review established study selection criteria based on the PICOS framework. Studies meeting all the following criteria were included:
Study Design: RCTs with parallel-group or crossover designs.
Participants: Peri-or post-menopausal women.
Perimenopause: Irregular menses in women aged 40–60 years, transitioning toward menopause (endocrine criteria: FSH ≥ 25 IU/L).
Natural Postmenopause: ≥12 months of amenorrhea in women aged ≥ 40 years, confirmed by FSH > 30 IU/L and E2 < 20 pg/mL.
-
3.
Interventions: Structured physical activity or exercise training protocols with standardized descriptions of exercise type (e.g., aerobic, resistance), intensity (e.g., %HR < sub > max, METs), and frequency (sessions/week).
-
4.
Outcomes: Pre- and post-intervention depression severity data measured using validated scales (e.g., Hamilton Depression Rating Scale (HAM-D), Beck Depression Inventory (BDI)).
-
5.
Data Completeness: Sufficient raw or extractable data (means, standard deviations, sample sizes, exact p-values) to calculate effect sizes (e.g., standardized mean differences).
-
6.
Language: Full-text articles published in English.
Exclusion criteria
Studies were excluded if they met any of the following criteria:
Study Design: Observational studies (e.g., cross-sectional studies, case-control studies, cohort studies).
Participants: Studies involving premenopausal women, non-menopausal women (including undefined status), chemotherapy/radiation therapy-induced menopause, or participants aged < 40 years were excluded.
Interventions: Physical activity interventions with insufficient protocol details (e.g., unquantified intensity/duration) or combined interventions (e.g., exercise with dietary supplements) where exercise effects could not be isolated.
Study Type: Qualitative studies, reviews, dissertations, or conference proceedings.
Incomplete Data: Missing critical outcome data or non-extractable data (e.g., descriptive statistics without numerical values).
Ethical Concerns: Violations of ethical standards (e.g., lack of informed consent, disproportionate risk-benefit ratio).
Language: Non-English publications.
Data extraction
To ensure the reliability of the literature search and selection process, two researchers (HYW and DL) independently reviewed the titles, abstracts, and full texts following the completion of the search. The inter-rater reliability (Cohen’s kappa) was then calculated for both screening stages: the initial phase involving title and abstract review, and the subsequent phase of full-text screening. The level of consistency was categorized as follows: fair consistency (0.40–0.59), good consistency (0.60–0.74), and excellent consistency (> 0.75) [27].
The systematic review established literature selection, inclusion, and exclusion criteria based on the PICOS framework. Data extraction was independently performed by two investigators using a standardized protocol. Discrepancies encountered during this process were resolved through consensus-based discussions with the research team. The following data were extracted from each included study:
Descriptive information: First author, publication year, and country of origin.
Participant characteristics: Age range, sex distribution, and sample size.
Intervention details: Temporal parameters (session duration), frequency (sessions per week), and intervention duration (total weeks).
Outcome measures: Depression severity metrics specific to menopausal populations, including validated psychometric scales.
For studies presenting intervention parameters or outcome data exclusively in graphical format without numerical clarification, the Engauge Digitizer software (v12.1) was employed for precise data extraction. In trials with multiple follow-up assessments, only immediate post-intervention data were included to standardize temporal comparability. Where standard deviations (SDs) were unreported, they were computed from 95% confidence intervals (CIs) of group means using the formula.
Quality assessment
We employed the Cochrane Risk of Bias (RoB2) assessment tool to evaluate study quality across five domains: [1] randomization process; [2] deviations from intended interventions; [3] missing outcome data; [4] measurement of outcomes, and [5]selection of reported results. Based on these criteria, each study was systematically evaluated for overall bias and classified into one of three categories: low risk, high risk, or some concerns.
Statistical analysis
For continuous outcomes, we calculated the standardized mean difference (SMD) and its corresponding 95% CIs. To assess statistical heterogeneity, we used the P-value from the Chi-squared test and evaluated the I² statistic, where an I² value exceeding 50% is typically considered moderate heterogeneity, and values above 75% are considered high heterogeneity. To address scale heterogeneity across studies (e.g., divergent measurement tools and intervention protocols), we implemented a random-effects model with inverse variance weighting, explicitly accounting for between-study variability through τ² estimation. This conservative approach preserved methodological parsimony while enhancing comparative consistency. In accordance with PRISMA-NMA specifications, a frequentist framework was prioritized over Bayesian alternatives to optimize interpretability and avoid computational complexities associated with Markov chain Monte Carlo convergence. The analytical workflow encompassed three core components: network configuration using Stata 15.1’s ‘network’ package generated evidence diagrams where node diameters scaled with study sample sizes and connecting line thickness reflected trial counts per comparison; effect size synthesis via maximum likelihood estimation in multivariate meta-regression for integrating direct-indirect evidence; and consistency validation through node-splitting tests quantifying disagreement between direct and indirect comparisons (with p > 0.05 indicating statistical consistency). To visually contextualize effect estimates and heterogeneity patterns, forest plots were constructed using the network graphs suite, horizontally aligning study-specific odds ratios with 95% CIs against a vertical midline representing the pooled effect. Diamond-shaped summary markers at the plot base quantified overall treatment effects, whose width inversely correlated with estimation precision. This graphical framework enabled simultaneous assessment of individual study weights, between-study variance contributions, and subgroup effect deviations through visual inspection of interval overlaps and outlier positioning. The integrated architecture balanced statistical rigor with analytical transparency, leveraging Stata’s computational infrastructure to maintain tractable parameter estimation while ensuring compatibility with established reporting standards. The tripartite design strategically aligned methodological simplicity with robust variance accounting, thereby achieving rigorous synthesis of heterogeneous evidence networks without compromising interpretative clarity. To assess the effectiveness of various interventions, we calculated the SUCRA and presented the results in a probability ranking table. SUCRA values are expressed as percentages, indicating the effectiveness of the interventions; the higher the percentage, the more effective the treatment. Potential publication bias was assessed through funnel plots, supported by Begg’s and Egger’s statistical tests. We further applied the trim-and-fill method to adjust for potential bias impacts.
Results
Trial selection
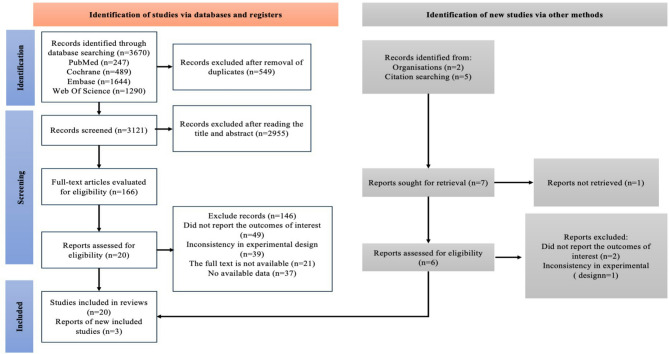
In the initial phase of the search, a thorough search was performed across four electronic databases, covering the period from their inception up to April 4, 2025. This process resulted in the identification of 3,670 articles. After removing duplicate studies (n = 549), a total of 3,121 articles were retained. Following the screening of titles and abstracts, 2,955 articles were excluded, leaving 166 articles for full-text review. At this stage, the inter-rater reliability between the two assessors was considered “good,” with a Cohen’s kappa value of 0.73 (≥ 0.6 indicates substantial agreement, critical for minimizing subjective bias in screening). Following the full-text review, 146 articles were further excluded, with the reasons being: no reported outcomes (n = 49), inconsistent experimental designs (n = 39), unavailable full text (n = 21), andno usable data (n = 37). As a result, the initial search yielded 20 studies, screening the references of these studies identified an additional 3 that met the criteria (Fig. 1). At this point, the inter-rater reliability between the two assessors was rated as “excellent,” with a Cohen’s kappa value of 0.84 (> 0.8 reflects near-perfect consensus, enhancing confidence in final study inclusion).
