Abstract
DNA vaccination elicits humoral and cellular immune responses and has been shown to confer protection against several viral, bacterial, and parasitic pathogens. Here we report that optimized codon usage of an injected DNA sequence considerably increases both humoral and cellular immune responses. We recently generated a synthetic human immunodeficiency virus type 1 gp120 sequence in which most wild-type codons were replaced with codons from highly expressed human genes (syngp120). In vitro expression of syngp120 is considerably increased in comparison to that of the respective wild-type sequence. In BALB/c mice, DNA immunization with syngp120 resulted in significantly increased antibody titers and cytotoxic T-lymphocyte reactivity, suggesting a direct correlation between expression levels and the immune response. Moreover, syngp120 is characterized by rev-independent expression and a low risk of recombination with viral sequences. Thus, synthetic genes with optimized codon usage represent a novel strategy to increase the efficacy and safety of DNA vaccination.
It has been shown that “naked” DNA administered to animals is taken up by cells and expressed (21, 30, 66). Inoculated plasmid DNAs expressing foreign genes induce humoral and cellular immune responses and thus can be used for vaccination (46, 58, 67). In animal models, DNA vaccination has been shown to induce protective immunity against a variety of viral, bacterial, and parasitic pathogens. Inoculation of plasmid DNAs conferred protection against challenges with influenza virus (46, 61), rabies virus (68), herpes simplex virus (37), papillomavirus (16), lymphocytic choriomeningitis virus (72), and flavivirus (44) but also against tuberculosis (28, 59), leishmaniasis (69), and malaria (17, 54). Plasmid DNAs expressing genes derived from simian immunodeficiency virus or human immunodeficiency virus (HIV) were recently shown to induce humoral and cellular immune responses in rodents (64, 65), in nonhuman primates (6, 12, 34, 42, 71), and in phase I and II studies with humans (unpublished data). Although these constructs were able to induce an immune response, both circulating antibody titers and HIV type 1 (HIV-1)-specific cytotoxic T-lymphocyte (CTL) levels were transient and low. As there are several lines of evidence for a correlation among protection, clinical course, and immune responses from previous studies on mother-to-infant transmission of HIV (24, 48, 49), repeatedly HIV-exposed but uninfected individuals from high-risk groups (50), human long-term survivors (8, 43), and vaccination trials with nonhuman primates (7, 26), we sought to increase the efficacy of DNA vaccines expressing HIV genes. We generated a synthetic gp120 sequence in which codon usage was optimized for expression in human cells (syngp120) (25). In this study, the syngp120 sequence induced a considerably higher immune response than did the respective wild-type sequence, suggesting that the efficacy of DNA vaccines can be significantly improved by optimization of translational signals.
MATERIALS AND METHODS
Plasmid constructs.
The syngp120 sequence was previously generated by use of eight long synthetic oligonucleotides, which were amplified by a PCR with overlapping primers with unique restriction sites; the oligonucleotides were subsequently subcloned into a pCdm7-derived plasmid with a suitable polylinker 3′ to the CD5 signal peptide sequence (25). A wild-type gp120 sequence from either HIV-1 LAI (gp120LAI) or HIV-1 MN (gp120MN) were similarly expressed in pCdm7 under control of the human cytomegalovirus immediate-early promoter (55). In some experiments, wild-type gp120 sequences in which the endogenous signal sequence was replaced with the CD5 signal sequence were used (see Fig. 4 and 5) (25). There was no difference in antibody induction between constructs with the endogenous signal sequence and the CD5 signal sequence. syngp120v3LAI, used for immunization of mice tested in CTL assays, was generated by subcloning of a 111-mer oligonucleotide adapter into the MluI and XbaI restriction sites of syngp120. gp120MNrre, gp120LAIrre, syngp120rre, and syngp120v3LAIrre were generated by inserting a 0.24-kb fragment containing the HIV-1 rev-responsive element (RRE) into the NotI restriction site of the respective plasmids. pCMV-rev was kindly provided by the National Institutes of Health AIDS repository.
FIG. 4.
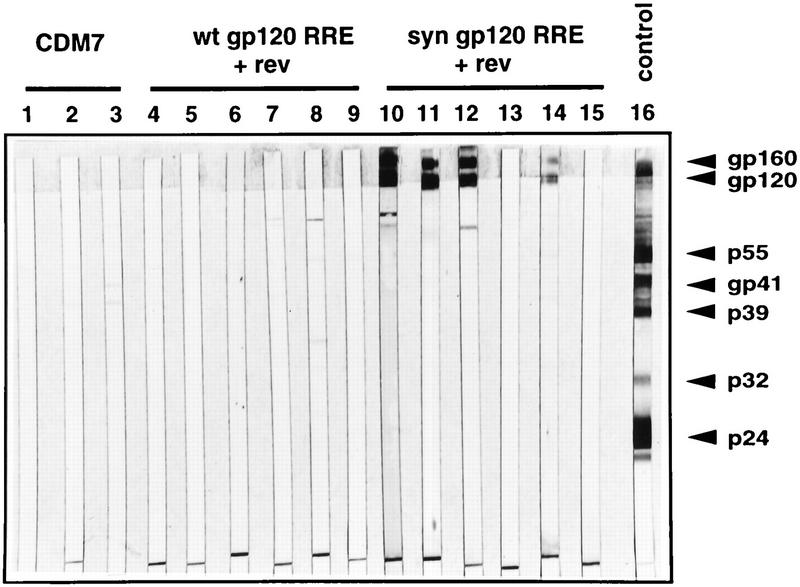
Increased humoral immune responses of BALB/c mice immunized with syngp120 independently of the regulatory protein Rev. Western blot analysis of sera from mice immunized with pCdm7 (lanes 1 to 3), gp120MNrre and pCMV-rev (lanes 4 to 9), or syngp120v3LAIrre and pCMV-rev (lanes 10 to 15) is shown. Western blot strips prepared with the HIV-1 MvP899 isolate were reacted with sera derived from either DNA-injected mice (lanes 1 to 15) or an HIV-1-infected individual (lane 16). Mice were immunized twice, and serum was collected 12 weeks after the initial immunization. wt, wild type.
FIG. 5.
