Abstract
Although the adeno-associated virus type 2 (AAV)-based vector system has gained attention as a potentially useful alternative to the more commonly used retroviral and adenoviral vectors for human gene therapy, the single-stranded nature of the viral genome, and consequently the rate-limiting second-strand viral DNA synthesis, significantly affect its transduction efficiency. We have identified a cellular tyrosine phosphoprotein, designated the single-stranded D sequence-binding protein (ssD-BP), which interacts specifically with the D sequence at the 3′ end of the AAV genome and may prevent viral second-strand DNA synthesis in HeLa cells (K. Y. Qing et al., Proc. Natl. Acad. Sci. USA 94:10879–10884, 1997). In the present studies, we examined whether the phosphorylation state of the ssD-BP correlates with the ability of AAV to transduce various established and primary cells in vitro and murine tissues in vivo. The efficiencies of transduction of established human cells by a recombinant AAV vector containing the β-galactosidase reporter gene were 293 > KB > HeLa, which did not correlate with the levels of AAV infectivity. However, the amounts of dephosphorylated ssD-BP which interacted with the minus-strand D probe were also as follows: 293 > KB > HeLa. Predominantly the phosphorylated form of the ssD-BP was detected in cells of the K562 line, a human erythroleukemia cell line, and in CD34+ primary human hematopoietic progenitor cells; consequently, the efficiencies of AAV-mediated transgene expression were significantly lower in these cells. Murine Sca-1+ lin− primary hematopoietic stem/progenitor cells contained predominantly the dephosphorylated form of the ssD-BP, and these cells could be efficiently transduced by AAV vectors. Dephosphorylation of the ssD-BP also correlated with expression of the adenovirus E4orf6 protein, known to induce AAV gene expression. A deletion mutation in the E4orf6 gene resulted in a failure to catalyze dephosphorylation of the ssD-BP. Extracts prepared from mouse brain, heart, liver, lung, and skeletal-muscle tissues, all of which are known to be highly permissive for AAV-mediated transgene expression, contained predominantly the dephosphorylated form of the ssD-BP. Thus, the efficiency of transduction by AAV vectors correlates well with the extent of the dephosphorylation state of the ssD-BP in vitro as well as in vivo. These data suggest that further studies on the cellular gene that encodes the ssD-BP may promote the successful use of AAV vectors in human gene therapy.
Adeno-associated virus type 2 (AAV), a nonpathogenic human parvovirus, requires coinfection with a helper virus, such as adenovirus (Ad), for optimal replication and lytic growth (3, 4). In the absence of a helper virus, the wild-type (wt) AAV genome has been shown to integrate into human chromosome 19q13.3-qter to establish a latent infection (17, 18, 38). AAV can infect a wide variety of cell types, including differentiated and nondividing primary cells (4, 6, 24). Its nonpathogenic nature, coupled with its broad host range, has motivated researchers to develop a recombinant AAV-based vector system for its potential use in human gene therapy (4, 24). To date, a number of studies have reported AAV-mediated transduction and expression of genes of interest in vitro as well as in vivo (5–10, 12–16, 29–32, 39, 41–43, 45). However, because AAV is a single-stranded-DNA-containing virus, a major obstacle in AAV-mediated high-efficiency transduction has been the conversion of the single-stranded viral genome to a transcriptionally active double-stranded intermediate (7, 8). Although a variety of biological, chemical, and physical events, such as coinfection of target cells with Ad, expression of the Ad E4orf6 protein (7, 8), treatment with hydroxyurea (HU) or etoposide (35), or UV or X-ray irradiation (2, 7), can greatly enhance the efficiency of AAV-mediated transduction, presumably by catalyzing the genomic conversion, the precise mechanism(s) by which these agents facilitate viral second-strand DNA synthesis still remains unknown (33).
Recent studies by members of our laboratory have documented the existence of a host cell protein, which we have designated the single-stranded D sequence-binding protein (ssD-BP), which interacts specifically and preferentially with the D sequence at the 3′ end of the AAV genome (33). The ssD-BP is phosphorylated at tyrosine residues, and we have hypothesized that the phosphorylated form of the ssD-BP prevents viral second-strand DNA synthesis and subsequently blocks AAV-mediated transgene expression (33). This hypothesis is supported by the fact that inhibition of cellular protein tyrosine kinases by genistein, a potent inhibitor of protein tyrosine kinases (1), results in dephosphorylation of the ssD-BP, leading not only to enhancement of the transgene expression of recombinant AAV but also to autonomous replication of the wt AAV genome (33). It appears, therefore, that the host cell ssD-BP plays a pivotal role in the life cycle of AAV. However, all of the above-mentioned experiments were carried out with cells of the HeLa line, an established human cervical carcinoma cell line. It remains unclear what role the ssD-BP plays in other human cell types, both established and primary, in AAV-mediated transduction in vitro. Furthermore, since members of our laboratory (28–31, 41) and others (8–10, 12, 13, 15, 16, 22, 39, 45) have observed wide variations in AAV-mediated transduction of different cell types in vivo as well, it remains to be determined whether these variations are the consequence of the involvement of the ssD-BP. In the present studies, we have carried out systematic analyses of the ssD-BP–D sequence interactions in several different human and murine cell types (both established and primary cells) in vitro and in murine tissues in vivo.
Levels of dephosphorylation of the ssD-BP correlate with the efficiencies of AAV-mediated transduction in established and primary cells in vitro.