Fig. 1.
A summary of the evidence searches and selection process
Trial characteristics
Table 1 summarizes the characteristics of the studies included in this analysis. The studies were published over a period from 2006 to 2025. Notably, the United States contributed the largest number of publications, with a total of 5 articles. The sample size in the intervention group varied from 13 to 117 participants, encompassing a total of 1,202 postmenopausal women. In the control group, the sample size ranged from 13 to 142 participants, with an overall total of 953 postmenopausal women. In both groups, the average age of the women exceeded 45 years.
Table 1.
Summary table of included reviews
| Study | Country | N (IG; CG) | Age (IG; CG) | Intervention (IG) | Intervention (CG) | Population | Outcome measures | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention content | Intervention time, frequency, period | Type | Intervention content | Intervention time, frequency, period | Type | Otcome | ||||||
| Carmen Villaverde Gutie´rrez et al.,2012 [50] | Spain | 30;30 | 60–70 | Programme of physical exercise |
50–60 min, 2–3 weekly 6 months |
Multi mode motion | NI | NR | NI |
IG1:Moderate depression Postmenopausal women IG2:Severe depression Postmenopausal women |
GDS | Multi mode motion reduced depressive symptoms. |
| Steriani Elavsky et al., 2007[29] | American |
IG1:63; 39 IG2:62; 39 |
42–58 |
IG1:Walking IG2: Yoga |
IG1:60 min 3 weekly 4 months IG2:90 min 2 weekly 4 months |
IG1:Aerobic exercise IG2:Mind-body exercise |
NI | NR | NI | Postmenopausal women | GCS | All interventions worked; aerobic exercise was more effective. |
| Agustín Aibar-Almazán et al., 2019 [41] | Spain | 55; 55 |
69.15 ± 8.94; 69.15 ± 8.94 |
Pilates |
60 min, 2 weekly, 12 weeks |
Mind-body exercise | NI | NR | NI | Postmenopausal women | HADS | Pilatesr educed depressive symptoms. |
| Yanghee Pang et al., 2021 [30] | Korea |
IG1:23; 16 IG2:13; 16 |
60.89 ± 6.62; 59.33 ± 6.54 |
IG1:Calendar training and exercise IG2:Exercise |
12 weeks |
IG1:Multi mode motion IG2:Aerobic exercise |
NI | NR | NI | Postmenopausal women | GDS | All interventions worked; aerobic exercise was more effective. |
| Maryam Abdoshahi et al.,2023 [42] | Iran | 16; 16 | 50–55 | Pilates |
2 weekly 3 mouths |
Mind-body exercise | NI | NR | NI | Postmenopausal women | DASS-21 | Pilatesr educed depressive symptoms. |
| Masaki Takahashi et al., 2019 [51] | Singapore | 19; 19 | 70.2 ± 3.9 | Daily physical activity |
75–150 min, 3–5 weekly, 8 weeks |
Multi mode motion | NI | NR | NI | Postmenopausal women | GDS | Daily physical activity educed depressive symptoms. |
| Deborah J Bowen et al., 2006 [31] | American | 86; 86 | 50–75 | Moderate-to-vigorous intensity aerobic exercise |
45 min 5 weekly 12 months |
Aerobic exercise | Stretching exercise |
45 min 5 weekly 12 months |
NI | Postmenopausal women | BSI | Aerobic exercise reduced depressive symptoms. |
| Ikuyo Imayama et al., 2011 [32] | American | 117; 87 | 50–75 | Moderate-to-vigorous intensity aerobic exercise |
45 min 5 weekly 12 months |
Aerobic exercise | NI | NR | NI | Postmenopausal women | BSI-18 | Aerobic exercise reduced depressive symptoms. |
| Rui Ferreira Afonso et al., 2012 [43] | Brazil |
IG1:14; 15 IG2:15; 15 |
50–65 |
IG1:Passive stretching IG2:Yoga |
60 min 2 weekly 4 months |
IG1:Stretching exercise IG2:Mind-body exercise |
NI | NR | NI | Postmenopausal women | BDI | All interventions worked; stretching exercise was more effective. |
| P. Bernard et al., 2015 [33] | France | 61; 60 | 57–75 | Moderate intensity walking |
45 min 3 weekly 6 months |
Aerobic exercise | NI | NR | NI | Postmenopausal women | BDI | Aerobic exercise reduced depressive symptoms. |
| Lei Gao et al., 2016 [48] | China | 32;28 | 45–55 | Square dance exercise |
60–90 min, 5 weekly, 3 mouths |
Stretching exercise | NI | NR | NI | perimenopausal women | SDS | Stretching exercise reduced depressive symptoms. |
| Liang Hu et al., 2016 [34] | China | 40; 40 | 45–65 | Walking | 4 mouths | Aerobic exercise | NI | NR | NI | Postmenopausal women | BDI | Aerobic exercise reduced depressive symptoms. |
| Keqiang Li et al., 2022 [44] | Poland | 17; 15 | 57.94(9.38); 58.23༈11.81) | Baduanjin exercise | 8 weeks | Mind-body exercise | Drug Therapy | NR | NI | Postmenopausal women | SDS | Mind-body exercise reduced depressive symptoms. |
| Barbara Sternfeld et al., 2014 [35] | American | 106; 142 | 40–62 | Exercise program |
3 weekly, 12 weeks |
Aerobic exercise | NI | NR | NI | perimenopausal and postmenopausal women | PHQ-8 | Aerobic exercise reduced depressive symptoms. |
| Katherine M. Newton et al., 2014 [45] | American | 107; 142 | 40–62 | Yoga |
90 min, 12 weekly, 12 weeks |
Mind-body exercise | NI | NR | NI | perimenopausal and postmenopausal women | PHQ-8 | Yoga reduced depressive symptoms. |
| P. Abedi et al., 2015 [36] | Iran | 53; 53 |
52.4 ± 3.8; 53 ± 4.1 |
Walking |
7 weekly, 12 weeks |
Aerobic exercise | NI | NR | NI | Postmenopausal women | BDI | Aerobic exercise reduced depressive symptoms. |
| Marwa M. Elsayed et al., 2022 [37] | Egypt | 30; 30 |
58.79 ± 2.81; 58.79 ± 2.81 |
Aerobic exercise |
3 weekly, 12 weeks |
Aerobic exercise | balanced-restricted diet program | NR | NI | Postmenopausal women | SDS | Aerobic exercise reduced depressive symptoms. |
| María del Carmen Carcelén-Fraile, et al., 2022 [46] | Spain | 63; 62 |
69.70 ± 6.15; 69.75 ± 6.76 |
Qigong |
60 min, 2 weekly, 12 weeks |
Mind-body exercise | NI | NR | NI | Postmenopausal women | HADS | Qigong reduced depressive symptoms. |
