Abstract
Background
Plant-pathogen interactions are characterized by evolutionary arms races. At the molecular level, fungal effectors can target important plant functions, while plants evolve to improve effector recognition. Rapid evolution in genes encoding effectors can be facilitated by transposable elements (TEs). In Magnaporthe oryzae, the causal agent of blast disease in several cereals and grasses, TEs play important roles in chromosomal evolution as well as the gain or loss of effector genes in host specialized lineages. However, a global understanding of TE dynamics driving effector evolution at population scale and across lineages is lacking.
Results
Here, we focus on 16 AVR effector loci assessed across a global sampling of 11 reference genomes and 447 newly generated draft genome assemblies from publicly available short-read sequencing data across all major M. oryzae lineages and outgroups. We classified each effector based on evidence for duplication, deletion and translocation processes among lineages. Next, we determined AVR gain and loss dynamics across lineages allowing for a broad categorization of effector dynamics. Each AVR was integrated in a distinct genomic niche determined by the TE activity profile contributing to the diversification at the locus. We quantified TE contributions to effector niches and found that TE identity helped diversify AVR loci. We used the large genomic dataset to recapitulate the evolution of the rice blast AVR1-CO39 locus.
Conclusions
Taken together, our work demonstrates how TE dynamics are an integral component of M. oryzae effector evolution, likely facilitating escape from host recognition. In-depth tracking of effector loci is a valuable tool to predict the durability of host resistance.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12915-025-02385-7.
Keywords: Magnaporthe oryzae, AVR effectors, Transposable elements, Rice blast, Wheat blast
Background
Plant-pathogen interactions are characterized by continuous evolutionary arms races, where hosts adapt to resist infection while pathogens evolve to overcome host defenses [1]. Effector-receptor dynamics are crucial for this process, where pathogens deploy effectors (i.e. avirulence factors, AVR) to manipulate host immunity [2, 3], while host receptors are responsible to detect them and trigger defense responses [4, 5]. Genes encoding such effectors are among the most rapidly evolving genes in pathogen genomes, driven by high mutation rates and strong selection pressure [6], enabling pathogens to evade host resistance and posing a challenge for effective disease control. Effector genes are often encoded in subtelomeric chromosomal regions, which are repeat-rich regions and evolve more rapidly than regions encoding housekeeping genes [7, 8]. These genomic regions are also frequently associated with transposable element (TE) activity, which can disrupt coding sequences or promote regulatory changes [9–14]. Moreover, these chromosomal regions are also exhibiting a higher propensity for stochastic epigenetic regulation [15–17], and chromosomal rearrangements resulting in rapid effector gene evolution [18–20].
Plant pathogens capable of infecting both wild host plants and cultivated crops are of particular concern given their propensity to switch to new hosts [21]. Magnaporthe oryzae can infect over 50 wild and cultivated grass species including major cereal crops such as rice (Oryza sativa) and wheat (Triticum aestivum) [22]. Despite the wide host range, M. oryzae genotypes are grouping into host-specialized forms and recognized as different pathotypes [23–25]. The most studied pathotypes include M. oryzae Oryza (MoO), M. oryzae Triticum (MoT), and M. oryzae Lolium (MoL), causing blast disease on rice, wheat and ryegrass, respectively. The origin of new pathotypes was triggered by host jumps including the emergence of wheat and rice blast disease [26, 27]. Wheat blast emerged from a host jump from ryegrass (Lolium spp.) to Brazilian wheat cultivars lacking the RWT3 gene, which is the resistance gene expressing the receptor recognizing the PWT3 effector. While both PWT3 and PWT4 effector genes are found in the Lolium pathotype, cultivation of rwt3 wheat cultivars (lacking the ability to recognize PWT3) allowed the emergence of a new Triticum pathotype isolates carrying the PWT3 effector but losing PWT4, an effector that would be recognized due to the presence of its complementary resistance gene (RWT4) in wheat cultivars. Subsequent loss-of-function mutations arose due to the nearby cultivation of wheat cultivars carrying RWT3, along with the spread of pathogens to common wheat varieties [26]. These findings support the hypothesis that host specialization was mainly driven by genetic changes at effector gene loci including gain or loss of effector functions [28].