In order to investigate the efficiency of AAV-mediated transduction in different cell types, HeLa (a human cervical carcinoma cell line), KB (a human nasopharyngeal carcinoma cell line), and 293 (an Ad-transformed human embryonic kidney cell line) cells were infected with the recombinant AAV vector vCMVp-lacZ, containing the β-galactosidase gene under the control of the cytomegalovirus immediate-early promoter. High titers (∼1011 to 1012 particles/ml) of the recombinant vector were generated with the recombinant AAV helper plasmid pAAV/Ad (37) and the recombinant AAV vector construct pCMVp-lacZ and purified on CsCl equilibrium density gradients as previously described (19, 27–32, 40, 43). Recombinant AAV vectors were used to infect cells at a multiplicity of infection (MOI) of 20 (∼104 particles/cell) under identical conditions. Briefly, cells were incubated with the virus in a volume of 100 μl of serum-free Iscove modified Dulbecco medium for 2 h at 37°C. Forty-eight hours postinfection (p.i.), expression of the transduced lacZ gene was analyzed by 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) staining and enumeration of blue cells under a Nikon inverted light microscope as previously described (29, 30). The results are shown in Fig. 1. It was determined that whereas the recombinant-AAV transduction efficiencies were approximately 2% for HeLa cells (Fig. 1A) and approximately 11% for KB cells (Fig. 1B), the transduction efficiency was significantly higher, approximately 52%, for 293 cells (Fig. 1C). Although all three cell lines are permissive for AAV infection, it remained possible that the observed differences were due to differential susceptibilities of these cells to the virus, presumably because the putative membrane receptors for AAV in 293 cells far outnumber those in HeLa and KB cells. This possibility was ruled out by performing virus-binding assays as follows. 35S-labeled AAV particles were used in binding assays with intact HeLa, KB, and 293 cells. Tran35S-label (specific activity, 1,206 Ci/mmol; ICN Pharmaceuticals Inc., Irvine, Calif.) was used to generate radiolabeled AAV as previously described (19). Briefly, 293 cells were coinfected with wt AAV (200 particles/cell) and Ad type 2 (10 PFU/cell) and maintained in the presence of the radiolabel (35 μCi/ml) in cysteine- and methionine-free Eagle’s minimum essential medium supplemented with 10% fetal bovine serum and 1% l-glutamine. Radiolabeled AAV particles were also purified on CsCl equilibrium density gradients as described above. AAV-binding experiments with HeLa, KB, and 293 cells were carried out as described by Mizukami et al. (22), with the following modifications. Briefly, [35S]methionine-labeled wt AAV (108 particles) was mixed with 105 cells in triplicate in serum-free Iscove modified Dulbecco medium containing 1% bovine serum albumin and incubated at 4°C for 1 h. Mock-infected cells were also included in each assay. Cells of the M07e line, a human megakaryocytic leukemia cell line known to be resistant to AAV infection (30), were used as a negative control under identical conditions. Cells were washed three times with 1× phosphate-buffered saline and resuspended in scintillation fluid, and the bound radioactive counts were determined with a Beckman LS3801 liquid scintillation counter. In competition experiments, a 30-fold excess of unlabeled wt AAV particles was added along with the 35S-labeled virus, and binding assays were performed as described above. The results are shown in Fig. 2. It is evident that binding of radiolabeled AAV to KB cells was the highest, followed by that to HeLa cells and that to 293 cells. No binding to M07e cells occurred, as expected (28). Virus binding was specific, since a 30-fold excess of unlabeled AAV could significantly compete for binding with the radiolabeled virus (data not shown). Thus, differential susceptibilities to infection could not account for the observed differences in the efficiency of AAV transduction of these cell types. We hypothesized, therefore, that the observed differences in efficiency of recombinant-AAV transduction of these cell lines might be due to functional differences in the host cell ssD-BP (33).
FIG. 1.

Comparative analyses of transduction efficiencies of vCMVp-lacZ in established human cell lines. Approximately equivalent numbers of HeLa (A), KB (B), and 293 (C) cells were infected with vCMVp-lacZ at an MOI of 20 under identical conditions. Forty-eight hours p.i., cells were fixed and stained with X-Gal, and blue cells were enumerated as described in the text. Magnification, ×100.
FIG. 2.
Analysis of binding of AAV to HeLa, KB, and 293 cells. Equivalent numbers of cells from each cell line, along with a negative (M07e) control, were analyzed in virus-binding assays with 35S-radiolabeled AAV as described in the text.
To test this hypothesis, whole-cell extracts (WCE) were prepared from HeLa, KB, and 293 cells under identical conditions according to the method described by Muller (23) and used in electrophoretic mobility shift assays (EMSA) with the minus-strand D sequence [D(−)] probe, as described previously (33, 43, 44). Briefly, DNA-binding reactions were performed in a volume of 20 μl with 2 μg of poly(dI-dC), 2 μg of bovine serum albumin, and 12% glycerol in HEPES buffer (pH 7.9). Ten micrograms of protein from each WCE was preincubated for 10 min at 25°C followed by the addition of 10,000 cpm of 32P-labeled D(−) sequence synthetic oligonucleotide (5′-AGGAACCCCTAGTGATGGAG-3′) in the reaction mixture. In some experiments, EMSA were also carried out with 32P-labeled double-stranded D sequence [D(±)] oligonucleotide which was prepared by annealing the complementary D(−) and D(+) sequences (5′-CTCCATCACTAGGGGTTCCT-3′) followed by purification on 10% polyacrylamide gels as previously described (43). The binding reaction was allowed to proceed for 30 min at 25°C, and the bound complexes were separated from the unbound probe on low-ionic-strength 4% polyacrylamide gels with recirculating Tris-acetate-EDTA (TAE) buffer (pH 7.9) containing 6.72 mM Tris-HCl, 3.3 mM sodium acetate, and 1 mM EDTA as previously described (33, 43, 44). Gels were dried in vacuo and autoradiographed at −70°C. The results are shown in Fig. 3. It is interesting that whereas KB cells (Fig. 3, lane 3) contained both the phosphorylated and the dephosphorylated forms of the ssD-BP, HeLa cells contained predominantly the phosphorylated form (Fig. 3, lane 2). 293 cells, on the other hand, contained predominantly the dephosphorylated form of the ssD-BP (Fig. 3, lane 4). Repeat analyses of these assays consistently yielded similar results, although some autophosphorylation and autodephosphorylation of the ssD-BP were observed in cryopreserved WCE. The ratios of the dephosphorylated form to the phosphorylated form of the ssD-BP in HeLa, KB, and 293 cells, determined by densitometric scanning of multiple autoradiograms with a Digital Imaging System Alphaimager (Alpha Innotech Co., San Leandro, Calif.), are indicated in Table 1. These data strongly suggest that the extent of AAV-mediated transgene expression correlates with the amount of dephosphorylated cellular ssD-BP. In addition, K562 cells, derived from a human erythroleukemia cell line, contained predominantly the phosphorylated form of the ssD-BP (Table 1), which explains why the efficiency of transgene expression by the same recombinant AAV has previously been shown to be significantly lower in these cells (30, 41). Thus, it appears that the more dephosphorylated ssD-BP present in the host cell, the greater the efficiency of AAV transduction.