| Sema Arslan Kabasakal et al., 2025 [47] | Turkey | 13; 13 |
59.45 ± 11.52; 59.45 ± 11.52 |
Pilates |
60 min, 2 weekly, 6 weeks |
Mind-body exercise | NI | NR | NI | Postmenopausal women | BDI-PC | Pilates reduced depressive symptoms. |
|
Riitta Luoto et al., 2012 [38] |
Finland | 88; 88 |
54.5 ± 3.8; 54.2 ± 3.7 |
Aerobic exercise |
50 min, 4 weekly, 6 mouths |
Aerobic exercise | NI | NR | NI | Postmenopausal women | WHQ | Aerobic exercise reduced depressive symptoms. |
| Yuko Kai et al.,2016 [49] | Japan | 20; 20 |
51.0 ± 7.0; 51.2 ± 7.9 |
Stretching exercise |
10 min, 7 weekly, 3 weeks |
Stretching exercise | NI | NR | NI | perimenopausal and postmenopausal women | SDS | Stretching exercise reduced depressive symptoms. |
| EunHee Noh et al., 2020 [39] | Korea | 21; 19 | 50–65 | Walking |
60 min, 3 weekly, 12 weeks |
Aerobic exercise | Routine medical | NR | Routine medical | Postmenopausal women | SCL-95-R | Aerobic exercise reduced depressive symptoms. |
| Ekin Ilke Sen e al., 2020 [40] | Turkey |
IG1:38; 20 IG2:38; 20 |
40–65 |
IG1:Wbv IG2:High-intensity exercise |
IG1:20–40 min, 3 weekly, 24 weeks IG2:10–30 min, 5 weekly, 12 weeks |
IG1:Multi mode motion IG2:Aerobic exercise |
NI | NR | NI | Postmenopausal women | BDI |
All interventions worked; multi mode motion was more effective. |
IG intervention group, CG control group, N number, NA not available, NR no report, NI no intervention, GDS geriatric depression scale, HADS hospital anxiety and depression scale, DASS-21 depression anxiety stress scale-21, GCS greene climacteric scale, BSI brief symptom inventory, BSI-18 brief symptom inventory-18, BDI beck depression inventory, SDS self-rating depression scale, PHQ-8 Patient Health Questionnaire-8, BDI-PC beck depression inventory for primary care, WHQ women’s health questionnaire, SCL-95-R Korea symptom-checklist-90-revision
To investigate whether distinct types of physical activities exert different effects on depression in perimenopausal and postmenopausal women, we categorized these activities into four types [28, 29] based on shared characteristics and prior research: namely, aerobic exercise (12 studies), involving rhythmic engagement of large muscle groups to elevate heart rate and respiration for enhanced cardiovascular function (e.g., walking, running, cycling) [30–41]; mind-body exercises (8 studies), characterized by physical activity integrated with mental regulation to emphasize mind-body unity and coordination, typically including practices such as yoga and Pilates that focus on breathing, postures, meditation, and relaxation techniques to promote psychosomatic balance [30, 42–48]; stretching exercises (4 studies), primarily aimed at improving flexibility, posture, and reducing muscle tension through elongating various muscle groups, often involving static or dynamic stretches targeting specific body parts to relax muscles and increase joint range of motion [42, 44, 49, 50]; and multi mode exercise programs (4 studies), which combine various exercise forms (e.g., aerobic and stretching exercises) into a comprehensive regimen designed to enhance overall physical fitness and health through diverse training methodologies [31, 41, 51, 52].
Non-physical activity interventions include no intervention and routine care. Commonly used measurement tools for depression in postmenopausal women include the Geriatric Depression Scale (GDS), Hospital Anxiety and Depression Scale (HADS), Depression Anxiety Stress Scale-21 (DASS-21), Greene Climacteric Scale (GCS), Brief Symptom Inventory (BSI), Brief Symptom Inventory-18 (BSI-18), Beck Depression Inventory (BDI), Self-Rating Depression Scale (SDS), Patient Health Questionnaire-8 (PHQ-8), Beck Depression Inventory for Primary Care (BDI-PC), Women’s Health Questionnaire (WHQ), and Simplified Mental Health Test II (Korea Symptom-Checklist-90-Revision, SCL-95-R), among others.
Risk of bias
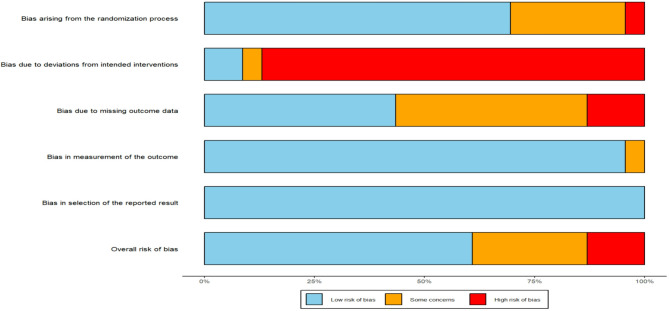
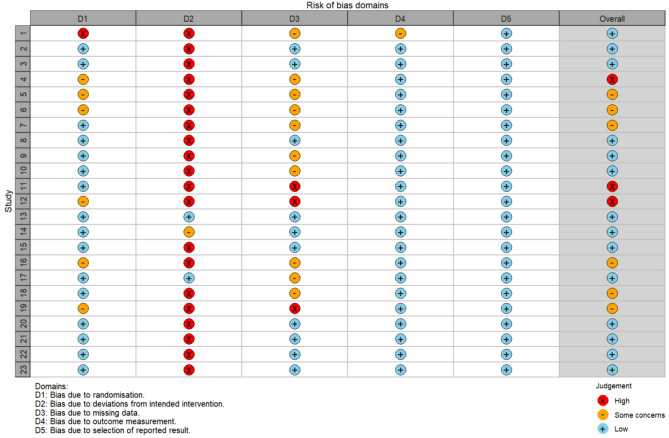
Among the 23 included studies, 16 were rated as having a low risk of bias in the randomization process, one had a high risk, and six did not provide specific details regarding their randomization procedures. Regarding the risk of bias in the measurement of outcomes, 22 studies were assessed as low risk, while one study was classified as high risk. In terms of selective reporting bias, all 23 studies were deemed to be at low risk, with no studies showing a high risk. Based on the five criteria, the overall risk of bias for the 23 studies was categorized as follows: 14 studies were identified as having a low overall risk of bias, six were assessed as having a high overall risk, and three were assessed as having “some concerns”.
The detailed risk of bias assessment results are presented in Fig. 2 and Fig. 3, which document the ratings and classifications for each study according to the various risk of bias criteria.
Fig. 2.
Risk of bias of included studies
Fig. 3.
Risk of bias assessment of included studies
Network meta-analysis
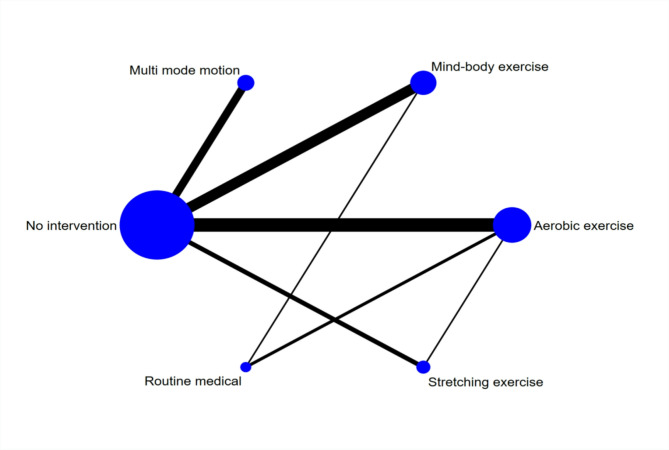
Figure 4 presents the network meta-analysis plot. The three interventions with the largest sample sizes in the experimental group were traditional aerobic exercise, mind-body exercises, and multi mode motion. In contrast, the control group had the largest sample size in the no-intervention group. The most commonly examined comparisons involve traditional aerobic exercise versus no intervention, mind-body exercise versus no intervention, and multi modal exercise versus no intervention.
Fig. 4.