Numerous M. oryzae AVRs were cloned and characterized for their interaction with plant resistance factors [29]. Among these, AVR-ACE1 is involved in the production of secondary metabolites and activates the rice resistance factor Pi33 [30, 31]. AVR-Piz-t is recognized by Piz-t and suppresses pathogen-associated molecular pattern (PAMP)-triggered immunity [32, 33]. PWL effectors are primarily encoded by MoO and are rapidly evolving, small, glycine-rich secreted proteins [34, 35]. Several AVRs (i.e., AVR-Pi54, Pib, Pik and Pia) are classified as Magnaporthe AVRs and ToxB-like (MAX) effectors, sharing a conserved structural fold despite low sequence similarity and being recognized by different R proteins [36, 37]. Beyond functional differences in their encoded proteins, AVR genes exhibit a high degree of genetic instability and are often localized in telomeric regions [38, 39]. Such high rates of sequence changes are likely increasing their adaptative potential to evade host recognition. Sequence diversification occurred mostly through simple point mutations as reported for AVR-Pita and AVR-Pik [40–42], or sequence rearrangements causing segmental deletion of coding sequences as for AVR-Pita and AVR-Pib [43, 44] associated with gains in virulence. There is also evidence for horizontal transfer of PWT4 from M. pennisetigena to an Avena isolate from Brazil [45]. There is strong evidence that TEs impacted AVR loci and facilitate rearrangements. TEs facilitated virulence gains of MoO through loss-of-function mutations such as the insertion of a Mg-SINE into the AvrPi9 coding sequence [46] or gains of virulence linked to AVR-Pita and AVR-Pib due to a Pot3 TE insertion [11, 47]. TEs were also likely facilitating the translocations observed for several AVR genes including AVR-Pita [9]. The loss of telomeric ends resulting in the elimination of AVR-Pita and AVR-Pii [9, 48] were likely also favored by the repetitive nature of subtelomeric regions. Overall, the rapid evolution of M. oryzae AVRs to evade host recognition is likely facilitated by TE dynamics. TEs have specifically expanded in MoO compared to MoT and MoL [49, 50]. Furthermore, TE insertions also mediated the divergence of M. oryzae populations infecting different rice subspecies [49, 50]. However, a population genomics perspective on TE impacts on effectors leveraging the vast available genomic datasets on M. oryzae lineages is lacking.
Here, we used extensive genomic datasets covering all major M. oryzae host-associated pathotypes to recapitulate insertion dynamics near 16 AVRs and included M. grisea and M. pennisetigena as outgroups. We used reference-quality genomes to recapitulate chromosomal rearrangements affecting AVR loci across the M. oryzae pathotypes MoO, MoT, and MoL. We expanded AVR loci investigations at the population-scale by generating draft assemblies from available short-read sequencing datasets in order to assess AVR gain/loss patterns in conjunction with TE insertion dynamics.