FIG. 3.

EMSA with WCE prepared from human HeLa, KB, and 293 cells. Equivalent amounts of WCE prepared from each cell type were used in EMSA with the D(−) probe as described in the text. The phosphorylated and dephosphorylated forms of the ssD-BP are indicated by the arrow and the arrowhead, respectively.
TABLE 1.
Relative amounts of phosphorylated and dephosphorylated ssD-BP in human and murine cells
| Cell type | Ratio of the dephosphorylated form to the phosphorylated form of the ssD-BPa |
|---|---|
| Established cell lines | |
| HeLa | 0.3 ± 0.1 |
| KB | 0.9 ± 0.4 |
| 293 | 2.4 ± 1.1 |
| K562 | 0.3 ± 0.1 |
| Primary cells | |
| Human CD34+ | 0.1 |
| Mouse Sca-1+lin− | 8.0 |
These ratios (means ± standard errors of the means) were derived from densitometric scanning of autoradiograms as described in the text. Data for established cell lines are from three separate experiments with three different preparations of WCE. Data for primary cells are from one experiment each, due to availability of limited numbers of these cells.
We next investigated whether the cellular protein that interacts with the D(±) probe (dsD-BP) (43) showed a pattern similar to that of the D(−) probe in the three cell types. This investigation was carried out by EMSA with WCE prepared from the cells with D(−) and D(±) probes under identical conditions. The results, shown in Fig. 4A, indicate that although some autophosphorylation and autodephosphorylation occurred in cryopreserved WCE, a pattern of distribution of the ssD-BP in the three cell types roughly similar to that seen in Fig. 3 was obtained when the D(−) probe was used (Fig. 4A, lanes 2 to 4). However, the pattern of complex formation with the D(±) probe (Fig. 4A, lanes 6 to 8) did not appear to be significantly different in these cell types. Thus, all subsequent studies were performed with the D(−) probe. We also wished to examine whether alteration in the extent of phosphorylation of the ssD-BP in HeLa cells correlated with AAV-mediated transgene expression. This was carried out by using EMSA with WCE prepared from HeLa cells treated with either 150 μM genistein (a specific inhibitor of protein tyrosine kinases [1]) or 1 mM sodium orthovanadate (NaOV) (a specific inhibitor of protein phosphatases) for 2 h (33). The results, shown in Fig. 4B, once again indicate that treatment with genistein (ratio of dephosphorylated form to phosphorylated form of the ssD-BP = 1.6), but not with NaOV (ratio = 0.5), resulted in dephosphorylation of the ssD-BP that interacted with the D(−) probe. Under identical conditions, the dsD-BP that interacted with the D(±) probe remained unaffected (Fig. 4B). Interestingly, replicate HeLa cell cultures that were either mock treated or treated with genistein or NaOV, transduced with vCMVp-lacZ, and analyzed for transgene expression 48 h p.i. also indicated that, consistent with our recent observations (33), treatment with genistein, but not with NaOV, resulted in a significant increase in the efficiency of recombinant-AAV-mediated transduction (data not shown). Thus, it is clear that a strong correlation between the level of dephosphorylation of the cellular ssD-BP and AAV-mediated-transduction efficiency exists.
FIG. 4.
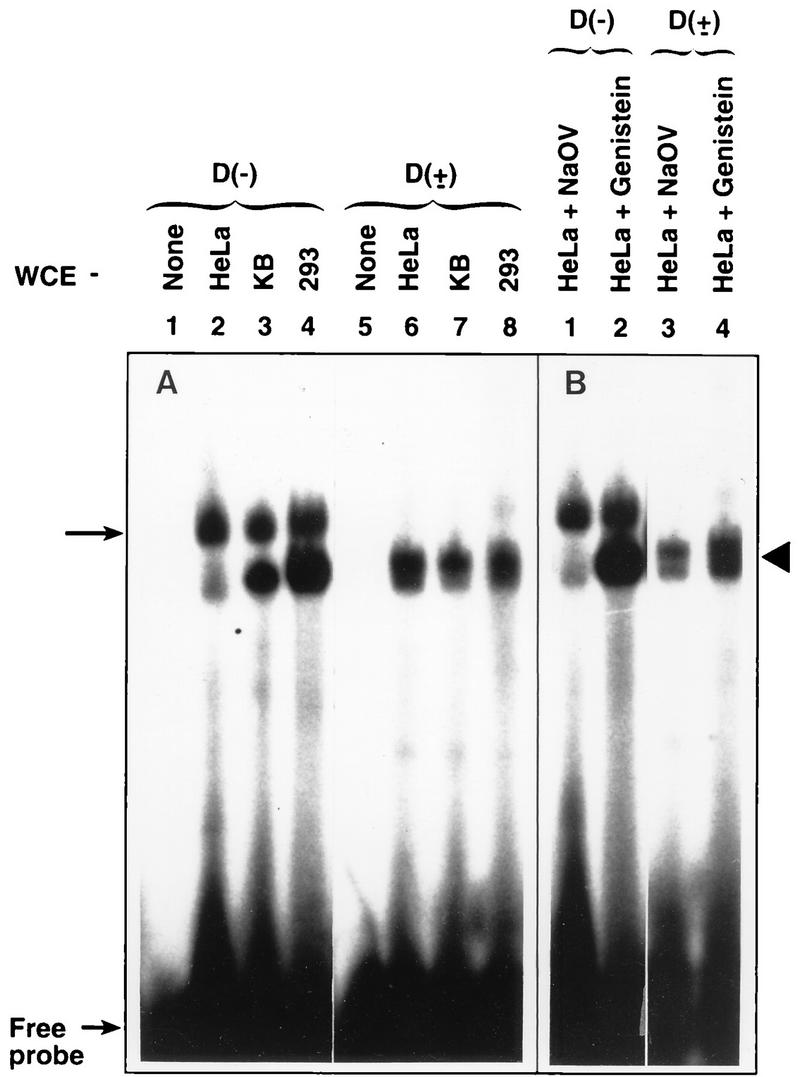
EMSA with the D(−) and D(±) probes and effect of treatment with NaOV and genistein on the ssD-BP and the dsD-BP. (A) WCE prepared from the indicated cell types were used in EMSA with the D(−) probe and the D(±) probe as described in the text. (B) HeLa cells were treated either with 1 mM NaOV or with 150 μM genistein, and equivalent amounts of WCE prepared from these cells were used in EMSA with the D(−) probe and the D(±) probe as described in the text.