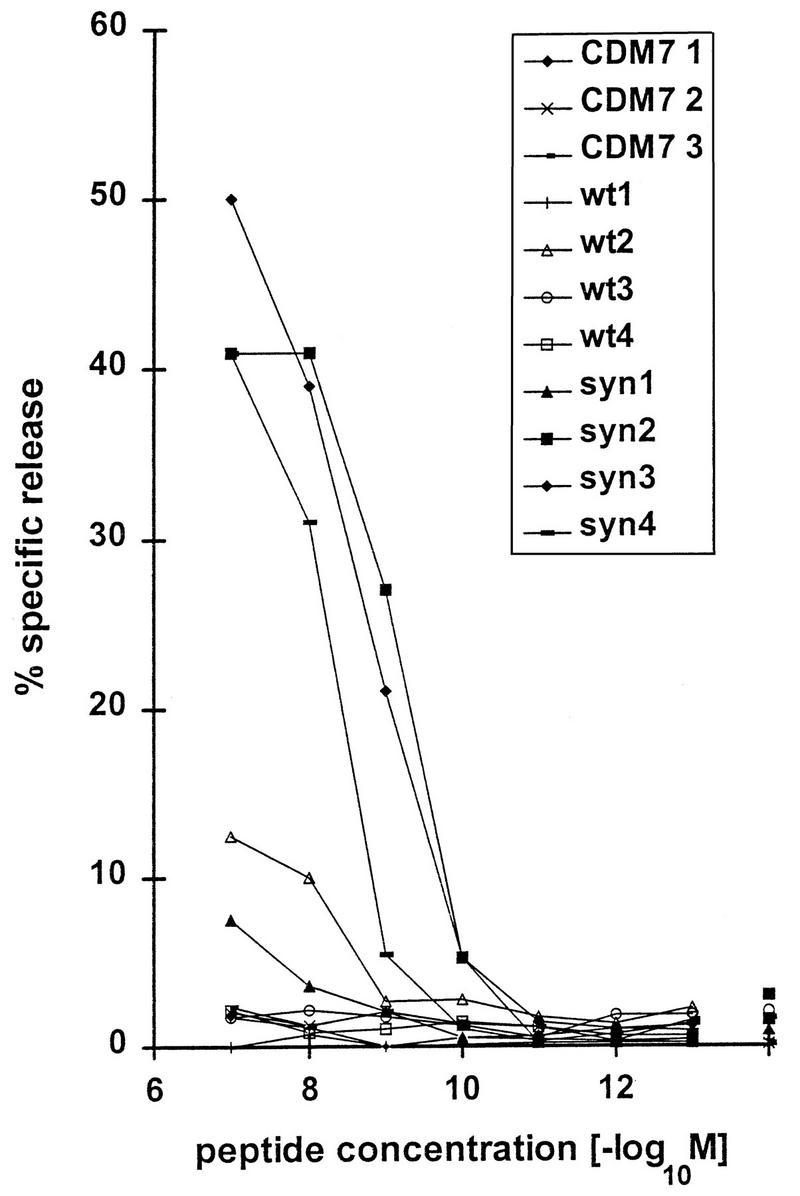
Increased cellular immune responses of BALB/c mice immunized with syngp120. CTL assay of spleen cells from DNA-injected mice against target cells loaded with titrated amounts of the nonamer peptide GPGRAFVTI. BALB/c mice were immunized with 40 μg of either pCDM7 (CDM71 to CDM73; negative control), syngp120v3LAIrre (syn1 to syn4), or gp120LAIrre (wt1 to wt4) and 10 μg of pCMV-rev, boosted twice after 6 and 30 weeks, and sacrified after an additional 3 weeks. Spleen cells were restimulated with interleukin 2 and peptide-loaded B7 cells in vitro. Data are mean percentages of three replicate cultures. Killing was antigen specific and not caused by NK cells, as wells without peptide did not show any chromium release (<5% specific release in all mice).
Cell lines.
293T (adenovirus-transformed human kidney cells), HeLa (human cervical carcinoma cells), NIH 3T3 (murine fibroblasts), and COS-7 (African green monkey kidney cells) were maintained in Dulbecco’s modified Eagle medium (Life Technologies, Paisley, Scotland) supplemented with 10% heat-inactivated fetal calf serum (FCS), 100 IU of penicillin per ml, 100 μg of streptomycin per ml, and 2 mM l-glutamine. P815 (murine mastocytoma cells) and B7, a P815-derived cell line expressing the costimulatory molecule B7, were maintained in RPMI medium supplemented with 5% FCS, 100 IU of penicillin per ml, 100 μg of streptomycin per ml, and 0.05 mM 2-mercaptoethanol.
Immunoprecipitation.
Cells were transfected by calcium phosphate coprecipitation with 10 μg of plasmid DNA per 6-cm tissue culture dish according to standard protocols (51). In brief, precipitated DNA was incubated with cells at approximately 70% confluence for 8 to 12 h. After 100 μM chloroquine was added for an additional 4 h, the culture medium was exchanged. At 3 days posttransfection, cells were metabolically labelled with 200 μCi of [35S]Cys/Met per dish. Supernatants were immunoprecipitated with human antiserum 95-1 from an HIV-infected patient and analyzed by reducing sodium dodecyl sulfate (SDS)–7% polyacrylamide gel electrophoresis (PAGE).
Nucleic acid immunization.
BALB/c mice (Jackson Laboratories) were immunized with 50 μg of plasmid DNA (in 50 μl of phosphate-buffered saline [PBS]) in both anterior tibial muscles that had been pretreated with cardiotoxin (50 μl of a 10 μM cardiotoxin solution) from Naja nigricollis (Latoxan, Rosans, France) as reported previously (52). Sera were drawn from the tail vein after various intervals.
Cytotoxicity assay.
CTLs were prepared from spleen cells of sacrified mice by culturing in alpha minimal essential medium (Life Technologies) supplemented with 10 mM HEPES buffer, 0.05 mM 2-mercaptoethanol, 100 IU of penicillin per ml, 100 μg of streptomycin per ml, and 10% heat-inactivated FCS. After 5 days, interleukin 2 was added at a concentration of 100 U/ml; after an additional 2 days, peptide-loaded B7 cells irradiated with 80,000 rads were added at a ratio of 1:2. Cytotoxic effector cells were restimulated every 2 weeks and harvested after various intervals. Cytolytic activity was measured with a standard 51Cr release assay. In brief, 103 51Cr-labelled P815 target cells per well were incubated for 1 h at 37°C with titrated amounts (10−7 to 10−13 M) of the nonamer peptide GPGRAFVTI, constituting the crown of the HIV-1 LAI v3 loop. Subsequently, 104 effector cells were added to each well and incubated for 4 h at 37°C. Finally, 100 μl of supernatant was harvested from each well and analyzed in a Canberra Packard microplate scintillation counter. Specific release was calculated with the formula [(experimental release − spontaneous release)/(total release − spontaneous release)] × 100. All data are means of results for triplicate cultures.
ELISA.
Sera from immunized mice were tested for antibodies directed against HIV-1 gp120 by either an enzyme-linked immunosorbent assay (ELISA) or Western blotting. ELISA microtiter plates were coated with 1 μg of a CD4-immunoglobulin G (IgG) fusion protein (kindly provided by Behring, Marburg, Germany) per well overnight and washed four times; subsequently, blocking was done with PBS–0.2% Tween for 2 h. After removal of the blocking solution, 100 μl of supernatant from 293T cells transfected with syngp120 was added and incubated for 90 min. The supernatant was discarded, and 100 μl of prediluted mouse serum was added and incubated for 1 h. Microtiter plates were washed four times and incubated with a secondary, peroxidase-coupled anti-mouse IgG antibody (Jackson Laboratories). Finally, ELISA plates were washed, 200 μl of 2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) substrate (Boehringer GmbH, Mannheim, Germany) was added per well, and the optical density at 405 nm was measured after 15 min. The optical density of control wells without mouse serum was between 0 and 0.2 (in most cases, below 0.1).
Western blotting.
Virus stocks were isolated from supernatants of H9 cells infected with HIV-1 MvP899, purified by sucrose density gradient centrifugation, and subjected to denaturing SDS–12% PAGE. The gel was blotted onto a polyvinylidene difluoride membrane (Millipore, Bedford, Mass.), blocking was done with 1% nonfat dry milk powder, and the gel was cut into strips. Mouse sera were diluted 1:100 in PBS and reacted with individual strips for 1 h. Subsequently, strips were washed four times with Tris-buffered saline–0.2% Tween, reacted with a peroxidase-coupled antiserum against mouse IgG (Jackson Laboratories), and incubated with diaminobenzidine substrate (Sigma, St. Louis, Mo.).
Statistical analysis.