Results
Samples distribution, genome assembling and reference genomes
To unravel M. oryzae AVR evolution, we assembled a collection of genomic datasets for 458 Magnaporthe spp. isolates collected across continents and diverse hosts (Fig. 1A) [51–54]. Most isolates were collected in Asia (59%) and predominantly infecting O. sativa (65%) (Fig. 1A), reflecting the high incidence of rice blast disease in this region. Isolates infecting cereals such as Triticum sp. as well as grasses such as Lolium sp. and Eleusine sp. across continents were also included (Fig. 1A). From the 458 analyzed isolates, 448 belonged to M. oryzae (Additional file 2: Table S1). Genomic data included 441 short read sequencing datasets and 7 reference-quality genomes from MoO, MoL and MoT (Fig. 1B; Additional file 2: Table S2). Ten outgroup genomes from two distinct Magnaporthe species (M. grisea and M. pennisetigena) were also included in the analysis of which four genomes were of reference quality (Additional file 2: Table S2). The selected outgroup Magnaporthe species M. grisea and M. pennisetigena clustered separately from each other and separated well from M. oryzae in the phylogenomic tree analysis (Fig. 1B). To complement the available reference genomes, we either accessed or assembled draft genomes for 447 additional isolates. Assembly genome sizes ranged from 39.1 Mbp in isolates infecting Leersia spp. and 42.9 Mbp in isolates infecting Cenchrus sp. (Fig. 1C). Assembled genomes for this study showed acceptable contiguity for the purpose of analyzing coding regions with the N50 (Length of the shortest contig for which longer Length contigs cover at least 50% of the assembly) averaging between 19,433 bp in isolates infecting Pennisetum sp. and 103,607 bp in isolates infecting Setaria sp. (Fig. 1C). Assembly genome sizes were not meaningfully correlated with the assembly contiguity (i.e. N50), which supports the notion that the totality of the genome is reasonably well covered despite variation in assembly quality (Additional file 1: Fig. S1). The reference-quality genomes showed BUSCO completeness scores ranging from 96.5% in PM1 (M. pennisetigena) to 97.9% in M. oryzae isolates infecting Oryza spp. (Fig. 1D). All draft genome assemblies similarly exhibited > 95% BUSCO completeness and are hence comparable to the reference genomes in terms of gene content. A small number of Oryza-infecting isolates showed slightly lower BUSCO completeness scores compared to the reference genomes, yet the assembly genome sizes were similar.
Fig. 1.
Global panel of analyzed Magnaporthe spp. genomes. A Geographical distribution of the Magnaporthe spp. isolates. The color identifies the reported host genus, and the size defines the number of samples from the same location. B Phylogenomic tree of reference-quality genomes. The host genus is reported in parentheses. Bootstrap confidence values > 80% are displayed. C De novo draft assembly genome size for genomes assembled from short-read sequencing data. Genomes are grouped by reported host genus. D Analyses of genome completeness based on BUSCO completeness percentages for reference-quality genomes (orange on top) and de novo draft assembled genomes
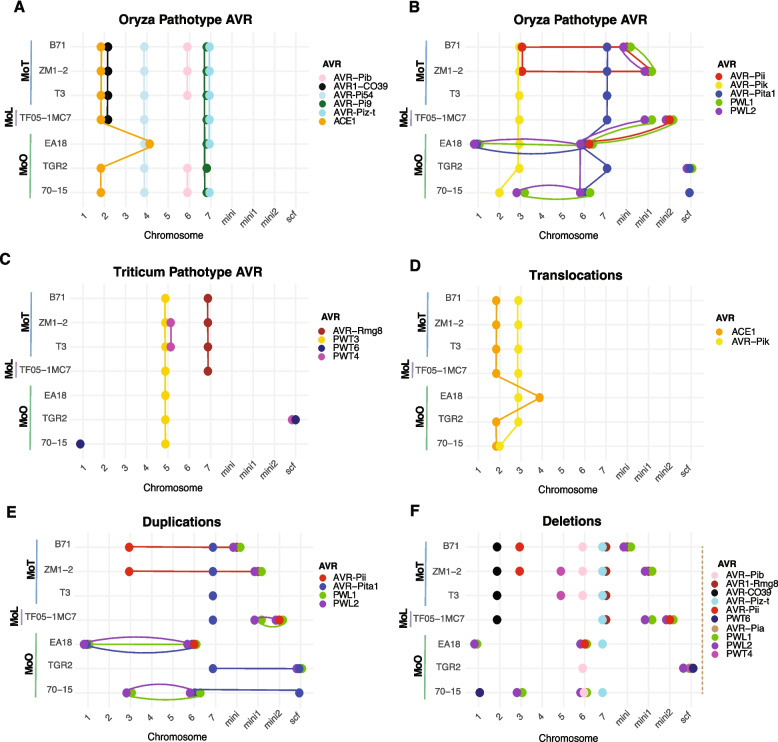
Rearrangements of AVRs among M. oryzae reference genomes