Network diagram
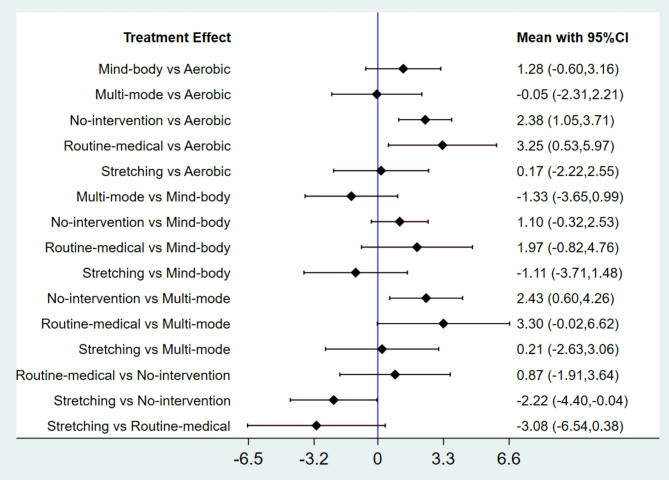
Forest plot comparing standardized mean differences (95% CIs) of physical activity interventions for depressive symptoms in perimenopausal and postmenopausal women, with direct and indirect analyses (Fig. 5). Aerobic and multimodal exercise demonstrate superior efficacy over control conditions; negative SMD values indicate greater treatment benefit.
Fig. 5.
Forest plot
In all 23 RCTs [29–51], various physical activity interventions were shown to reduce depressive symptoms compared with control groups (no intervention or routine care). Aerobic exercise (such as walking and cycling) consistently improved depression scores across intervention durations ranging from 8 weeks to 12 months. Mind-body interventions (including yoga, pilates, and qigong) exerted beneficial effects, though their short-term efficacy was often less pronounced than that of aerobic exercise. Stretching programs and multimodal interventions (combining aerobic exercise, strength training, and flexibility exercises) also led to significant alleviation of depressive symptoms.
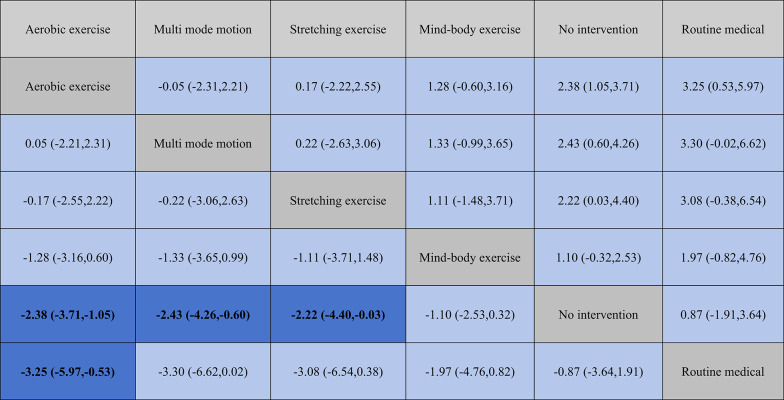
The following results directly report the effect size magnitudes (expressed as SMD with 95% CIs) comparing the efficacy of different physical activity interventions: Aerobic exercise demonstrates greater improvement compared to stretching exercises (SMD = −0.17 [95% CI: −2.55, 2.22]) and mind-body exercises (SMD = −1.28 [−3.16, 0.60]), although there is no significant difference when compared to multi mode motion (SMD = 0.05 [95% CI: −2.21, 2.31]) (Table 2). Multi mode motion is more effective than stretching exercises (SMD = −0.22 [−3.06, 2.63]) and also more effective than mind-body exercises (SMD = −1.33 [95% CI: −3.65, 0.99]). Stretching exercises are more effective than mind-body exercises (SMD = −1.11 [95% CI: −3.71, 1.48]). These SMD values quantify the magnitude of difference in depressive symptom reduction between intervention pairs, with larger absolute values indicating more pronounced effects (negative values indicate greater benefit for the intervention listed first in the comparison).
Table 2.
League table on interventions
Regarding the probability of different interventions impacting depression, SUCRA rankings quantify each approach’s likelihood of being the optimal treatment, guiding clinicians through clear probabilistic metrics. aerobic exercise ranks first (SUCRA = 78.7%), followed by multi mode motion (SUCRA = 78.1%). Stretching exercises and mind-body exercises rank third and fourth, respectively (SUCRA = 72.6% and 45.4%). The probability percentages for the “best treatment” show that multi mode motion (37.4%) and aerobic exercise (29.6%), as well as stretching exercises (30.8%), stand out. Together, these three interventions account for 97.8%, with specific results shown in Fig. 6.
Fig. 6.
SUCRA plot
Publication bias
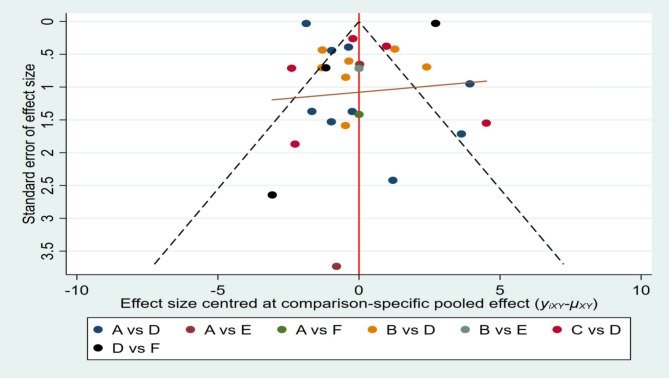
As shown in Fig. 7, we first assessed publication bias using a funnel plot. The funnel plot shows a generally symmetrical distribution of the studies, and a visual examination did not indicate any clear evidence of publication bias. We subsequently conducted both the Begg and Egger tests to assess potential bias in the studies. The Begg test revealed no significant bias (z = −0.55, p = 0.581), while the Egger test showed significant bias (bias term: t = 3.21, p = 0.002), as presented in Table 3 and Online Appendix C1. These tests were selected because they address publication bias through different statistical approaches: Begg’s test (rank-based) is less sensitive to small sample heterogeneity, whereas Egger’s test (regression-based) may detect bias more effectively in larger datasets. Their conflicting results likely reflect differences in statistical power and methodological assumptions.
Fig. 7.
Funnel plot on publication bias
Table 3.
Begg’s and egger’s test results for publication bias
| Test | Statistic | Value | Std. Err. | t -value | P -value | 95%CI |
|---|---|---|---|---|---|---|
| Begg’s Test | Kendall’s Score (P-Q) | −78 | - | |||
| Std. Dev. of Score | 141.48 | - | ||||
| z -value | −0.55 | 0.581 | - | |||
| z-value(continuity corrected) | 0.54 | 0.586 | - | |||
| Egger’s Test | Slope bias | 0.1349 | 0.4141 | 0.33 | 0.746 | −0.6953, 0.9650 |
| Intercept | 16.9316 | 5.2736 | 3.21 | 0.002 | 6.3587, 27.5045 |
To evaluate publication bias more comprehensively, we performed a trim-and-fill analysis using a random-effects model. The findings indicated that after adjusting for publication bias through the trim-and-fill method, the estimated effect size slightly decreased, though the change was minimal. This approach complements funnel plot and statistical tests by empirically estimating hypothetical missing studies, thereby addressing limitations of purely visual or parametric methods. The estimated effect size remained statistically significant, further supporting the robustness of the study’s findings, as demonstrated in Online Appendix C2.
Additionally, a sensitivity analysis, revealed no significant changes to the overall findings, as outlined in Online Appendix C3. This indicates that the core results including the relative efficacy rankings of physical activity interventions (aerobic exercise, multi mode motion, stretching exercises, and mind-body exercises) and their comparative effects on depressive symptoms are not unduly influenced by any single study, reinforcing the stability of the conclusions. Combining these methods mitigates overreliance on a single technique, ensuring a balanced interpretation of potential bias risks while strengthening confidence in the conclusion’s stability.