Statistical analysis was done with the Kruskal-Wallis test and SAS release 6.08 TS407 software (SAS Inc., Cary, N.C.).
RESULTS
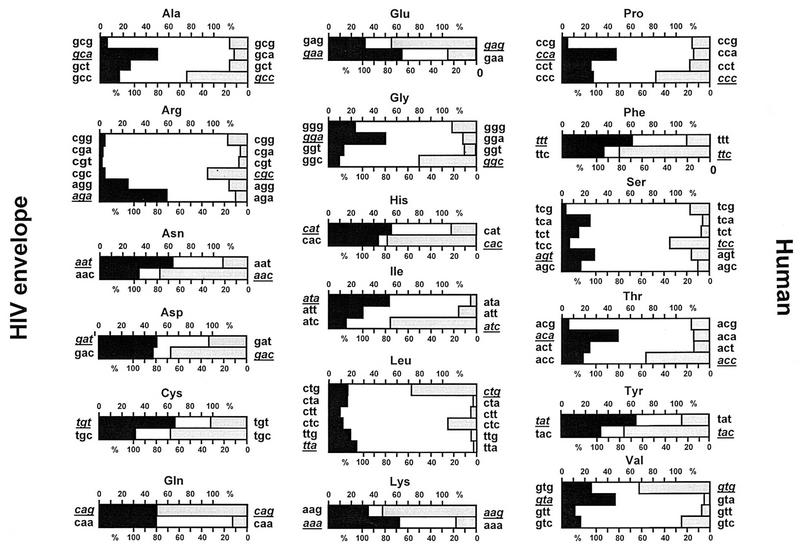
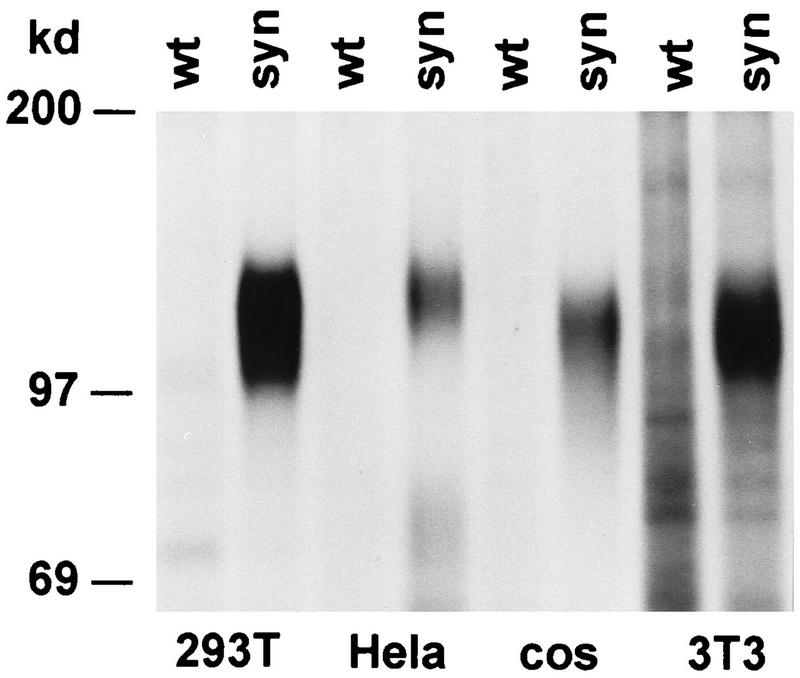
The expression of cloned HIV-1 env sequences in eukaryotic expression plasmids is inefficient due to poorly characterized negative sequence elements, which can be found throughout the HIV-1 genome (11, 18, 40, 47, 53). Inhibition appears to be partly caused by elements mediating nuclear mRNA retention, which can be reversed by Rev, a regulatory HIV-1 protein promoting the export of unspliced viral RNA into the cytoplasm (18–20, 36). However, even HIV-1 transcripts located in the cytoplasm are insufficiently expressed due to low translational efficiency caused by a highly distinct codon bias for adenine and thymine at the third codon position. Codon usage in env is very similar in all primary and laboratory HIV-1 isolates, is independent of subtype and phenotype, and is strikingly divergent from that of highly expressed human genes, in which predominantly codons with cytosine and guanine at the third codon position are found (Fig. 1). To test the effect of codon preference in env, we generated syngp120, which has an amino acid sequence 100% identical to that of the HIV-1 MN isolate (subtype B) and in which all wild-type codons are replaced by codons used in highly expressed human genes (25). Levels of expression of the syngp120 sequence are considerably increased in comparison to that of the wild-type gp120 sequence and, moreover, are independent of the Rev regulatory protein. In various mammalian cell lines, significantly more glycoprotein could be immunoprecipitated from supernatants of cells transiently transfected with the syngp120 sequence than with the wild-type gp120 sequence (Fig. 2). The difference in expression levels between the synthetic and the wild-type constructs depends on the expression system and the tissue transfected. In 293T cells transiently transfected with the eukaryotic expression vector pCdm7, there is usually a 10- to 50-fold increase in expression levels with the synthetic gene.
FIG. 1.
Codon bias in the HIV-1 envelope gene. Codon frequencies in the HIV-1 envelope gene (black boxes) and in highly expressed human genes (shaded boxes) were calculated by use of standard software from the University of Wisconsin Genetics Computer Group and sequences derived from the Los Alamos National Library database. Codon frequencies were tabulated with 24 different HIV-1 envelope sequences from the following isolates: Ada, Ant70, Br0141, Br0259, JFL, JRCSF, JY1, LAI, M12199, MaI, MN, MVP5180, RF, Rw0914, SF2, SF162, SF33, T8659, Th1412, Ug0205, Ug0317, Ug0378, ZAM20, and Z6649. Codon frequencies from highly expressed cellular genes are listed according to the work of Cherry (10). The most frequently used codon for every amino acid is underlined.
FIG. 2.
Increased expression of syngp120 in various mammalian cell lines in vitro. 293T (adenovirus-transformed human kidney cells), HeLa (human cervix carcinoma cells), NIH 3T3 (mouse fibroblasts), and COS-7 (African green monkey kidney cells) were transiently transfected by calcium phosphate coprecipitation with plasmids carrying genes coding for either wild-type gp120MN (wt) or syngp120 (syn). Culture supernatants of radioactively labelled cells were immunoprecipitated with a human antiserum derived from an HIV-1-infected individual and analyzed on a reducing SDS–7% PAGE.
In DNA inoculation, the expression of injected genes is influenced by the promoter used (9, 38). The immune response was shown to be modulated in some but not in all cases by the promoter (4, 62, 73). We thus speculated that codon usage modulates the immune response and compared results with plasmids carrying genes expressing the syngp120 sequence and the respective wild-type gp120 sequence. Plasmid DNA encoding either gene subcloned in an identical vector backbone was inoculated into the anterior tibial muscles of BALB/c mice and tested for antibody and CTL induction. A total of 75 BALB/c mice (17 controls, 29 receiving wild-type gp120, and 29 receiving syngp120) were immunized in six separate experiments. Mice immunized with the syngp120 gene developed considerably higher antibody titers than mice injected with the wild-type gp120 gene (Kruskal-Wallis test, P < 0.00479). One prototypic experiment is shown in Fig. 3. In this experiment, injection of plasmid DNA containing syngp120 resulted in high concentrations of anti-gp120 antibodies in three of four mice, whereas one mouse developed no measurable antibody response. In contrast, all mice inoculated with a plasmid containing wild-type gp120 developed no or only barely detectable antibodies with this immunization schedule. Similar results were achieved with gp120LAI, indicating that the low production of antibodies was due to neither the particular virus strain nor the plasmid construct used (data not shown). Since in vitro in transiently transfected cells the expression of wild-type gp120 but not of syngp120 can be increased when the posttranslational transactivator Rev is supplied in trans and the RRE is supplied in cis, we coinjected plasmid pCMV-rev expressing HIV-1 Rev with plasmids containing an RRE 3′ to the gp120 coding region and tested for antibody induction (Fig. 4). We were able to detect antibodies directed against gp120/gp160 in four of six mice immunized with the syngp120 sequence but in none injected with the wild-type gp120 sequence, indicating that even in the presence of Rev and the RRE the induction of antibodies was increased with the synthetic sequence.