These analyses were further extended to include human and murine primary hematopoietic stem/progenitor cells. Human CD34+ primitive hematopoietic stem/progenitor cells were isolated from low-density bone marrow cells obtained from hematologically normal volunteer donors as previously described (28). Murine Sca-1+ lin− primitive hematopoietic stem/progenitor cells were isolated from C57BL6/J mice as previously described (29). These protocols were approved by the Institutional Laboratory Animals and Human Subjects Committees, respectively, of Indiana University School of Medicine. Our recent studies have shown that in CD34+ cells from approximately 50% of the donors, the efficiency of AAV-mediated transduction ranges between 15 and 80% (28), and when a WCE prepared from such cells from one donor was examined by EMSA with the D(−) probe, predominantly the phosphorylated form of the ssD-BP was detected. In contrast, we have documented a transduction efficiency of murine Sca-1+ lin− hematopoietic stem/progenitor cells exceeding 95% by the same recombinant AAV vector (29, 31), and, consistent with our hypothesis, when EMSA were carried out under identical conditions, predominantly the dephosphorylated form of the ssD-BP was detected in WCE prepared from these cells. These results are summarized in Table 1. In these experiments, the autoradiograms were scanned densitometrically to determine the ratios of the dephosphorylated form to the phosphorylated form of the ssD-BP in each cell type. These data indicate that the same or similar ssD-BPs exist in human and in murine cells and that once again, the ability of recombinant AAV vectors to transduce different cell types strongly correlates with the extent of dephosphorylation of the ssD-BP that interacts with the D(−) probe.
Dephosphorylation of the ssD-BP also leads to increased stable integration and long-term expression of the AAV-borne transgene in human cells.
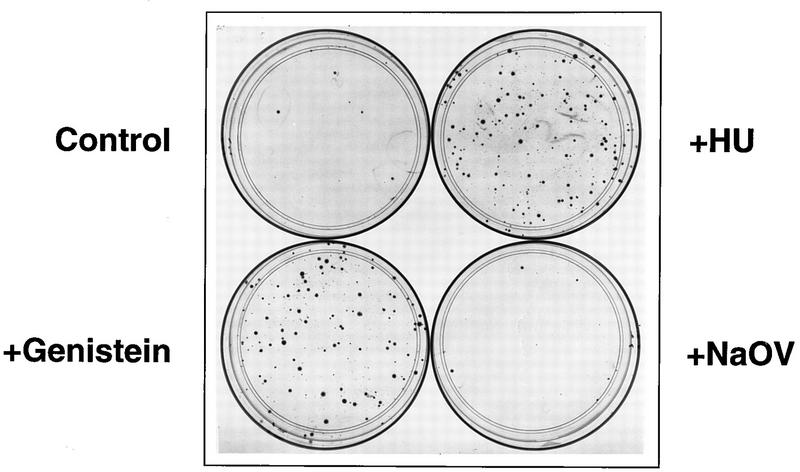
Although our previous studies have indicated that dephosphorylation of the ssD-BP leads to significant enhancement of the efficiency of transient transduction by recombinant AAV, we also wished to explore whether the efficiency of long-term expression, and whether stable integration in particular, of the AAV-borne transgene could be enhanced by simple dephosphorylation of the ssD-BP. HeLa cells were treated with genistein as previously described (33) and infected with a recombinant AAV vector, vTc-Neo (containing the herpesvirus thymidine kinase promoter-driven gene for resistance to neomycin [25]), at an MOI of 20. Mock-treated cells or cells treated with HU (known to increase AAV’s transduction efficiency) (7, 33, 35) or with NaOV were used as appropriate controls. Each treatment was carried out in duplicate. Forty-eight hours p.i., cells were exposed to the drug G418, a structural analog of neomycin, at a final active concentration of 400 μg/ml, and G418-resistant colonies were enumerated 14 days p.i. following staining with methylene blue as previously described (11). The results are shown in Fig. 5. The numbers of G418-resistant colonies in duplicate from each treatment were as follows: mock treatment, 7 and 6; NaOV treatment, 9 and 8; genistein treatment, 96 and 91; and HU treatment, 84 and 90. It is evident that treatment with HU or genistein increased the efficiency of stable transduction by the recombinant AAV to nearly the level attained during transient transduction (33). Southern blot analysis (40) of total genomic DNA isolated from each of the clones (36) following digestion with XbaI (no site in the proviral genome) or BamHI (one site in the proviral genome) by using the 32P-labeled DNA probe specific for the neo gene (27) indicated that the transduced neo gene was stably integrated into the chromosomal DNA of transduced cells (data not shown). The integration patterns of the proviral DNA were distinct in all of the clones examined. Using PCR assays with a neo- and chromosome 19-specific primer pair (27, 32, 38), we did not observe proviral genomic integration at the chromosome 19 target site previously characterized for the wt AAV genome (17, 18, 38) (data not shown). These results are consistent with previously published reports by members of our laboratory (27, 32) and others (14) which state that unlike the wt AAV genome, recombinant AAV genomes integrate randomly in human cells.
FIG. 5.
Transduction efficiency of recombinant vTc-Neo in HeLa cells following treatment with HU, genistein, or NaOV. Equivalent numbers of cells were infected with the recombinant vector at an MOI of 20 following either no treatment or treatment with the indicated compounds. Forty-eight hours p.i., G418 was added, and G418-resistant colonies were enumerated 14 days p.i. following staining with methylene blue as described in the text.
A deletion mutation in the Ad E4orf6 gene fails to augment AAV-mediated transgene expression or facilitate dephosphorylation of the ssD-BP.