Discussion
This network meta-analysis comprehensively assessed the impact of various physical activity interventions on reducing depressive symptoms in women during menopause. Based on a comprehensive analysis of 23 RCTs, our findings indicate that aerobic exercise, multi mode motion, and stretching exercises are the most effective non-pharmacological interventions for alleviating depressive symptoms in this population. Notably, aerobic exercise emerged as the intervention with the highest probability of being the most optimal (SUCRA = 78.7%), followed closely by multi mode motion (SUCRA = 78.1%), reflecting its synergistic potential in addressing the complex pathophysiological mechanisms of menopausal depression. Stretching exercises ranked third (SUCRA = 72.6%) (Table 4).
Table 4.
Ranking of SUCRA probabilities
| Intervention Name | SUCRA (%) | Probability of Best (%) | Rank |
|---|---|---|---|
| Aerobic exercise | 78.7 | 29.6 | 1 |
| Multi mode motion | 78.1 | 37.4 | 2 |
| Stretching exercise | 72.6 | 30.8 | 3 |
| Mind-body exercise | 45.4 | 1.9 | 4 |
| No intervention | 16.6 | 0 | 5 |
| Routine medical | 8.6 | 0.3 | 6 |
The menopausal transition is a natural and unavoidable phase in the aging process of women, resulting from the depletion of ovarian follicles and the interplay between neuroendocrine changes and psychosocial factors [53]. Fluctuations in estrogen levels are a key factor; during the transition to menopause and postmenopause, estrogen levels significantly decline, affecting the balance of neurotransmitters such as serotonin and dopamine, thereby leading to disruptions in mood regulation [6]. Psychosocially, changes in family roles—such as children becoming independent and taking on the responsibility of caring for elderly relatives—along with issues like job stress and retirement, require women to adjust their life focus and sense of self-worth [1, 3]. This often leads to feelings of loneliness, anxiety, and depression [2]. The risk of developing depressive symptoms or depression during the menopausal transition is elevated by two to four times [54]. Physical activity can maintain the levels of estrogen and progesterone in the body while also enhancing cardiovascular, respiratory, and neurological functions [55]. Exercise impacts both the physiological and psychological states of individuals and is considered an effective behavioral intervention for treating depression [56]. Existing evidence suggests that physical activity helps in the prevention of depression [19, 57]. In a cohort study, Kim et al. observed that moderate physical activity led to a reduction in the incidence of depressive symptoms [19]. In a similar study, Li et al. demonstrated that running exercise helped protect astrocytes in the hippocampus, reduced the formation of new astrocytes, and, as a result, prevented central nervous system damage while reducing the onset of depressive symptoms [57].
Aerobic exercise effectively eases menopausal depression by addressing estrogen decline-related neurotransmitter disruptions through multiple pathways. The benefits of aerobic exercise in alleviating depressive symptoms are consistent with existing neuroendocrine evidence [58]. Menopausal depression is closely associated with a decline in estrogen levels [59, 60], and fluctuations in estrogen are considered one of the primary triggers of perimenopausal depression [60]. Estrogen plays a critical role in the central nervous system through its receptors, including the regulation of neurotransmitter synthesis, transport, and receptor expression [61]. A decrease in estrogen levels impairs the function of the serotonin and norepinephrine systems, which in turn triggers depressive symptoms [61, 62]. However, aerobic exercise can improve this condition through multiple mechanisms [63]. For instance, exercise can increase serum estradiol levels [64] and alleviate depressive symptoms by enhancing estrogen signaling pathways. Moreover, exercise can reduce pro-inflammatory cytokine levels and improve sleep patterns, further alleviating depression [65]. Notably, aerobic exercise not only positively affects estrogen levels but also improves depressive symptoms through other mechanisms [21]. For example, exercise can increase serum endorphin levels, a neurotransmitter associated with positive emotions and well-being [66]. Additionally, exercise can regulate cortisol levels and reduce inflammatory responses, thereby improving mood [67]. These combined effects suggest that aerobic exercise is an effective, multifaceted intervention for improving depressive symptoms [68].
Multimodal exercise is effective as it combines various exercises and includes social interactions; mind-body exercises rank lower because their regulatory effects are slower, with less obvious short-term results. The effectiveness of multimodal interventions may stem from their multidimensional regulation of menopausal physiology [69], allowing for the combination of various types of exercise to reduce the monotony of a single activity. Additionally, such programs often include social interactions (e.g., group classes), which can address psychosocial risk factors such as feelings of isolation and the loss of social roles—common triggers for depressive episodes during the menopausal transition [70]. Surprisingly, mind-body exercises (such as yoga, Pilates, and Baduanjin) ranked lower (SUCRA = 45.4%). These exercises focus on holistic regulation (e.g., relaxation response, parasympathetic activation), and their hormonal regulatory effects may be slower or more indirect, making it difficult to demonstrate significant differences in the short term [71].
Compared to traditional pharmacological treatments, physical activity interventions offer numerous significant advantages. While pharmacological treatments can alleviate symptoms, they are often associated with individual variability and potential risks [72]. HRT may increase the risk of breast cancer [12], and some antidepressants have limited efficacy in treating hormone fluctuation-related depression, in addition to potential side effects [73]. In contrast, physical activity interventions not only regulate estrogen metabolism to improve mood [63–65], but also offer benefits such as minimal side effects, cost-effectiveness, and high patient adherence [74]. Exercise therapy can serve as an independent treatment modality or be combined with pharmacotherapy or psychotherapy to enhance overall treatment outcomes [75]. This multidimensional therapeutic advantage makes it an ideal choice for the treatment of menopausal depression.
Research has shown that the effectiveness of physical activity interventions is influenced by various factors. Exercise type, intensity, frequency, and duration all play a role in determining outcomes [7, 8]. Different types of exercise have distinct mechanisms of action. Aerobic exercise primarily alleviates depression by improving cardiovascular function and regulating neurotransmitters [38, 63], while mind-body exercises such as yoga, Pilates, and Baduanjin focus on holistic regulation of both the body and mind [45–47]. In terms of exercise intensity, moderate intensity is more effective in triggering adaptive responses in the body, promoting overall health, whereas excessive intensity may lead to fatigue and injury, thus compromising the intervention’s effectiveness [76]. Frequency and duration are also critical; regular and long-term exercise interventions are necessary for sustained improvements in mood, while short-term exercise may not yield optimal results [77]. Furthermore, individual differences, such as age, physical condition, psychological status, and exercise habits, also influence the intervention’s efficacy [78, 79]. Older women, those with poor physical condition, or those lacking exercise habits may face greater challenges in adapting to and maintaining an exercise regimen, thus requiring more personalized exercise plans [80].
Strengths and limitations
This study has several significant strengths. First, this study marks the inaugural network meta-analysis examining the effects of physical activity on menopausal depression, providing crucial scientific insights that can guide the selection of effective physical activity interventions for women during menopause. Secondly, incorporating a substantial number of studies strengthens the reliability and precision of the findings. Thirdly, limiting the analysis to RCTs while excluding observational and cross-sectional studies further enhances the reliability of the results. Despite these strengths, our network meta-analysis also presents certain limitations. For example, individual variations among women experiencing peri-and post-menopausal depression—such as age, baseline depression severity, and physical fitness may contribute to outcome differences. More importantly, the differing intensities, frequencies, and durations of physical activity across studies posed challenges to result interpretation. For instance, aerobic exercise interventions varied from moderate-intensity walking (45–60 minutes, 3–5 times/week for 12 weeks) to high intensity interval training (20–30 minutes, 5 times/week for 8 weeks), while multi mode motion programs combined aerobic and stretching exercises with inconsistent scheduling (e.g., 2–3 sessions/week for 6 months vs. daily sessions for 3 weeks). Such disparities in ‘exercise dosage’ (e.g., intensity defined by %HRmax or METs, frequency, and total intervention duration) may have obscured the true comparative efficacy of modalities.