FIG. 3.
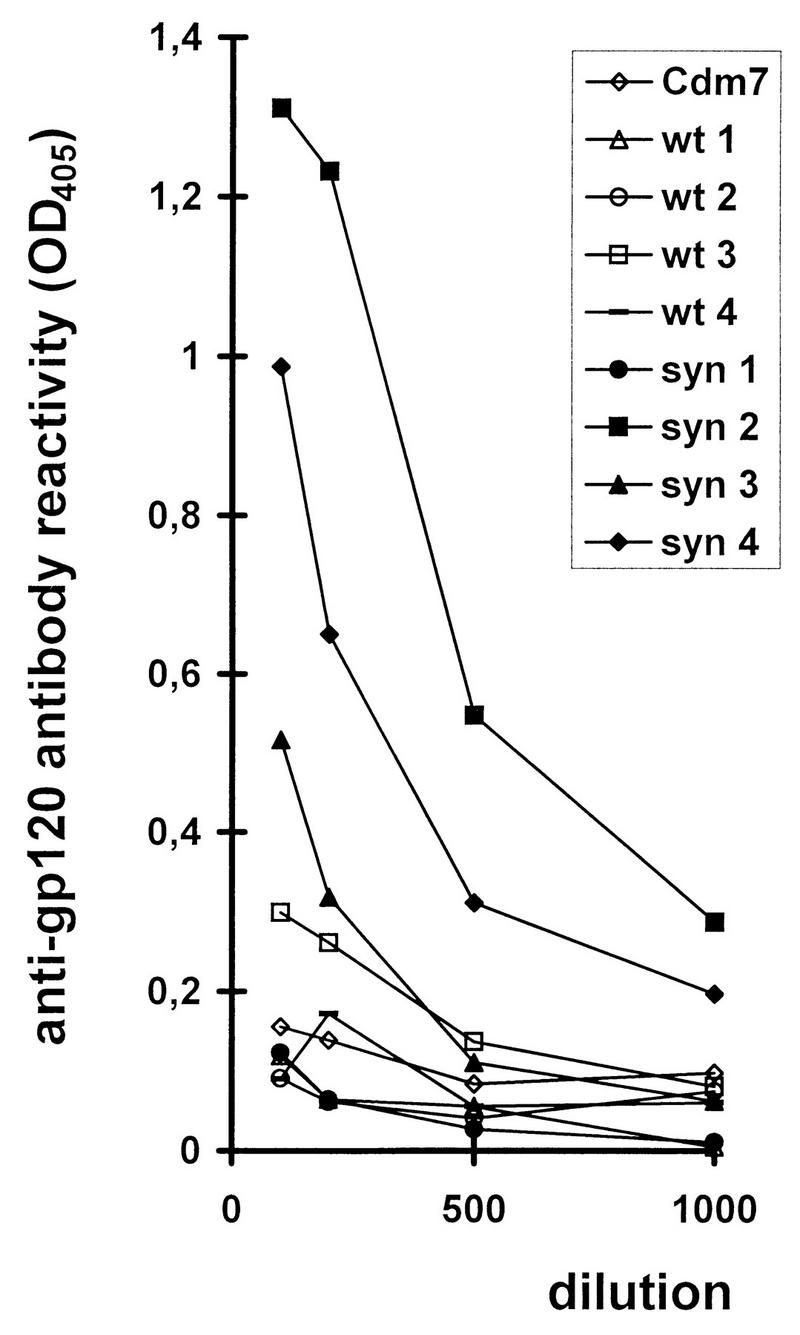
Increased humoral immune responses of BALB/c mice immunized with syngp120. ELISA analysis of sera from mice immunized with plasmid DNA encoding either syngp120 (filled symbols) or wild-type gp120 MN (open symbols). IgG antibody reactivity against gp120 in sera from DNA-inoculated mice was measured by an ELISA. Mice were either immunized once (wt 1, wt 2, syn 1, and syn 2) or immunized and boosted three times after 2, 4, and 6 weeks (wt 3, wt 4, syn 3, and syn 4). Sera were drawn from the tail vein 3 (wt 1, wt 2, syn 1, and syn 2) or 10 (wt 3, wt 4, syn 3, and syn 4) weeks after the initial immunization. OD405, optical density at 405 nm; wt, wild type; syn, syngp120.
The induction of CTLs was tested with spleen cells isolated from DNA-immunized mice as effector cells and peptide-loaded P815 mouse mastocytoma cells as target cells. The GPGRAFVTI nonamer peptide used in this assay is known to constitute a Dd-restricted CTL epitope in mice. For evaluation of the cellular immune response, a total of 16 mice (four controls, 6 receiving wild-type gp120, and 6 receiving syngp120) were tested in two experiments. Like antibody production, CTL responses in mice immunized with syngp120 were significantly higher than those in mice injected with wild-type gp120 (Kruskal-Wallis test with 10−7 M peptide concentration, P < 0.01; with 10−7 to 10−9 M peptide concentration, P < 0.001). In the experiment shown in Fig. 5, the cellular immune response was increased in mice immunized with the syngp120 sequence and again was barely detectable in mice immunized with the wild-type gp120 sequence. Differences in the immune responses of mice treated with wild-type gp120 and syngp120 constructs were not caused by a delayed time course in mice inoculated with the wild-type sequence (Fig. 6). Even multiple boosts with the wild-type sequence over a period of 5 months did not significantly increase antibody production.
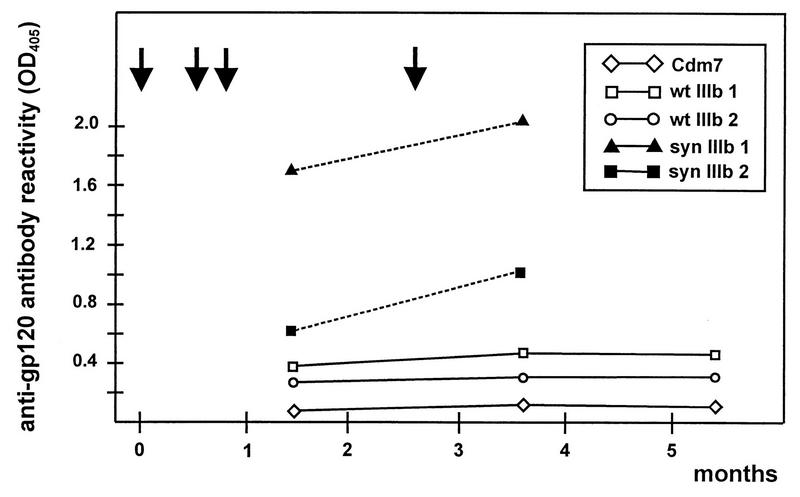
FIG. 6.