Some M. oryzae AVR underwent chromosomal translocations. To comprehensively track AVR localization among isolates, we focused on 16 cloned and characterized AVR effectors (Table 1) in seven reference M. oryzae genomes from MoO, MoL and MoT. We use the term “AVR pathotype” to identify in which host–pathogen interaction the AVR was first reported. However, this does not exclude the possibility that additional pathotypes or hosts share the AVR or receptor, respectively. We expected MoO effectors to be shared among most MoO reference genomes. However, from the 12 MoO AVR effectors (Table 1), only seven were shared among all MoO reference genomes and only four were found in all M. oryzae reference genomes (Fig. 2A, B). These four AVRs include AVR-Pi9 and AVR-Pi54 being at conserved chromosomal locations within pathotypes, while ACE1 and AVR-Pik showed translocations among MoO isolates (Fig. 2D). AVR-Pita1, PWL1 and PWL2 were present in all MoO reference genomes but have undergone duplication events (Fig. 2E). We also identified deletions of two MoO AVRs (AVR-Piz-t and AVR-Pib) in at least one MoO reference genome (Fig. 2F). AVR-Pii was absent in two MoO reference genomes (Fig. 2F) but exhibited a duplication in two MoT reference genomes (Fig. 2E). AVR-Pii was the only MoO effector that does not exhibit a duplication in a reference genome for its associated pathotype but in other M. oryzae pathotype reference genomes. AVR1-CO39 and AVR-Pia suffered the most dramatic loss, being absent in all MoO reference genomes (Fig. 2F). AVR1-CO39 is shared among all MoL and MoT reference genomes though (Fig. 2F). MoT effectors showed similar loss patterns to MoO effectors. Two MoT effectors were shared among the MoT reference genomes (Fig. 2C). PWT3 is shared among all M. oryzae reference genomes and located at a conserved position on chromosome 5 (Fig. 2C). PWT4 is absent in most M. oryzae reference genomes except MoT. PWT6 exhibited the most pronounced pattern of effector loss with the AVR being retained in only two MoO reference genomes (Fig. 2F). Overall, AVR localizations are highly dynamic among the seven reference genomes with contributions by translocations, deletions and duplication events.
Table 1.
Overview of Magnaporthe oryzae AVR effectors analyzed in this study. The AVR pathotype identifies the M. oryzae pathotype in which the effector was first characterized
| AVR gene | Corresponding resistance gene | AVR pathotype 1 | Sequence NCBI identifier | References |
|---|---|---|---|---|
| AVR-Pi9 | Pi9 | MoO | MW288376.1 | [46] |
| AVR-Pi54 | Pi54, Pi54th, Pi54of | MoO | KY441415.1 | [55] |
| ACE1 | Pi33 | MoO | AJ704622.1 | [30] |
| AVR-Piz-t | Piz-t | MoO | LC175951.1 | [32] |
| PWL2 | MLA3 | MoO | MN072512.1 | [56, 57] |
| AVR-Pib | Pib | MoO | KM887844.1 | [44] |
| AVR-Pik | Pik | MoO | AB498876.1 | [58] |
| AVR-Pita1 | Ptr | MoO | FJ842897.1 | [59] |
| AVR-Pii | Pii | MoO | LC175996.1 | [58] |
| AVR-Pia | Pia (RGA4/RGA5) | MoO | AB498873.1 | [58, 60] |
| PWL1 | Unknown | MoO | MT669814.1 | [34] |
| AVR1-CO39 | Pi-CO39 (RGA4/RGA5) | MoO | AF463528.1 | [60, 61] |
| AVR-Rmg8 | Rmg8 | MoT | LC223814.1 | [62] |
| PWT3 | Rmg6 (Rwt3) | MoT | LC202652.1 | [26] |
| PWT4 | Rmg1 (Rwt4) | MoT | LC202656.1 | [26] |
| PWT6 | Rmg9 (Rwt6) | MoT | LC574008.12 | [63] |
1MoO represents the Oryza pathotype, and MoT the Triticum pathotype
2Sequence from MoE (Eleusine pathotype)
Fig. 2.