Future research could address several areas.Firstly, it would be valuable to investigate personalized physical activity interventions that are customized to address the unique characteristics of women suffering from menopausal depression. For instance, when determining suitable physical activity interventions, the content and type of activities should be aligned with the particular needs of the target population. Customizing interventions based on factors such as age, depression history, onset timing, and depression severity could potentially increase the effectiveness of physical activity interventions. Second, further investigation into the optimal “dose” of physical activity—considering frequency, duration, and intensity—could refine the precision of interventions aimed at alleviating menopausal depression.
Practical implications
The findings offer actionable guidance for clinical practice, community health, and individual management to alleviate depressive symptoms in peri- and post-menopausal women. For clinicians, aerobic exercise (SUCRA = 78.7%) should be the first-line recommendation, with specific suggestions like brisk walking (30–60 min, 3–5 times/week) or low-impact options (e.g., water aerobics) for those with limitations. Multimodal programs (SUCRA = 78.1%), combining aerobic and stretching, are ideal for patients seeking variety, while stretching exercises (SUCRA = 72.6%) suit those with pain or mobility issues. Mind-body exercises (SUCRA = 45.4%) can complement care for women with high stress, though short-term effects are less pronounced. Baseline assessments (depression severity, fitness) are key to personalization, with multi mode motion and psychological interventions for severe cases. Community programs should prioritize group-based aerobic or multimodal classes to foster social support, offering flexible scheduling and childcare to reduce barriers. Diversifying offerings (e.g., dance-based aerobics) and linking with healthcare via “exercise prescriptions” can boost participation. Educational campaigns should highlight that even short activity bouts aid symptom reduction, dispelling myths about exercise worsening menopausal symptoms. For individuals, starting with 15–20 min of aerobic exercise 3 times/week, pairing activity with daily routines (e.g., post-dinner walks), tracking mood improvements, and joining support groups can enhance adherence. For those with severe depression or comorbidities, supervised programs or mind-body exercises as a stepping stone are advisable, while low-cost options (community walks, bodyweight stretches) work in resource-limited settings. Overall, a collaborative approach—clinicians providing personalized plans, communities offering accessible programs, and individuals adopting sustainable habits—can leverage aerobic, multimodal, and stretching exercises to improve menopausal mental health, reducing reliance on pharmacology and empowering women through the transition.
Conclusion
This study examined the impact of various types of physical activity on menopausal depression. Based on the results, we suggest that women experiencing menopause engage in aerobic exercises, multi-modal movements, and stretching routines to more effectively prevent or alleviate symptoms of menopausal depression. Additionally, prior to initiating physical activity interventions, it is crucial to conduct a thorough evaluation of the unique characteristics of each woman affected by menopausal depression to ensure the selection of the most suitable type of exercise and intervention dosage. Healthcare providers can use these findings to give personalized exercise advice, matching each woman’s needs to boost results. Communities can run group sessions for these exercises, making them easier to join and fostering support. For women, these routines ease depression, improve health, and with help from providers and communities, make managing menopause feel more manageable. Furthermore, community and healthcare providers, as well as family members, should actively maintain regular communication with menopausal women, encouraging them to continue their exercise routines and offering support to mitigate the negative emotional challenges that may accompany menopause. Future studies should investigate personalized physical activity interventions tailored to the specific needs of women with menopausal depression. Research focused on determining the optimal frequency, duration, and intensity of physical activity interventions could further refine and improve the effectiveness of treatments for menopausal depression.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
All authors were involved in the conceptualization and design of the study. DL, HYW were responsible for the initial conception and design. Data collection was carried out by DL, HYW, QQH, and YZ. The data analysis and interpretation were performed by YZ, SL, and HYW. The manuscript was initially drafted by HYW, DL, KLG, and SL, while subsequent revisions were made by DL, HYW, and SL. All authors have reviewed the final manuscript and approved its publication.
Funding
No funding.
Data availability
All datasets generated for this study are included in the article/supplementary material.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hongyu Wang and Shuang Li have contributed equally.
Contributor Information
Ke-Lei Guo, Email: guokelei20040328@163.com.
Dong Li, Email: Lidong58999@163.com.
References
- 1.Cohen LS, Soares CN, Vitonis AF, Otto MW, Harlow BL. Risk for new onset of depression during the menopausal transition - the Harvard study of moods and cycles. Arch Gen Psychiatry. 2006;63(4):385–90. [DOI] [PubMed] [Google Scholar]
- 2.Tang RY, Luo M, Li JY, Peng YJ, Wang YC, Liu B, Liu GF, Wang YP, Lin SQ, Chen R. Symptoms of anxiety and depression among Chinese women transitioning through menopause: findings from a prospective community-based cohort study. Fertil Steril. 2019;112(6):1160–71. [DOI] [PubMed] [Google Scholar]
- 3.Tangen T, Mykletun A. Depression and anxiety through the climacteric period: an epidemiological study (HUNT-II). J Psychosom Obstet Gynaecol. 2008;29(2):125–31. [DOI] [PubMed] [Google Scholar]
- 4.Greendale GA, Lee NP, Arriola ER. The menopause. Lancet. 1999;353(9152):571–80. [DOI] [PubMed] [Google Scholar]
- 5.Goodman NF, Cobin RH, Ginzburg SB, et al. American association of clinical endocrinologists medical guidelines for clinical practice for the diagnosis and treatment of menopause. Endocr Pract. 2011;17:1–25. [DOI] [PubMed] [Google Scholar]
- 6.Nanette Santoro C, Roeca BA Peters, Neal-Perry G. The menopause transition: signs, symptoms, and management options. Journal Clin Endocrinol Metab. 2021;106(1):1–15. [DOI] [PubMed] [Google Scholar]
- 7.Jia Y, Zhou Z, Xiang F, Hu W, Cao X. Global prevalence of depression in menopausal women: a systematic review and meta-analysis. J Affect Disord. 2024;358:474–82. 10.1016/j.jad.2024.05.051. [DOI] [PubMed] [Google Scholar]
- 8.Li J, Liu F, Liu Z, Li M, Wang Y, Shang Y, et al. Prevalence and associated factors of depression in postmenopausal women: a systematic review and meta-analysis. BMC Psychiatry. 2024;24:431. 10.1186/s12888-024-05875-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moret C. Combination/augmentation strategies for improving the treatment of depression[J]. Neuropsychiatr Dis Treat. 2005;1(4):301–9. [PMC free article] [PubMed] [Google Scholar]
- 10.Ibeachu CP, Uahomo PO. Quality of life in menopausal women: effects of sociodemographic factors and Symptoms[J]. South Asian Res J Med Sci. 2024;6(5):178–88. [Google Scholar]