Time course analysis of sera from mice immunized with either wild-type gp120 or syngp120 sequences. Time course of antibody reactivity against gp120 in sera from BALB/c mice immunized with either syngp120 (syn IIIb 1 and syn IIIb 2) or wild-type gp120 (wt IIIb 1 and wt IIIb 2). Mice were immunized and boosted three times after 2, 4, and 11 weeks with 50 μg of plasmid DNA in both anterior tibial muscles (arrows). Serum was drawn 6 and 15 (wt IIIb 1, wt IIIb 2, syn IIIb 1, and syn IIIb 2) and 23 (wt IIIb 1 and wt IIIb 2) weeks after the initial immunization from the tail vein of immunized mice. IgG antibody reactivity against gp120 in sera from DNA-inoculated mice was measured by an ELISA and is shown for a serum dilution of 1:100. OD405, optical density at 405 nm.
DISCUSSION
In this study, we showed that DNA immunization with a synthetic sequence with optimized codon usage resulted in considerably increased humoral and cellular immune responses. Protection against HIV or simian immunodeficiency virus has been achieved in some animal models, with different vaccines. In primate models in which a rather avirulent virus challenge was used, vaccines inducing a limited immune response (like that induced by subunit vaccines, recombinant vectors, and peptides) were protective (3, 5, 56); however, in more pathogenic animal models, only live attenuated virus was successful (1, 13). These results suggest that a potential vaccine meeting safety requirements for humans can only be successful if strategies to increase immunogenicity are developed.
Our in vitro data with transfected cells lines suggest that the difference between the syngp120 and wild-type gp120 sequences is caused by different expression levels in muscle cells. In a previous study, we showed that there is no difference in cytoplasmic RNA levels in cells transfected with either wild-type gp120 or syngp120, indicating that the difference in protein levels is most likely a purely translational effect (25). However, an alternative explanation for the enhanced immune response might be the increase in CpG motifs in the synthetic gene administered. Recently, several groups were able to show that DNA containing unmethylated CpG motifs, such as bacterial DNA, is able to trigger B-cell activation (33, 45, 70). Moreover, Klinman and colleagues suggested that unmethylated CpG motifs might also contribute to the immunogenicity of gene vaccines (32). As a considerable number of CpG motifs, 92, have been introduced into the gp120 sequence by codon exchange, it is possible that they contribute to the increased immunogenicity of the syngp120 sequence.
With the wild-type gp120 sequence, we obtained low or undetectable antibody and CTL activities, in contrast to results in some of the earlier reports on DNA immunization with HIV-1-derived genes (12, 22, 35, 64, 65, 71). In in vitro transfection experiments, we are able to detect gp120 expressed from wild-type sequences when gels were exposed longer (25). However, in Fig. 2, a rather short exposure is shown because otherwise the autoradiography would have been overexposed due to the strong signal of syngp120. Moreover, as can be seen in Fig. 3 and 5, at least in some animals there was low but specific antibody (wt 3; Fig. 3) and CTL (wt2; Fig. 5) induction by the wild-type constructs that we used, indicating that gp120 is expressed from these constructs. Thus, low or undetectable immune responses with wild-type gp120 sequences can be explained not by cloning artifacts (e.g., by PCR cloning) but rather by the fact that we tried to improve neither our inoculation protocols nor the assays measuring antibody and CTL responses and consequently used both inefficient delivery conditions and detection assays. For example, the number of nonresponders, which might be reflective of the vector or the immunization technique used, could probably be decreased with gene-gun DNA delivery (23). Further improvement might be achieved with cytokine or cytokine-plasmid adjuvants, as has been shown previously (27, 29, 31, 73). The use of vectors coexpressing both gp120/gp160 and Rev or vectors with rev-independent gp120 expression might be helpful as well, although there are no hints in the literature suggesting that they induce a higher immune response than do coinjected env- and rev-expressing plasmids (6, 22, 34, 42, 64, 65). Moreover, in view of the potential interference with other cellular genes and potential use in humans, the introduction of genes coding for regulatory proteins is disadvantageous, and expression systems which are rev independent are preferable. Another major advantage of syngp120 is the low homology to viral sequences on the nucleic acid level, which considerably reduces the risk of homologous recombination with latent or defective viral genomes. Although relatively little is known about sequence requirements for homologous recombination in mammalian cells, it appears that DNA sequence mismatching presents a considerable barrier to homologous recombination in a wide variety of systems (14, 41, 57, 60, 63). As almost every third nucleotide has been exchanged in the syngp120 sequence and the longest stretch of identical nucleotides has a length of only 14 bp, it is conceivable that the risk of recombination is considerably decreased. Thus, in terms of safety issues, there are at least two aspects which argue in favor of the approach presented here.
The antibody response was tested by means of either an ELISA or Western blotting. The Western blot strips were prepared with HIV-1 isolates MvP899 (Fig. 4) and LAI (data not shown). Interestingly, sera from mice immunized with the syngp120 gene with the HIV-1 isolate MN amino acid sequence cross-reacted with both MvP899 and LAI strips. In the influenza virus system, there is evidence that DNA vaccines induce a broader immunity than subunit or inactivated-virus vaccines (15, 39). The induction of cross-reactive immunity might be further increased with a multigene DNA immunization strategy, as has been shown previously with malaria (17) and mycoplasma (2). The synthetic sequence presented here appears to be suitable for a similar multigene approach, since unique restriction sites have been introduced into the synthetic env sequence over an approximately 100-bp distance, allowing the generation of chimeric molecules with exchanged domains from other virus isolates. Alternatively, it might be useful to increase the number of potential epitopes by coinjection of DNA plasmids carrying other HIV genes, as was shown in a previous report, in which two vectors expressing env and gag/pol conferred protection against a heterologous HIV-1 challenge in chimpanzees (5).
In summary, codon optimization of the HIV-1 env gene appears to be advantageous because of (i) increased humoral and cellular immune responses, (ii) rev-independent expression, (iii) a low risk of recombination due to little homology to viral sequences, and (iv) potential use in a multigene approach. The putative superiority of this method needs to be addressed in future protection studies with macaques and humans by use of a single-gene or multigene approach. In more general terms, the data presented here suggest a similar strategy for genes from other pathogens used in DNA vaccination and for cellular genes used in gene therapy.
ACKNOWLEDGMENTS
We acknowledge the expert technical assistance of A. Weiss and the help of I. Crnkovic and J. Reimann with the immunization of mice and of H. Hengel and M. Eggers with the cytotoxicity assay. Plasmid pCMV-rev was kindly provided by M.-L. Hammarskjöld through the AIDS Research and Reference Reagent Program. Recombinant CD4:IgG1 fusion protein was kindly provided by Behring (Marburg, Germany).
This work was supported by the AIDS scholar program of the BMBF, grant PMG94/17 of EU programme EVA, and grant HA1754/2-1 of the Deutsche Forschungsgemeinschaft.