Recent studies from two independent laboratories have shown that the Ad E4orf6 gene product catalyzes the synthesis of AAV second-strand DNA, leading to significant enhancement in AAV’s transduction efficiency (7, 8). In order to firmly establish the correlation between Ad E4orf6-mediated enhancement of transgene expression and dephosphorylation of the ssD-BP, the following approach was taken. Studies by members of our laboratory have demonstrated that expression of the Ad E4orf6 gene product is necessary and sufficient to cause dephosphorylation of the ssD-BP in HeLa cells (33). We hypothesized that a deletion mutation in this gene would not only fail to augment AAV-mediated transgene expression but also result in failure to catalyze dephosphorylation of the ssD-BP. To determine whether this was the case, HeLa cells were either mock transfected or transfected with plasmid pAdE4orf6 (containing the gene for the Ad E4orf6 protein) (7) or pKY-4 (containing a deletion mutation in the Ad E4orf6 gene), which was constructed by deletion of a 130-bp fragment between the EcoRV and BbsI sites within the E4orf6 gene followed by religation by standard techniques (36). Forty-eight hours posttransfection, cells were either mock infected or infected with vCMVp-lacZ at an MOI of 4 under identical conditions. Blue cells were enumerated as described above. Whereas the recombinant-AAV transduction efficiency in HeLa cells increased to approximately 18% following transfection with pAdE4orf6, compared with 2% in mock-transfected cells, the transduction efficiency following transfection with pKY-4 was only 3%, nearly the same as that of mock-transfected HeLa cells (data not shown). Since these results confirm that expression of the Ad E4orf6 protein, known to facilitate second-strand viral DNA synthesis in AAV-infected cells, increases AAV-mediated transgene expression, and that a deletion mutation in the Ad E4orf6 gene abolishes this function, we next examined whether this deletion mutation in the Ad E4orf6 gene also abrogated the ability of this gene product, either directly or indirectly, to dephosphorylate the ssD-BP. WCE prepared from replicate cultures of HeLa cells that were either mock transfected or transfected with plasmids pAdE4orf6 or pKY-4 as described above were used in EMSA with the D(−) probe. The results are presented in Fig. 6. As expected, whereas the ratio of the dephosphorylated form to the phosphorylated form of the ssD-BP was low in mock-transfected HeLa cells (Fig. 6, lane 2; ratio = 0.3), the level of dephosphorylation of the ssD-BP correlated well with expression of the Ad E4orf6 protein in these cells (Fig. 6, lane 3; ratio = 4.6). A deletion mutation in this gene indeed resulted in a failure to catalyze dephosphorylation of the ssD-BP (Fig. 6, lane 4; ratio = 0.5). Taken together, these data establish a strong correlation between AAV-mediated transgene expression and the level of phosphorylation of the cellular ssD-BP in vitro.
FIG. 6.

EMSA with WCE prepared from HeLa cells expressing the Ad E4orf6 protein. Equivalent amounts of WCE prepared from mock-transfected cells or cells transfected with plasmid pAdE4orf6 or plasmid pKY-4 were used in EMSA with the D(−) probe as described in the text. The phosphorylated and dephosphorylated forms of the ssD-BP are indicated by the arrow and the arrowhead, respectively.
The efficiency of transduction by recombinant AAV correlates with the extent of dephosphorylation of the ssD-BP in murine tissues in vivo.
Finally, we wished to evaluate whether a similar strong correlation between AAV’s transduction efficiency and the level of phosphorylation of the cellular ssD-BP exists in intact organs and tissues in vivo. This is of particular interest, since a number of investigators have identified specific organs or tissues, such as muscle (9, 15, 45), brain (12, 21), liver (16, 29, 39), lung (5, 10), and heart (13, 26), as exhibiting high-efficiency transduction by recombinant AAV vectors, although the precise mechanism underlying this phenomenon remains unknown. Since the ssD-BPs detected in WCE prepared from murine Sca-1+ lin− hematopoietic cells appeared to be indistinguishable from those detected in WCE prepared from human cells, we systematically analyzed various murine organs or tissues obtained from three animals each, prepared protein extracts, and used them in EMSA with the D(−) probe under conditions identical to those described for human and murine cells. The ratio of the dephosphorylated form to the phosphorylated form of the ssD-BP for each of the organs or tissues was determined from EMSA autoradiograms by densitometric scanning. The results are summarized in Table 2. Compared with results from EMSA carried out with WCE prepared from either human or murine cells, ssD-BPs, either in the phosphorylated or in the dephosphorylated form, or both, could be readily detected in most of the murine organs and tissues analyzed. However, the most striking observation was that the ratio of the dephosphorylated form to the phosphorylated form of the ssD-BP was highest in murine skeletal-muscle tissues, followed by the brain, lung, liver, and heart, and these organs and tissues have been shown, roughly in that order, to allow high-efficiency AAV-mediated transduction in vivo. Kidney tissues also showed a high ratio, indicating that they contain predominantly the dephosphorylated form of the ssD-BP; therefore, they would be expected to exhibit a reasonably high efficiency of AAV-mediated transduction. Other organs, such as the spleen and thymus, contain more of the phosphorylated form of the ssD-BP (data not shown), and thus far, it has been difficult to document AAV-mediated transgene expression in these tissues (29, 31). Thus, these results establish a strong correlation between AAV’s transduction efficiency and the level of dephosphorylation of the cellular ssD-BP in vivo.
TABLE 2.
Relative amounts of phosphorylated and dephosphorylated ssD-BP in murine tissues
| Tissue sourcea | Ratio of the dephosphorylated form to the phosphorylated form of the ssD-BPb |
|---|---|
| Brain | 4.0 ± 1.9 |
| Heart | 2.5 ± 1.5 |
| Kidney | 1.4 ± 0.1 |
| Liver | 2.7 ± 1.6 |
| Lung | 2.9 ± 1.4 |
| Muscle | 8.2 ± 1.5 |
For each tissue source, tissues from three C57BL6/J animals were obtained and pulverized separately with the MSK cell homogenizer according to the preparation protocol supplied by the vendor (B. Braun Biotech Inc., Allentown, Pa.) and analyzed separately in two independent experiments. Total protein concentrations for WCE were determined with the protein assay kit (Bio-Rad Laboratories, Hercules, Calif.), and the extracts were frozen in liquid N2 and stored at −70°C.