Overview of AVR effector synteny among M. oryzae reference genomes from MoO, MoL and MoT. Chromosomal localizations of AVRs are reported for (A, B) MoO and (C) MoT. Overview of AVRs showing evidence for (D) translocation among chromosomes, as well as (E) duplication and (F) deletion events. Lines indicate different types of rearrangements, with their type defined by the plot context. Colored lines connect AVR localizations between reference genomes or chromosomes of the same genome (i.e. duplications)
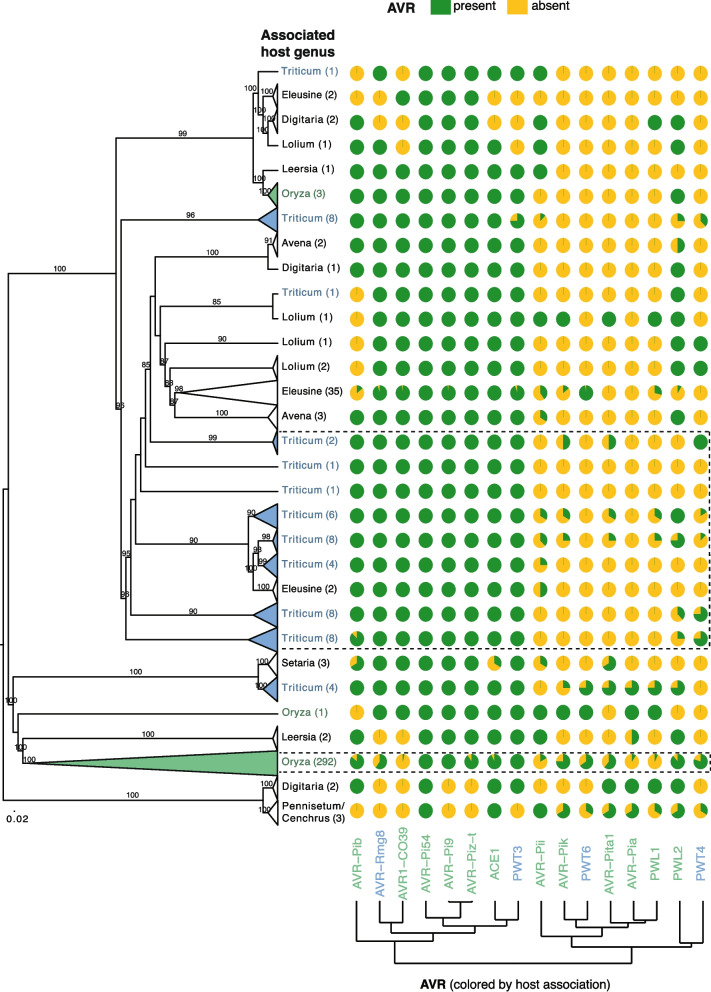
Effector gain and loss dynamics across global M. oryzae lineages
To comprehensively map the evolution trajectory of AVRs across M. oryzae Lineages, we searched for effector homologs across a dataset of 458 M. oryzae genomes (Additional file 2: Table S3). Isolates infecting Cenchrus and Pennisetum sp. were used as outgroups to clarify gains and loss patterns, since no other reference isolate contains all the investigated AVRs. Relationships among isolates were assessed based on a phylogenomic tree including 300 single-copy orthologs focusing only on the most frequently sampled host-associated lineages to reduce complexity. The phylogenetic grouping is consistent with previous studies of M. oryzae lineage diversification (Fig. 3). Isolates belonging to the Avena, Lolium and Triticum pathotypes clustered together. Among those, isolates collected from Triticum sp. were the most dispersed across the tree, corroborating the high genetic diversity reported for MoT pathotype isolates [106]. We verified consistency of phylogenetic placements for 24 isolates overlapping with a previous phylogenomic analysis [54]. Three isolates reported as collected on Oryza sp. leaves (A-PHL-64, ARG-60 and ARG-61) [65] did not cluster with the remaining MoO isolates. Furthermore, four MoT isolates were not clustering as closely with MoL and MoA (M. oryzae Avena) as expected but rather with MoS (M. oryzae Setaria) (Fig. 3).