REFERENCES
- 1.Almond N, Kent K, Cranage M, Rud E, Clarke B, Stott E J. Protection by attenuated simian immunodeficiency virus in macaques against challenge with virus-infected cells. Lancet. 1995;345:1342–1344. doi: 10.1016/s0140-6736(95)92540-6. [DOI] [PubMed] [Google Scholar]
- 2.Barry M A, Lai W C, Johnston S A. Protection against mycoplasma infection using expression-library immunization. Nature. 1995;377:632–635. doi: 10.1038/377632a0. [DOI] [PubMed] [Google Scholar]
- 3.Berman P W, Gregory T J, Riddle L, Nakamura G R, Champe M A, Porter J P, Wurm F M, Hershberg R D, Cobb E K, Eichberg J W. Protection of chimpanzees from infection by HIV-1 after vaccination with recombinant glycoprotein gp120 but not gp160. Nature. 1990;345:622–625. doi: 10.1038/345622a0. [DOI] [PubMed] [Google Scholar]
- 4.Boehm W, Kuhroeber A, Paier T, Mertens T, Reimann J, Schirmbeck R. DNA vector constructs that prime hepatitis B surface antigen-specific cytotoxic T lymphocyte and antibody responses in mice after intramuscular injection. J Immunol Methods. 1996;193:29–40. doi: 10.1016/0022-1759(96)00035-x. [DOI] [PubMed] [Google Scholar]
- 5.Boyer J D, Ugen K E, Wang B, Agadjanyan M, Gilbert L, Bagarazzi M L, Chattergoon M, Frost P, Javadian A, Williams W V, Refaeli Y, Ciccarelli R B, McCallus D, Coney L, Weiner D. Protection of chimpanzees from high-dose heterologous HIV-1 challenge by DNA vaccination. Nat Med. 1997;3:526–532. doi: 10.1038/nm0597-526. [DOI] [PubMed] [Google Scholar]
- 6.Boyer J D, Wang B, Ugen K E, Agadjanyan M, Javadian A, Frost P, Dang K, Carrano R A, Ciccarelli R, Coney L, Williams W V, Weiner D B. In vivo protective anti-HIV immune responses in non-human primates through DNA immunization. J Med Primatol. 1996;25:242–250. doi: 10.1111/j.1600-0684.1996.tb00022.x. [DOI] [PubMed] [Google Scholar]
- 7.Bruck C, Thiriart C, Fabry L, Francotte M, Pala P, Van Opstal O, Culp J, Rosenberg M, DeWilde M, Heidt P, Heeney J. HIV-1 envelope-elicited neutralizing antibody titres correlate with protection and virus load in chimpanzees. Vaccine. 1994;12:1141–1148. doi: 10.1016/0264-410x(94)90185-6. [DOI] [PubMed] [Google Scholar]
- 8.Cao Y, Qin L, Zhang L, Safrit J, Ho D D. Virologic and immunologic characterization of long-term survivors of human immunodeficiency virus type 1 infection. N Engl J Med. 1995;332:201–208. doi: 10.1056/NEJM199501263320401. [DOI] [PubMed] [Google Scholar]
- 9.Cheng L, Zieglhoffer P R, Yang N-S. In vivo promoter activity and transgene expression in mammalian somatic tissues evaluated by using particle bombardment. Proc Natl Acad Sci USA. 1993;90:4455–4459. doi: 10.1073/pnas.90.10.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cherry M. Codon usage and frequency of codon occurrence. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1992. pp. A1.8–A1.9. [Google Scholar]
- 11.Cochrane A W, Jones K S, Beidas S, Dillon P J, Skalka A M, Rosen C A. Identification and characterization of intragenic sequences which repress human immunodeficiency virus structural gene expression. J Virol. 1991;65:5305–5313. doi: 10.1128/jvi.65.10.5305-5313.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coney L, Wang B, Ugen K E, Boyer J, McCallus D, Srikantan V, Agadjanyan M, Pachuk C J, Herold K, Merva M, et al. Facilitated DNA inoculation induces anti-HIV-1 immunity in vivo. Vaccine. 1994;12:1545–1550. doi: 10.1016/0264-410x(94)90082-5. [DOI] [PubMed] [Google Scholar]
- 13.Daniel M C, Kirchhoff F, Czajak S C, Sehgal P K, Desrosiers R C. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science. 1992;258:1938–1941. doi: 10.1126/science.1470917. [DOI] [PubMed] [Google Scholar]
- 14.Deng, C., and M. R. Capecchi. 1992. Reexamination of gene targeting frequency as a function of the extent of homology between the targeting vector and the target locus. Mol. Cell. Biol. 3365–3371. [DOI] [PMC free article] [PubMed]
- 15.Donnelly J J, Friedman A, Martinez D, Montgomery D L, Shiver J W, Motzel S L, Ulmer J B, Liu M A. Preclinical efficacy of a prototype DNA vaccine: enhanced protection against antigenic drift in influenza virus. Nat Med. 1995;1:583–587. doi: 10.1038/nm0695-583. [DOI] [PubMed] [Google Scholar]
- 16.Donnelly J J, Martinez D, Jansen K U, Ellis R W, Montgomery D K, Liu M A. Protection against papillomavirus with a polynucleotide vaccine. J Infect Dis. 1996;713:314–320. doi: 10.1093/infdis/173.2.314. [DOI] [PubMed] [Google Scholar]
- 17.Doolan D L, Sedehah M, Hedstrom R C, Hobart P, Charoenvit Y, Hoffman S L. Circumventing genetic restriction of protection against malaria with multigene DNA immunization: CD8+ T cell-, interferon gamma-, and nitric oxide-dependent immunity. J Exp Med. 1996;183:1739–1746. doi: 10.1084/jem.183.4.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Emerman M, Vazeux R, Peden K. The rev gene product of the human immunodeficiency virus affects envelope-specific RNA localization. Cell. 1989;57:1155–1165. doi: 10.1016/0092-8674(89)90053-6. [DOI] [PubMed] [Google Scholar]
- 19.Felber B, Hadzopoulou-Cladaras M, Cladaras C, Copeland T, Pavlakis G N. Rev protein of human immunodeficiency virus type 1 affects the stability and transport of the viral mRNA. Proc Natl Acad Sci USA. 1989;86:1495–1499. doi: 10.1073/pnas.86.5.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischer U, Meyer S, Teufel C, Lührmann R, Rautmann G. Evidence that HIV-1 rev directly promotes the nuclear export of unspliced RNA. EMBO J. 1994;13:4105–4112. doi: 10.1002/j.1460-2075.1994.tb06728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fleckenstein B, Daniel M D, Hunt R D, Werner R D, Falk L A, Mulder C. Tumour induction with DNA of oncogenic primate herpesviruses. Nature. 1978;274:57–59. doi: 10.1038/274057a0. [DOI] [PubMed] [Google Scholar]
- 22.Fuller D H, Haynes J R. A qualitative progression in HIV type 1 glycoprotein 120-specific cytotoxic cellular and humoral immune responses in mice receiving a DNA-based glycoprotein vaccine. AIDS Res Hum Retroviruses. 1994;10:1433–1441. doi: 10.1089/aid.1994.10.1433. [DOI] [PubMed] [Google Scholar]
- 23.Fynan E F, Webster R G, Fuller D H, Haynes J R, Santoro J C, Robinson H L. DNA vaccines: protective immunizations by parenteral, mucosal and gene-gun inoculations. Proc Natl Acad Sci USA. 1993;90:11478–11482. doi: 10.1073/pnas.90.24.11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goeddert J J, Mendez H, Drummond J E, Robert-Guroff M, Minkoff H L, Holman S, Stevens R, Rubinstein A, Blattner W A, Willoughby A, et al. Mother-to-infant transmission of human immunodeficiency virus type 1: association with prematurity or low anti-gp120. Lancet. 1989;ii:1351–1354. doi: 10.1016/s0140-6736(89)91965-x. [DOI] [PubMed] [Google Scholar]