These ratios (means ± standard errors of the means) were derived from densitometric scanning of autoradiograms as described in the text.
AAV-based vectors have proven to be safe and effective vehicles for gene delivery in vitro, and these vectors are now being developed and tested in preclinical and clinical stages for a wide range of potential applications in gene therapy, both ex vivo and in vivo. However, members of our laboratory (28–31, 41, 46) and others (5–10, 12, 13, 15, 16, 22, 39, 42, 45) have repeatedly observed wide variations in AAV’s transduction efficiency in different cells and tissues in vitro as well as in vivo. It seems reasonable to suggest that AAV’s transduction efficiency correlates with the number of the putative cell surface receptor, although the identity of this receptor still remains elusive (22). However, it has become clear from our present studies that such a correlation most probably does not exist, since 293 cells, which express relatively low numbers of this putative receptor, are transduced very efficiently, an observation consistent with previously published reports (7, 8). Our data clearly demonstrate that dephosphorylation of the cellular ssD-BP correlates strongly with AAV’s transduction efficiency. Dephosphorylation of the ssD-BP facilitates second-strand synthesis of the AAV genome delivered to target cells as a single-stranded DNA molecule, suggesting that manipulation of the phosphorylation state of this protein may be exploitable as one of the strategies for significantly improving the transduction efficiencies of recombinant AAV vectors. A strong correlation between the level of phosphorylation of the ssD-BP and the efficiency of transduction by AAV in murine organs and tissues in vivo also lends credence to this approach and indicates that ssD-BPs may be evolutionarily conserved.
The initial success of AAV-mediated efficient gene transfer and expression in the lung has already led to clinical trials for the treatment of cystic fibrosis (5, 10). Similarly, the prospects for treatment of muscular dystrophy by AAV-mediated delivery of the dystrophin gene to skeletal muscles, of Parkinson’s disease by tyrosine hydroxylase gene delivery to the brain, of hemophilia B by factor IX gene delivery to the liver, and potentially of myocardial infarction by delivery of the vascular endothelial growth factor gene to the heart appear promising, since AAV-mediated transgene expression in these organs has recently been shown to be highly efficient (8–10, 12, 13, 16, 21, 26, 39, 45). Since the data presented here support the hypothesis that high efficiency of recombinant-AAV transduction in these organs or tissues is most likely due to the presence of the dephosphorylated form of the ssD-BP, such an approach might also be useful in determining the potential for transduction of untested tissues and organs, especially those of human origin, by AAV vectors. For example, based on our data shown in Table 2, it appears that the kidney might be an additional organ of choice for AAV-mediated transduction, since the ratio of the dephosphorylated form to the phosphorylated form of the ssD-BP in these tissues is approximately 1.4, an observation consistent with results obtained with 293 cells, derived from human embryonic kidney.
Although our own interests are focused on high-efficiency AAV-mediated gene therapy of human hemoglobinopathies (41), such studies must await a better characterization of the underlying molecular mechanism of tyrosine phosphorylation of the cellular ssD-BP, in view of the relatively low ratio of the dephosphorylated form to the phosphorylated form of this protein in primitive human hematopoietic stem/progenitor cells. In the interim, the search for additional specific compounds that mediate dephosphorylation of the ssD-BP is on (20), in hopes of augmenting the transduction efficiencies of recombinant AAV vectors in primary hematopoietic stem/progenitor cells to allow their successful use in gene therapy of specific hematological disorders, such as sickle-cell anemia and β-thalassemia (31, 42, 46).
The mechanism by which dephosphorylation of the ssD-BP facilitates second-strand viral DNA synthesis still remains unclear. One of the possibilities, that dephosphorylated ssD-BP itself possesses a DNA polymerase-like activity, is currently being tested. Alternatively, dephosphorylation of the ssD-BP might activate a cellular DNA polymerase(s) necessary for host cell DNA synthesis or a DNA repair pathway by which the second-strand viral DNA synthesis is accomplished. Although a detailed characterization of the ssD-BP is currently under way (34), our recent studies with highly purified preparations of the ssD-BP indicate that this protein undergoes autophosphorylation followed by autodephosphorylation (data not shown), the significance of which is not clear. Characterization of the ssD-BP and determination of the availability of the cellular gene that encodes it remain high priorities for gaining an insight into its role not only in the host cell but also in the AAV life cycle in general and AAV-mediated gene transfer in particular. The elucidation of these relationships will hopefully facilitate successful use of AAV vectors in human clinical trials.
Acknowledgments
We thank Richard J. Samulski for generously providing the plasmid pAdE4orf6 and David A. Williams for supplying human bone marrow cells. We also thank Kelly Hiatt for expert technical assistance.
This research was supported in part by Public Health Service grants (HL-48342, HL-53586, and DK-49218, Centers of Excellence in Molecular Hematology) from the National Institutes of Health and by a grant from the Phi Beta Psi Sorority. A.S. was supported by an Established Investigator Award from the American Heart Association.