- 11.McIntyre RS, Konarski JZ, Grigoriadis S, et al. Hormone replacement therapy and antidepressant prescription patterns: a reciprocal relationship[J]. CMAJ. 2005;172(1):57–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of breast cancer: nested case-control studies using the QResearch and CPRD databases. BMJ. 2020;371:m3873. 10.1136/bmj.m3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soares CN. Depression in peri-and postmenopausal women: prevalence, pathophysiology and pharmacological management. Drugs Aging. 2013;30(9):677–85. [DOI] [PubMed] [Google Scholar]
- 14.Slavich GM, Sacher J. Stress, sex hormones, inflammation, and major depressive disorder: extending social signal transduction theory of depression to account for sex differences in mood disorders. Psychopharmacology. 2019;236(10):3063–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samami E, Shahhosseini Z, Elyasi F. The effects of psychological interventions on menopausal hot flashes: a systematic review. Int J Reprod Biomed. 2022;20(4):255–72. 10.18502/ijrm.v20i4.10898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conklin D, Carpenter JS, Whitney MS, DeLozier S, Ogede DO, Bazella C, McVoy M, Sajatovic M. Narrative analyses: cognitive behavior group therapy for women with menopause and bipolar or major depressive disorders. Womens Health Rep. 2021;2(1):430–42. 10.1089/whr.2021.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong Y, Zhang X, Zhao R, et al. The effects of mind-body exercise on anxiety and depression in older adults: a systematic review and network meta-analysis. Front Psychiatry. 2024;15: 1305295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li H, Xie P, Hou Y, et al. Preferences of patients with depression for exercises: a discrete choice experiment. BMC Public Health. 2025;25(1): 1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim SY, Park JH, Lee MY, et al. Physical activity and the prevention of depression: a cohort study. Gen Hosp Psychiatry. 2019;60:90–7. [DOI] [PubMed] [Google Scholar]
- 20.Goldstein KM, McDuffie JR, Shepherd-Banigan M, Befus D, Coeytaux RR, Van Noord MG, Goode AP, Masilamani V, Adam S, Nagi A, Williams JW Jr. Nonpharmacologic, nonherbal management of menopause-associated vasomotor symptoms: an umbrella systematic review (protocol). Syst Rev. 2016. 10.1186/s13643-016-0232-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie Y, Wu Z, Sun L, Zhou L, Wang G, Xiao L, Wang H. The effects and mechanisms of exercise on the treatment of depression. Front Psychiatry. 2021;12:705559. 10.3389/fpsyt.2021.705559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sani NA, Yusoff SSM, Norhayati MN, Zainudin AM. Tai Chi exercise for mental and physical well-being in patients with depressive symptoms: a systematic review and meta-analysis. Int J Environ Res Public Health. 2023;20(4): 2828. 10.3390/ijerph20042828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yue H, Yang Y, Xie F, Cui J, Li Y, Si M, Li S, Yao F. Effects of physical activity on depressive and anxiety symptoms of women in the menopausal transition and menopause: a comprehensive systematic review and meta-analysis of randomized controlled trials. Int J Behav Nutr Phys Act. 2025;22(1):13. 10.1186/s12966-025-01712-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han B, Duan Y, Zhang P, et al. Effects of exercise on depression and anxiety in postmenopausal women: a pairwise and network meta-analysis of randomized controlled trials. BMC Public Health. 2024;24(1): 1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Velentzis LS, Salagame U, Canfell K. Menopausal hormone therapy: a systematic review of cost-effectiveness evaluations. BMC Health Serv Res. 2017;17:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu R, Tang X. Effect of leisure-time physical activity on depression and depressive symptoms in menopausal women: a systematic review and meta-analysis of randomized controlled trials. Front Psychiatry. 2025;15: 1480623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sim J, Wright CC. The kappa statistic in reliability studies: use, interpretation, and sample size requirements. Phys Ther. 2005;85(3):257–68. [PubMed] [Google Scholar]
- 28.Mai S, Liu Y, He H, et al. The effects of different exercise programs on the prevention of perinatal depression: a systematic review and meta-analysis. Clin Exp Obstet Gynecol. 2023;50(1):9. [Google Scholar]
- 29.Daley AJ, Foster L, Long G, et al. The effectiveness of exercise for the prevention and treatment of antenatal depression: systematic review with meta-analysis. BJOG: An International Journal of Obstetrics & Gynaecology. 2015;122(1):57–62. [DOI] [PubMed] [Google Scholar]
- 30.Elavsky S, McAuley E. Physical activity and mental health outcomes during menopause: a randomized controlled trial. Ann Behav Med. 2007;33(2):132–42. [DOI] [PubMed] [Google Scholar]
- 31.Pang Y, Kim O. Effects of smartphone-based compensatory cognitive training and physical activity on cognition, depression, and self-esteem in women with subjective cognitive decline. Brain Sci. 2021;11(8): 1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bowen DJ, Fesinmeyer MD, Yasui Y, et al. Randomized trial of exercise in sedentary middle aged women: effects on quality of life. Int J Behav Nutr Phys Act. 2006;3:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Imayama I, Alfano CM, Kong A, et al. Dietary weight loss and exercise interventions effects on quality of life in overweight/obese postmenopausal women: a randomized controlled trial. Int J Behav Nutr Phys Act. 2011;8:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bernard P, Ninot G, Bernard PL, et al. Effects of a six-month walking intervention on depression in inactive post-menopausal women: a randomized controlled trial. Aging Ment Health. 2015;19(6):485–92. [DOI] [PubMed] [Google Scholar]
- 35.Hu L, Zhu L, Lyu J, et al. Benefits of walking on menopausal symptoms and mental health outcomes among Chinese postmenopausal women. Int J Gerontol. 2017;11(3):166–70. [Google Scholar]
- 36.Sternfeld B, Guthrie KA, Ensrud KE, et al. Efficacy of exercise for menopausal symptoms: a randomized controlled trial. Menopause. 2014;21(4):330–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abedi P, Nikkhah P, Najar S. Effect of pedometer-based walking on depression, anxiety and insomnia among postmenopausal women. Climacteric. 2015;18(6):841–5. [DOI] [PubMed] [Google Scholar]
- 38.Elsayed MM, El Refaye GE, Rabiee A, et al. Aerobic exercise with diet induces hormonal, metabolic, and psychological changes in postmenopausal obese women. Heliyon. 2022. 10.1016/j.heliyon.2022.e09165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luoto R, Moilanen J, Heinonen R, Mikkola T, Raitanen J, Tomas E, Ojala K, Mansikkamäki K, Clas-Håkan N. Effect of aerobic training on hot flushes and quality of life—a randomized controlled trial. Ann Med. 2012;44(6):616–26. 10.3109/07853890.2011.583674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noh E, Kim J, Kim M, Yi E. Effectiveness of sabang-dolgi walking exercise program on physical and mental health of menopausal women. Int J Environ Res Public Health. 2020;17: 6935. 10.3390/ijerph17186935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sen EI, Esmaeilzadeh S, Eskiyurt N. Effects of whole-body vibration and high impact exercises on the bone metabolism and functional mobility in postmenopausal women. J Bone Miner Metab. 2020;38:392–404. [DOI] [PubMed] [Google Scholar]
- 42.Aibar-Almazán A, Hita-Contreras F, Cruz-Díaz D, et al. Effects of pilates training on sleep quality, anxiety, depression and fatigue in postmenopausal women: a randomized controlled trial. Maturitas. 2019;124:62–7. [DOI] [PubMed] [Google Scholar]
- 43.Abdoshahi M. The impact of pilates training on mental health and happiness among untrained menopausal women. Womens Health Bull. 2023;10(2):96–103. [Google Scholar]
- 44.Afonso RF, Hachul H, Kozasa EH, et al. Yoga decreases insomnia in postmenopausal women: a randomized clinical trial[J]. Menopause. 2012;19(2):186–93. [DOI] [PubMed] [Google Scholar]