- 25.Haas J, Park E-C, Seed B. Codon usage limitation in the expression of HIV-1 envelope. Curr Biol. 1996;6:315–324. doi: 10.1016/s0960-9822(02)00482-7. [DOI] [PubMed] [Google Scholar]
- 26.Haynes B F, Pantaleo G, Fauci A S. Toward an understanding of the correlates of protective immunity to HIV infection. Science. 1996;271:324–328. doi: 10.1126/science.271.5247.324. [DOI] [PubMed] [Google Scholar]
- 27.Hengge U, Chan E F, Foster R A, Walker P S, Vogel J C. Cytokine gene expression in epidermis with biological effects following injection of naked DNA. Nat Genet. 1995;10:161–166. doi: 10.1038/ng0695-161. [DOI] [PubMed] [Google Scholar]
- 28.Huygen K, Content J, Denis O, Montgomery D L, Yawman A M, Deck R R, DeWitt C M, Orme I M, Baldwin S, D’Souza C, Drowart A, Lozes E, Vandenbussche P, Van Vooren J P, Liu M A, Ulmer J B. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat Med. 1996;2:893–898. doi: 10.1038/nm0896-893. [DOI] [PubMed] [Google Scholar]
- 29.Irvine K R, Rao J B, Rosenberg S A, Restifo N P. Cytokine enhancement of DNA immunization leads to effective treatment of established pulmonary metastases. J Immunol. 1996;156:238–245. [PMC free article] [PubMed] [Google Scholar]
- 30.Israel M A, Chan H W, Hourihan S L, Rowe W P, Martin M A. Biological activity of polyomavirus DNA in mice and hamsters. J Virol. 1979;29:990–996. doi: 10.1128/jvi.29.3.990-996.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim J J, Ayyavoo V, Bagarazzi M L, Chattergoon M A, Dang K, Wang B, Boyer J D, Weiner D B. In vivo engineering of a cellular immune response by coadministration of IL-12 expression vector with a DNA immunogen. J Immunol. 1997;158:816–826. [PubMed] [Google Scholar]
- 32.Klinman D M, Yamshchikov G, Ishigatsubo Y. Contribution of CpG motifs to the immunogenicity of DNA vaccines. J Immunol. 1997;158:3635–3639. [PubMed] [Google Scholar]
- 33.Krieg A, Yi A-K, Matson S, Waldschmidt T J, Bishop G A, Teasdale R, Koretzky G A, Klinman D M. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 34.Lu S, Arthos J, Montefiori D C, Yasutomi Y, Manson K, Mustafa F, Johnson E, Santoro J C, Wissink J, Mullins J I, Haynes J R, Letvin N L, Wyand M, Robinson H L. Simian immunodeficiency virus DNA vaccine trial in macaques. J Virol. 1996;70:3978–3991. doi: 10.1128/jvi.70.6.3978-3991.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu S, Santoro J C, Fuller D H, Haynes J R, Robinson H L. Use of DNAs expressing HIV-1 env and noninfectious HIV-1 particles to raise antibody responses in mice. Virology. 1995;209:147–154. doi: 10.1006/viro.1995.1238. [DOI] [PubMed] [Google Scholar]
- 36.Malim M H, Hauber J, Le S-Y, Maizel J V, Cullen B R. The HIV-1 rev trans-activator acts through a structural target sequence to activate nuclear export of unspliced viral mRNA. Nature. 1989;338:254–257. doi: 10.1038/338254a0. [DOI] [PubMed] [Google Scholar]
- 37.Manickan E, Rouse R J D, Yu Z, Wire W S, Rouse B T. Genetic immunization against herpes simplex virus. J Immunol. 1995;155:259–265. [PubMed] [Google Scholar]
- 38.Manthorpe M, Cornefert-Jensen F, Hartikka J, Felgner J, Rundell A, Margalith M, Dwarki V. Gene therapy by intramuscular injection of plasmid DNA: studies on firefly luciferase gene expression in mice. Hum Gene Ther. 1993;4:419–431. doi: 10.1089/hum.1993.4.4-419. [DOI] [PubMed] [Google Scholar]
- 39.Montgomery D L, Shiver J W, Leander K R, Perry H C, Friedman A, Martinez D, Ulmer J B, Donnelly J J, Liu M A. Heterologous and homologous protection against influenza A by DNA vaccination: optimization of DNA vectors. DNA Cell Biol. 1993;12:785–789. doi: 10.1089/dna.1993.12.777. [DOI] [PubMed] [Google Scholar]
- 40.Nasioulas G, Zolotukhin A S, Tabernero C, Solomin L, Cunningham C P, Pavlakis G N, Felber B K. Elements distinct from human immunodeficiency virus type 1 splice sites are responsible for the rev dependence of env mRNA. J Virol. 1994;68:2986–2993. doi: 10.1128/jvi.68.5.2986-2993.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Negritto M T, Wu X, Kuo T, Chu S, Bailis A M. Influence of DNA sequence identity on efficiency of targeted gene replacement. Mol Cell Biol. 1997;17:278–286. doi: 10.1128/mcb.17.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okuda K, Bukawa H, Hamajima K, Kawamoto S, Sekigawa K, Yamada Y, Tanaka S, Ishi N, Aoki I, Nakamura M. Induction of potent humoral and cell-mediated immune responses following direct injection of DNA encoding the HIV type 1 env and rev gene products. AIDS Res Hum Retroviruses. 1995;11:933–943. doi: 10.1089/aid.1995.11.933. [DOI] [PubMed] [Google Scholar]
- 43.Pantaleo G, Menzo S, Vaccarezza M, Graziosi C, Cohen O J, Demarest J F, Montefiori D, Orenstein J M, Fox C, Schrager L, Margolick J B, Buchbinder S, Biorgi J V, Fauci A S. Studies in subjects with long-term nonprogressive human immunodeficiency virus infection. N Engl J Med. 1995;332:209–216. doi: 10.1056/NEJM199501263320402. [DOI] [PubMed] [Google Scholar]
- 44.Phillpotts R J, Venugopal K, Brooks T. Immunisation with DNA polynucleotides protects mice against lethal challenge with St. Louis encephalitis virus. Arch Virol. 1996;141:743–749. doi: 10.1007/BF01718332. [DOI] [PubMed] [Google Scholar]
- 45.Pisetsky D S, Reich C, Crowley S D, Halpern M D. Immunological properties of bacterial DNA. Ann N Y Acad Sci. 1997;772:152–163. doi: 10.1111/j.1749-6632.1995.tb44740.x. [DOI] [PubMed] [Google Scholar]
- 46.Robinson H L, Hunt L A, Webster R G. Protection against a lethal influenza virus challenge by immunization with a haemagglutinin-expressing plasmid DNA. Vaccine. 1993;11:957–960. doi: 10.1016/0264-410x(93)90385-b. [DOI] [PubMed] [Google Scholar]
- 47.Rosen C A, Terwilliger E, Dayton A, Sodrowski J G, Haseltine W A. Intragenic cis-acting art gene-responsive sequences of the human immunodeficiency virus. Proc Natl Acad Sci USA. 1988;85:2071–2075. doi: 10.1073/pnas.85.7.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rossi P, Moschese V, Broliden P A, Fundaro C, Quinti I, Plebani A, Giaquinto C, Tovo P A, Ljunggren K, Rosen J, et al. Presence of maternal antibodies to human immunodeficiency virus 1 envelope glycoprotein gp120 epitopes correlates with the uninfected status of children born to seropositive mothers. Proc Natl Acad Sci USA. 1989;86:8055–8058. doi: 10.1073/pnas.86.20.8055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rowland-Jones S, Nixon D, Aldhous M. HIV-specific cytotoxic T-cell activity in an HIV-exposed but uninfected infant. Lancet. 1993;341:860–861. doi: 10.1016/0140-6736(93)93063-7. [DOI] [PubMed] [Google Scholar]