REFERENCES
- 1.Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- 2.Alexander I E, Russell D W, Spence A M, Miller A D. Effects of gamma irradiation on the transduction of dividing and nondividing cells in brain and muscle of rats by recombinant adeno-associated virus vectors. Hum Gene Ther. 1996;7:841–850. doi: 10.1089/hum.1996.7.7-841. [DOI] [PubMed] [Google Scholar]
- 3.Berns K I, Bohenzky R A. Adeno-associated viruses: an update. Adv Virus Res. 1987;32:243–307. doi: 10.1016/s0065-3527(08)60479-0. [DOI] [PubMed] [Google Scholar]
- 4.Berns K I, Giraud C. Biology of adeno-associated virus. Curr Top Microbiol Immunol. 1996;218:1–23. doi: 10.1007/978-3-642-80207-2_1. [DOI] [PubMed] [Google Scholar]
- 5.Carter B J, Flotte T R. Development of adeno-associated virus vectors for gene therapy of cystic fibrosis. Curr Top Microbiol Immunol. 1996;218:119–144. doi: 10.1007/978-3-642-80207-2_8. [DOI] [PubMed] [Google Scholar]
- 6.Chatterjee S, Lu D, Podsakoff G, Wong K K., Jr Strategies for efficient gene transfer into hematopoietic cells: the use of adeno-associated virus vectors in gene therapy. Ann N Y Acad Sci. 1995;770:79–90. doi: 10.1111/j.1749-6632.1995.tb31045.x. [DOI] [PubMed] [Google Scholar]
- 7.Ferrari F K, Samulski T, Shenk T, Samulski R J. Second-strand synthesis is a rate-limiting step for efficient transduction by recombinant adeno-associated virus vectors. J Virol. 1996;70:3227–3234. doi: 10.1128/jvi.70.5.3227-3234.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisher K J, Gao G-P, Weitzman M D, DeMatteo R, Burda J F, Wilson J M. Transduction with recombinant adeno-associated virus for gene therapy is limited by leading-strand synthesis. J Virol. 1996;70:520–532. doi: 10.1128/jvi.70.1.520-532.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher K J, Jooss K, Alston J, Yang Y, Haecker S E, High K, Pathak R, Raper S E, Wilson J M. Recombinant adeno-associated virus for muscle directed gene therapy. Nat Med. 1997;3:306–312. doi: 10.1038/nm0397-306. [DOI] [PubMed] [Google Scholar]
- 10.Flotte T R, Afione S A, Conrad C, McGrath S A, Solow R, Oka H, Zeitlin P L, Guggino B, Carter B J. Stable in vivo expression of the cystic fibrosis transmembrane conductance regulator with an adeno-associated virus vector. Proc Natl Acad Sci USA. 1993;90:10613–10617. doi: 10.1073/pnas.90.22.10613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghiringhelli P D, Romanowski V. Quick methylene blue staining for visualizing virus plaques in titration experiments. BioTechniques. 1994;17:464–465. [PubMed] [Google Scholar]
- 12.Kaplitt M G, Leone P, Samulski R J, Xiao X, Pfaff D W, O’Malley K L, During M J. Long-term gene expression and phenotypic correction using adeno-associated virus vectors in the mammalian brain. Nat Genet. 1994;8:148–153. doi: 10.1038/ng1094-148. [DOI] [PubMed] [Google Scholar]
- 13.Kaplitt M G, Xiao X, Samulski R J, Li J, Ojamaa K, Klein I L, Makimura H, Kaplitt M J, Strumpf R K, Diethrich E B. Long-term gene transfer in porcine myocardium after coronary infusion of an adeno-associated virus vector. Ann Thorac Surg. 1996;62:1669–1676. doi: 10.1016/s0003-4975(96)00946-0. [DOI] [PubMed] [Google Scholar]
- 14.Kearns W G, Afione S A, Fulmer S B, Pang M G, Erikson D, Egan M, Landrum M J, Flotte T R, Cutting G R. Recombinant adeno-associated virus (AAV-CFTR) vectors do not integrate in a site-specific fashion in an immortalized epithelial cell line. Gene Ther. 1996;3:748–755. [PubMed] [Google Scholar]
- 15.Kessler P D, Podsakoff G M, Chen X, McQuiston S A, Colosi P C, Matelis L A, Kurtzman G J, Byrne B J. Gene delivery to skeletal muscle results in sustained expression and systemic delivery of a therapeutic protein. Proc Natl Acad Sci USA. 1996;93:14082–14087. doi: 10.1073/pnas.93.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koeberl D D, Alexander I E, Halbert C L, Russell D W, Miller A D. Persistent expression of human clotting factor IX from mouse liver after intravenous injection of adeno-associated virus vectors. Proc Natl Acad Sci USA. 1997;94:1426–1431. doi: 10.1073/pnas.94.4.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kotin R M, Menninger J C, Ward D C, Berns K I. Mapping and direct visualization of a region-specific viral DNA integration site on chromosome 19q13-qter. Genomics. 1991;10:831–834. doi: 10.1016/0888-7543(91)90470-y. [DOI] [PubMed] [Google Scholar]
- 18.Kotin R M, Siniscalco M, Samulski R J, Zhu X D, Hunter L A, Laughlin C A, McLaughlin S K, Muzyczka N, Rocchi M, Berns K I. Site-specific integration by adeno-associated virus. Proc Natl Acad Sci USA. 1990;87:2211–2215. doi: 10.1073/pnas.87.6.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kube D M, Ponnazhagan S, Srivastava A. Encapsidation of adeno-associated virus type 2 Rep proteins in wild-type and recombinant progeny virions: Rep-mediated growth inhibition of primary human cells. J Virol. 1997;71:7361–7371. doi: 10.1128/jvi.71.10.7361-7371.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mah, C., K. Y. Qing, S. Ponnazhagan, X.-S. Wang, B. Khuntirat, D. M. Kube, M. C. Yoder, and A. Srivastava. 1997. Unpublished results.