Fig. 3.
Effectors gain and loss dynamics across Magnaporthe spp. Maximum Likelihood phylogenomic tree of 405 Magnaporthe genomes from the most frequently sampled pathotypes based on 300 protein sequence alignments of single-copy genes. Bootstrap confidence values > 80% are displayed. Numbers in parentheses indicate the number of genomes. Pie charts indicate the proportion of isolates carrying specific effectors per clade. AVRs are organized based on hierarchical clustering of presence/absence patterns. AVRs colored in green belong to the MoO AVR pathotype and in blue belong to the MoT AVR pathotype. A full-sized tree showing AVR presence/absence for each isolate is available from Zenodo (https://doi.org/10.5281/zenodo.15875042)
Although various genomic rearrangements were observed among reference genomes (Fig. 2), the analysis of the extended M. oryzae panel focused only on deletions given the limitations in contiguity of short-read assembled genomes. To categorize effector gain and loss dynamics, we assessed the frequency of effectors according to their reported host (Table 1). Most AVRs were present in at least one of the outgroups infecting Cenchrus and Pennisetum sp., respectively, suggesting that most M. oryzae effectors were present in the common ancestor to all extant M. oryzae lineages. Exceptions include AVR-Pi9, AVR-Piz-t, AVR1-CO39 and AVR-Rmg8 being absent in the outgroups. AVRs were mainly clustered into two groups: AVRs with largely stable frequencies among lineages and AVRs largely lost in most M. oryzae pathotypes, including AVRs largely lost in the pathotype reportedly linked to the AVR function (Fig. 3). MoO and MoT effectors were found among both main groups (Fig. 3). AVR-Pi9, AVR-Pi54, ACE1 and AVR-Piz-t are the most well conserved across the M. oryzae phylogeny (Fig. 3). The near fixation of the AVRs suggests that recognition by the host is not widely distributed among host varieties or that the AVR serves an additional function.
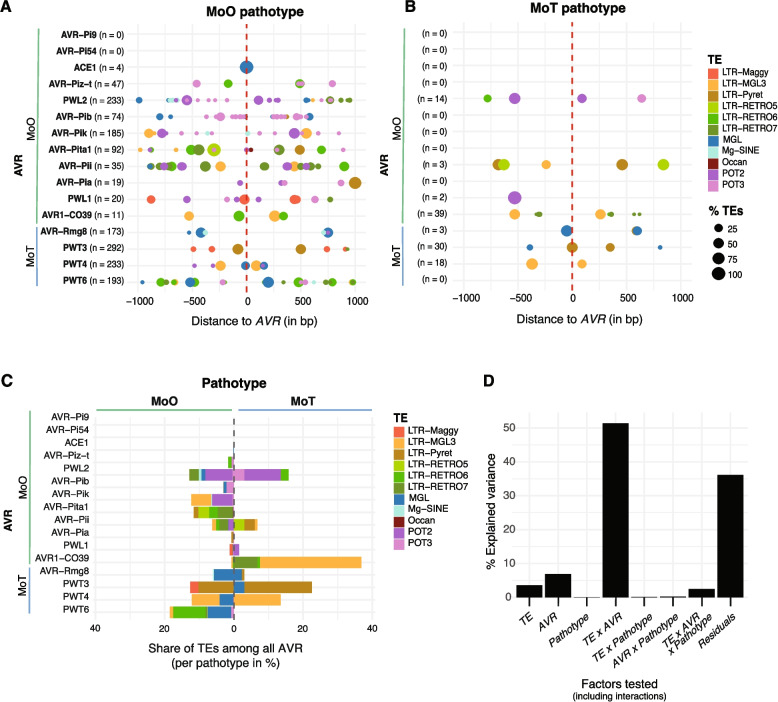
Transposable element colonization near M. oryzae effectors
The high frequency of rearrangements at AVR loci is consistent with high repetitive DNA content nearby. TE insertions are known to facilitate the creation of structural variation. Hence, we investigated patterns of TE dynamics surrounding AVRs and potential links to effector presence/absence variation. For this, we used only isolates clustering together with others from the same pathotype in Fig. 3. We annotated assembled contigs encoding the different AVRs for the presence of TE sequences considering a window of ± 1000 bp of the effector coding sequence (Additional file 1: Fig. S2; Additional file 2: Table S4). Here we found that the most frequent and conserved effectors (AVR-Pi9, AVR-Pi54, ACE1 and AVR-Piz-t) loci were devoid or nearly devoid of TEs in proximity in both MoO and MoT isolates (Fig. 4A and B). This suggests that the conservation of AVR effectors is facilitated by suppressed TE activity nearby. On the contrary, AVR1-CO39, an effector lost nearly