- 50.Rowland-Jones S, Sutton J, Ariyoshi K, Dong K, Dong T, Gotch F, McAdam S, Whitby D, Sabally S, Gallimore A, Corrah T, Takiguchi M, Schultz T, McMichael A, Whittle H. HIV-specific cytotoxic T-cells in HIV-exposed but uninfected Gambian women. Nat Med. 1995;1:59–64. doi: 10.1038/nm0195-59. [DOI] [PubMed] [Google Scholar]
- 51.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 52.Schirmbeck R, Böhm W, Ando K, Chisari F V, Reimann J. Nucleic acid vaccination primes hepatitis B virus surface antigen-specific cytotoxic T lymphocytes in nonresponder mice. J Virol. 1995;69:5929–5934. doi: 10.1128/jvi.69.10.5929-5934.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schwartz S, Felber B K, Pavlakis G N. Distinct RNA sequences in the gag region of human immunodeficiency virus type 1 decrease RNA stability and inhibit expression in the absence of Rev protein. J Virol. 1992;66:150–159. doi: 10.1128/jvi.66.1.150-159.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sedegah M, Hedstrom R, Hobart P, Hoffman S L. Protection against malaria by immunization with circumsporozoite protein plasmid DNA. Proc Natl Acad Sci USA. 1994;91:9866–9870. doi: 10.1073/pnas.91.21.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seed B, Aruffo A. Molecular cloning of the CD2 antigen, the T-cell erythrocyte receptor, by a rapid immunoselection procedure. Proc Natl Acad Sci USA. 1987;84:3365–3369. doi: 10.1073/pnas.84.10.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shafferman A, Jahrling P B, Benveniste R E, Lewis M G, Phipps T J, Eden-McCutchan F, Sadoff J, Eddy G A, Burke D S. Protection of macaques with a simian immunodeficiency virus envelope peptide vaccine based on conserved human immunodeficiency virus type 1 sequences. Proc Natl Acad Sci USA. 1991;88:7126–7130. doi: 10.1073/pnas.88.16.7126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smolik-Utlaut S, Petes T D. Recombination of plasmids into the Saccharomyces cerevisiae chromosomes is reduced by small amounts of sequence heterogeneity. Mol Cell Biol. 1983;3:1204–1211. doi: 10.1128/mcb.3.7.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tang D-C, DeVit M, Johnston S A. Genetic immunization is a simple method for eliciting an immune response. Nature. 1992;356:152–154. doi: 10.1038/356152a0. [DOI] [PubMed] [Google Scholar]
- 59.Tascon R E, Colston M J, Ragno S, Stavropoulos E, Gregory D, Lowrie D B. Vaccination against tuberculosis by DNA injection. Nat Med. 1996;2:888–892. doi: 10.1038/nm0896-888. [DOI] [PubMed] [Google Scholar]
- 60.te Riele H, Maandag R, Berns A. Highly efficient gene targeting in embryonic stem cells through homologous recombination with isogenic DNA constructs. Proc Natl Acad Sci USA. 1992;89:5128–5132. doi: 10.1073/pnas.89.11.5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ulmer J B, Donnelly J J, Suezanne E P, Rhodes G H, Felgner P L, Dwarki V J, Gromkowski S H, Deck R R, DeWitt C M, Friedman A, Hawe L A, Leander K R, Martinez D, Perry H C, Shiver J W, Montgomery D L, Liu M A. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 62.Wagener S, Norley S, zur Megede J, Kurth R, Cichutek K. Induction of antibodies against SIV antigens after intramuscular nucleic acid inoculation using complex expression constructs. J Biotechnol. 1996;44:59–65. doi: 10.1016/0168-1656(95)00093-3. [DOI] [PubMed] [Google Scholar]
- 63.Waldman A S, Liskay R M. Dependence of intrachromosomal recombination in mammalian cells on uninterrupted homology. Mol Cell Biol. 1988;8:5350–5357. doi: 10.1128/mcb.8.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang B, Boyer J, Srikantan V, Ugen K, Gilbert L, Phan C, Dang K, Merva M, Agadjanyan M G, Newman M, et al. Induction of humoral and cellular immune responses to the human immunodeficiency type 1 virus in nonhuman primates by in vivo DNA inoculation. Virology. 1995;211:102–112. doi: 10.1006/viro.1995.1383. [DOI] [PubMed] [Google Scholar]
- 65.Wang B, Ugen K E, Srikantan V, Agadjanyan M G, Dang K, Refaeli Y, Sato A I, Boyer J, Williams W V, Weiner D B. Gene inoculation generates immune responses against human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1993;90:4156–4160. doi: 10.1073/pnas.90.9.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Will H, Cattaneo R, Koch H-G, Darai G, Schaller H, Schellekens H, vanEerd P M C A, Deinhardt F. Cloned HBV DNA causes hepatitis in chimpanzees. Nature. 1982;299:740–742. doi: 10.1038/299740a0. [DOI] [PubMed] [Google Scholar]
- 67.Wolff J A, Malone R W, Williams P, Chong W, Acsadi G, Jani A, Felgner P L. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- 68.Xiang Z Q, Spitalnik S, Tran M, Wunner W, Cheng J, Ertl H C J. Vaccination with a plasmid vector carrying the rabies virus glycoprotein gene induces protective immunity against rabies virus. Virology. 1994;199:132–140. doi: 10.1006/viro.1994.1105. [DOI] [PubMed] [Google Scholar]
- 69.Xu D, Liew F Y. Protection against leishmaniasis by injection of DNA encoding a major surface glycoprotein, gp63, of L. major. Immunology. 1995;84:173–176. [PMC free article] [PubMed] [Google Scholar]
- 70.Yamamoto S, Yamamoto T, Shimada S, Kuramoto W, Yano O, Kataoka T, Tokunaga T. DNA from bacteria, but not vertebrates, induces interferons, activates NK cells and inhibits tumor growth. Microbiol Immunol. 1992;36:983. doi: 10.1111/j.1348-0421.1992.tb02102.x. [DOI] [PubMed] [Google Scholar]
- 71.Yasutomi Y, Robinson H L, Lu S, Mustafa F, Lekutis C, Arthos J, Mullins J I, Voss G, Manson K, Wyand M, Letvin N L. Simian immunodeficiency virus-specific cytotoxic T-lymphocyte induction through DNA vaccination of rhesus monkeys. J Virol. 1996;70:678–681. doi: 10.1128/jvi.70.1.678-681.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yokoyama M, Zhang J, Whitton J L. DNA immunization confers protection against lethal lymphocytic choriomenigitis virus infection. J Virol. 1995;69:2684–2688. doi: 10.1128/jvi.69.4.2684-2688.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ziang Z, Ertl H C. Manipulation of the immune response to a plasmid-encoded viral antigen by coinoculation with plasmids expressing cytokines. Immunity. 1995;2:129–135. doi: 10.1016/s1074-7613(95)80001-8. [DOI] [PubMed] [Google Scholar]