- 21.McCown T J, Xiao X, Li J, Breese G R, Samulski R J. Differential and persistent expression patterns of CNS gene transfer by an adeno-associated virus (AAV) vector. Brain Res. 1996;713:99–107. doi: 10.1016/0006-8993(95)01488-8. [DOI] [PubMed] [Google Scholar]
- 22.Mizukami H, Young N S, Brown K E. Adeno-associated virus type 2 binds to a 150-kilodalton cell membrane glycoprotein. Virology. 1996;217:124–130. doi: 10.1006/viro.1996.0099. [DOI] [PubMed] [Google Scholar]
- 23.Muller M T. Binding of the herpes simplex virus immediate-early gene product ICP4 to its own transcription start site. J Virol. 1987;61:858–865. doi: 10.1128/jvi.61.3.858-865.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muzyczka N. Use of adeno-associated virus as a general transduction vector for mammalian cells. Curr Top Microbiol Immunol. 1992;158:97–129. doi: 10.1007/978-3-642-75608-5_5. [DOI] [PubMed] [Google Scholar]
- 25.Nahreini P, Woody M J, Zhou S Z, Srivastava A. Versatile adeno-associated virus 2-based vectors for constructing recombinant virions. Gene. 1993;124:257–262. doi: 10.1016/0378-1119(93)90402-o. [DOI] [PubMed] [Google Scholar]
- 26.Ping P, Yang Q, Hammond H K. Altered beta-adrenergic receptor signaling in heart failure, in vivo gene transfer via adeno and adeno-associated virus. Microcirculation. 1996;3:225–228. doi: 10.3109/10739689609148292. [DOI] [PubMed] [Google Scholar]
- 27.Ponnazhagan S, Erikson D, Kearns W G, Zhou S Z, Nahreini P, Wang X-S, Srivastava A. Lack of site-specific integration of the recombinant adeno-associated virus 2 genomes in human cells. Hum Gene Ther. 1997;8:275–284. doi: 10.1089/hum.1997.8.3-275. [DOI] [PubMed] [Google Scholar]
- 28.Ponnazhagan S, Mukherjee P, Wang X-S, Qing K, Kube D M, Mah C, Kurpad C, Yoder M C, Srour E F, Srivastava A. Adeno-associated virus type 2-mediated transduction in primary human bone marrow-derived CD34+ hematopoietic progenitor cells: donor variation and correlation of transgene expression with cellular differentiation. J Virol. 1997;71:8262–8267. doi: 10.1128/jvi.71.11.8262-8267.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ponnazhagan S, Mukherjee P, Yoder M C, Wang X-S, Zhou S Z, Kaplan J, Wadsworth S, Srivastava A. Adeno-associated virus 2-mediated gene transfer in vivo: organ-tropism and expression of transduced sequences in mice. Gene. 1997;190:203–210. doi: 10.1016/s0378-1119(96)00576-8. [DOI] [PubMed] [Google Scholar]
- 30.Ponnazhagan S, Wang X-S, Woody M J, Luo F, Kang L Y, Nallari M L, Munshi N C, Zhou S Z, Srivastava A. Differential expression in human cells from the p6 promoter of human parvovirus B19 following plasmid transfection and recombinant adeno-associated virus 2 (AAV) infection: human megakaryocytic leukaemia cells are non-permissive for AAV infection. J Gen Virol. 1996;77:1111–1122. doi: 10.1099/0022-1317-77-6-1111. [DOI] [PubMed] [Google Scholar]
- 31.Ponnazhagan S, Yoder M C, Srivastava A. Adeno-associated virus type 2-mediated transduction of murine hematopoietic cells with long-term repopulating ability and sustained expression of a human globin gene in vivo. J Virol. 1997;71:3098–3104. doi: 10.1128/jvi.71.4.3098-3104.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qing K, Bachelot T, Mukherjee P, Wang X-S, Peng L, Yoder M C, Leboulch P, Srivastava A. Adeno-associated virus type 2-mediated transfer of ecotropic retrovirus receptor cDNA allows ecotropic retroviral transduction of established and primary human cells. J Virol. 1997;71:5663–5667. doi: 10.1128/jvi.71.7.5663-5667.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qing K Y, Wang X-S, Kube D M, Ponnazhagan S, Bajpai A, Srivastava A. Role of tyrosine phosphorylation of a cellular protein in adeno-associated virus 2-mediated transgene expression. Proc Natl Acad Sci USA. 1997;94:10879–10884. doi: 10.1073/pnas.94.20.10879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qing, K. Y., X.-S. Wang, S. Ponnazhagan, and A. Srivastava. 1997. Unpublished results.
- 35.Russell D W, Alexander I E, Miller A D. DNA synthesis and topoisomerase inhibitors increase transduction by adeno-associated virus vectors. Proc Natl Acad Sci USA. 1995;92:5719–5723. doi: 10.1073/pnas.92.12.5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 37.Samulski R J, Chang L-S, Shenk T. Helper-free stocks of recombinant adeno-associated viruses: normal integration does not require viral gene expression. J Virol. 1989;63:3822–3828. doi: 10.1128/jvi.63.9.3822-3828.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samulski R J, Zhu X, Xiao X, Brook J D, Houseman D E, Epstein N, Hunter L A. Targeted integration of adeno-associated virus (AAV) into human chromosome 19. EMBO J. 1991;10:3941–3950. doi: 10.1002/j.1460-2075.1991.tb04964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Snyder R O, Miao C H, Patijn G A, Spratt S K, Danos O, Nagy D, Gown A M, Winther B, Meuse L, Cohen L K, Thompson A R, Kay M A. Persistent and therapeutic concentrations of human factor IX in mice after hepatic gene transfer of recombinant AAV vectors. Nat Genet. 1997;16:270–276. doi: 10.1038/ng0797-270. [DOI] [PubMed] [Google Scholar]
- 40.Southern E M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975;98:503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- 41.Srivastava A, Wang X-S, Ponnazhagan S, Zhou S Z, Yoder M C. Adeno-associated virus 2-mediated transduction and erythroid lineage-specific expression in human hematopoietic progenitor cells. Curr Top Microbiol Immunol. 1996;218:93–117. doi: 10.1007/978-3-642-80207-2_7. [DOI] [PubMed] [Google Scholar]
- 42.Walsh C E, Liu J M, Miller J L, Nienhuis A W, Samulski R J. Gene therapy for human hemoglobinopathies. Proc Soc Exp Biol Med. 1993;204:289–300. doi: 10.3181/00379727-204-43665. [DOI] [PubMed] [Google Scholar]
- 43.Wang X-S, Ponnazhagan S, Srivastava A. Rescue and replication of adeno-associated virus type 2 as well as vector DNA sequences from recombinant plasmids containing deletions in the viral inverted terminal repeats: selective encapsidation of viral genomes in progeny virions. J Virol. 1996;70:1668–1677. doi: 10.1128/jvi.70.3.1668-1677.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang X-S, Qing K, Ponnazhagan S, Srivastava A. Adeno-associated virus type 2 DNA replication in vivo: mutation analyses of the D sequence in viral inverted terminal repeats. J Virol. 1997;71:3077–3082. doi: 10.1128/jvi.71.4.3077-3082.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiao X, Li J, Samulski R J. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J Virol. 1996;70:8098–8108. doi: 10.1128/jvi.70.11.8098-8108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou S Z, Li Q, Stamatoyannopoulos G, Srivastava A. Adeno-associated virus 2-mediated transduction and erythroid cell-specific expression of a human β-globin gene. Gene Ther. 1996;3:223–229. [PubMed] [Google Scholar]