- 45.Li K, Yu H, Lin X, et al. The effects of ER Xian Decoction combined with Baduanjin exercise on bone mineral density, lower limb balance function, and mental health in women with postmenopausal osteoporosis: a randomized controlled trial[J]. Evid-Based Complementary Altern Med. 2022;2022(1):8602753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Newton KM, Reed SD, Guthrie KA, et al. Efficacy of yoga for vasomotor symptoms: a randomized controlled trial. Menopause. 2014;21(4):339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.del Carmen Carcelén-Fraile M, Aibar-Almazán A, Martínez-Amat A, et al. Qigong for mental health and sleep quality in postmenopausal women: a randomized controlled trial. Medicine (Baltimore). 2022;101(39): e30897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arslan Kabasakal S. The effects of a 6-week pilates exercises on quality of life, depression, and musculoskeletal disorders in menopausal women. Eur Res J. 2025;11(2):296–303. 10.18621/eurj.1603630. [Google Scholar]
- 49.Gao L, Zhang L, Qi H, et al. Middle-aged female depression in perimenopausal period and square dance intervention[J]. Psychiatria Danubina. 2016;28(4):372–8. [PubMed] [Google Scholar]
- 50.Kai Y, Nagamatsu T, Kitabatake Y, et al. Effects of stretching on menopausal and depressive symptoms in middle-aged women: a randomized controlled trial. Menopause. 2016;23(8):827–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Villaverde Gutierrez C, Torres Luque G, Abalos Medina GM, et al. Influence of exercise on mood in postmenopausal women. J Clin Nurs. 2012;21(7–8):923–8. [DOI] [PubMed] [Google Scholar]
- 52.Takahashi M, Lim PJ, Tsubosaka M, et al. Effects of increased daily physical activity on mental health and depression biomarkers in postmenopausal women. J Phys Ther Sci. 2019;31(4):408–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Giannini A, Caretto M, Genazzani AR, et al. Neuroendocrine changes during menopausal transition. Endocrines. 2021;2(4):405–16. [Google Scholar]
- 54.Jia Y, Zhou Z, Wang F, et al. Global prevalence of depression in menopausal women: a systematic review and meta-analysis[J]. J Affect Disord. 2024. 10.1016/j.jad.2024.05.051. [DOI] [PubMed] [Google Scholar]
- 55.Guo Q, Chen C. Rehabilitation effect of rope skipping on patients with perimenopausal syndrome complicated with depressien. Chin Nurs Res. 2011;25:422–3. [Google Scholar]
- 56.Kandola A, Ashdown-Franks G, Hendrikse J, Sabiston CM, Stubbs B. Physical activity and depression: towards understanding the antidepressant mechanisms of physical activity. Neurosci Biobehav Rev. 2019;107:525–39. 10.1016/j.neubiorev.2019.09.040. [DOI] [PubMed] [Google Scholar]
- 57.Li Y, Luo Y, Tang J, Liang X, Wang J, Xiao Q, et al. The positive effects of running exercise on hippocampal astrocytes in a rat model of depression. Transl Psychiatry. 2021;11: 83. 10.1038/s41398-021-01216-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Archer T, Josefsson T, Lindwall M. Effects of physical exercise on depressive symptoms and biomarkers in depression. CNS & neurological Disorders-Drug targets (Formerly current drug targets-CNS & neurological Disorders). 2014;13(10):1640–53. [DOI] [PubMed] [Google Scholar]
- 59.Birkhäuser M. Depression, menopause and estrogens: is there a correlation? Maturitas. 2002;41:3–8. [DOI] [PubMed] [Google Scholar]
- 60.Turek J, Gąsior Ł. Estrogen fluctuations during the menopausal transition are a risk factor for depressive disorders. Pharmacol Rep. 2023;75(1):32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Soares CN, Cohen LS. The perimenopause, depressive disorders, and hormonal variability. Sao Paulo Med J. 2001;119:78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu Y, Fu X, Guan B, et al. The role and mechanism of estrogen in perimenopausal Depression[J]. Curr Neuropharmacol. 2025. 10.2174/011570159X371863250327073835. [DOI] [PubMed] [Google Scholar]
- 63.Zhou R, Wang Z, Zhou B, et al. Estrogen receptors mediate the antidepressant effects of aerobic exercise: a possible new mechanism. Front Aging Neurosci. 2022;14: 1040828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McTiernan A, Tworoger SS, Ulrich CM, et al. Effect of exercise on serum estrogens in postmenopausal women: a 12-month randomized clinical trial[J]. Cancer Res. 2004;64(8):2923–8. [DOI] [PubMed] [Google Scholar]
- 65.Zhao Y, Niu H, Liu S. Effects of aerobics training on anxiety, depression and sleep quality in perimenopausal women. Front Psychiatry. 2022;13: 102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.de Vries LP, van de Weijer MP, Bartels M. The human physiology of well-being: a systematic review on the association between neurotransmitters, hormones, inflammatory markers, the microbiome and well-being. Neurosci Biobehav Rev. 2022;139: 104733. [DOI] [PubMed] [Google Scholar]
- 67.Chen C, Nakagawa S. Recent advances in the study of the neurobiological mechanisms behind the effects of physical activity on mood, resilience and emotional disorders. Adv Clin Exp Med. 2023;32(9):937–42. [DOI] [PubMed] [Google Scholar]
- 68.Hossain MN, Lee J, Choi H, et al. The impact of exercise on depression: how moving makes your brain and body feel better. Physical Activity and Nutrition. 2024;28(2):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Anderson D, Mizzari K, Kain V, et al. The effects of a multimodal intervention trial to promote lifestyle factors associated with the prevention of cardiovascular disease in menopausal and postmenopausal Australian women. Health Care Women Int. 2006;27(3):238–53. [DOI] [PubMed] [Google Scholar]
- 70.Azizi M, Fooladi E, Abdollahi F, et al. Biopsychosocial risk factors of depression in the menopausal transition: a narrative review[J]. Iran J Psychiatry Behav Sci. 2018;12(4):e12928. [Google Scholar]
- 71.Yeung A, Chan JSM, Cheung JC, et al. Qigong and Tai-Chi for mood regulation[J]. Focus. 2018;16(1):40–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang CC, Wei IH, Chou YH, et al. Effect of age, gender, menopausal status, and ovarian hormonal level on rTMS in treatment-resistant depression. Psychoneuroendocrinology. 2008;33(6):821–31. [DOI] [PubMed] [Google Scholar]
- 73.Graziottin A, Serafini A. Depression and the menopause: why antidepressants are not enough? Menopause Int. 2009;15(2):76–81. [DOI] [PubMed] [Google Scholar]
- 74.Kolu P, Raitanen J, Nygård CH, et al. Cost-effectiveness of physical activity among women with menopause symptoms: findings from a randomised controlled trial. PLoS One. 2015;10(8): e0135099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pampallona S, Bollini P, Tibaldi G, et al. Combined pharmacotherapy and psychological treatment for depression: a systematic review. Arch Gen Psychiatry. 2004;61(7):714–9. [DOI] [PubMed] [Google Scholar]
- 76.Simpson RJ, Kunz H, Agha N, et al. Exercise and the regulation of immune functions[J]. Prog Mol Biol Transl Sci. 2015;135:355–80. [DOI] [PubMed] [Google Scholar]
- 77.Hearing CM, Chang WC, Szuhany KL, et al. Physical exercise for treatment of mood disorders: a critical review. Curr Behav Neurosci Rep. 2016;3:350–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bryan AD, Magnan RE, Nilsson R, et al. The big picture of individual differences in physical activity behavior change: a transdisciplinary approach. Psychol Sport Exerc. 2011;12(1):20–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sherwood NE, Jeffery RW. The behavioral determinants of exercise: implications for physical activity interventions. Annu Rev Nutr. 2000;20(1):21–44. [DOI] [PubMed] [Google Scholar]
- 80.McArthur D, Dumas A, Woodend K, et al. Factors influencing adherence to regular exercise in middle-aged women: a qualitative study to inform clinical practice[J]. BMC Womens Health. 2014;14:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.