entirely in MoO but remaining at high frequency in MoT isolates, showed the highest percentage of TEs among MoT isolates (Fig. 4C). Hence, the high rate of TE insertions nearby could have facilitated the loss of the effector. Interestingly, the frequency of TE families is associated with the AVR identity. The two DNA transposons POT2 and POT3 are at high frequency near PWT2, AVR-Pib and AVR-Pik loci (Fig. 4C). Retrotransposons were also abundant near specific AVRs. LTR-RETRO5 and LTR-RETRO7 were commonly found near the MoO effectors AVR-Pita1, AVR-Pii, and AVR1-CO39, while LTR-RETRO6 was more frequent near the MoT effector PWT6. Both LTR-RETRO7 and 6 show high sequence similarity with Inago1 and 2 retrotransposons respectively, which have been shown to be flank several M. oryzae AVRs [9, 66]. The LTR-MGL3 retrotransposon showed a high frequency near AVR1-CO39 and PWT4. Notably, LTR-Pyret was exclusively detected near PWT3 in both MoO and MoT isolates. Additionally, the MGL-LTR retrotransposon was most frequent near effectors first characterized in MoT (Fig. 4C). The distance between TEs and the nearby effector gene varied. Effectors in MoO had typically a higher distance to TEs than effectors in MoT isolates suggesting that sequence rearrangements could have affected the spacing between TEs and effectors (Fig. 4A and B).
Fig. 4.
Transposable element (TE) dynamics and diversification near M. oryzae AVR. A Presence of TEs in a ± 1000 bp window surrounding AVR loci in Oryza pathotype (MoO) isolates. The circle color reflects TE identity and classification. The circle size indicates the percentage of isolates carrying the respective AVR having a specific TE present. 100% refers to all isolates with the AVR sharing a specific TE at a specific position. Counts (n) indicate the number of isolates exhibiting at least one TE for a given AVR. Negative bp distance values represent AVR upstream regions. B Triticum pathotype (MoT) isolates. C Relative abundance of TEs among AVR loci shown separately for MoO and MoT isolates. Percentage calculations consider only isolates carrying the specific AVR. D Analyses of variance (ANOVA) of factors potentially explaining variation in TE abundance among AVRs. The percentage of variance explained by the different models based on factorial combinations for TE, AVR and pathotype (for which the AVR was first described) and their interactions
Given the heterogeneity in TE occupancy near AVRs across pathotypes, we sought to formally assess what factors explain best the variability in TE content. Using a multi-factorial ANOVA, we found that the TE identity (i.e. classification), the identity of the AVR and the pathotype explained a significant portion of the variance in TE occupancy (Fig. 4D; Additional file 2: Table S5). The largest proportion of variance (51%) in TE occupancy was explained by the interaction of AVR and TE identity. This suggests that AVR loci may have co-evolved with distinct TE families, reinforcing the idea that certain TEs have affinity for specific chromosomal regions.
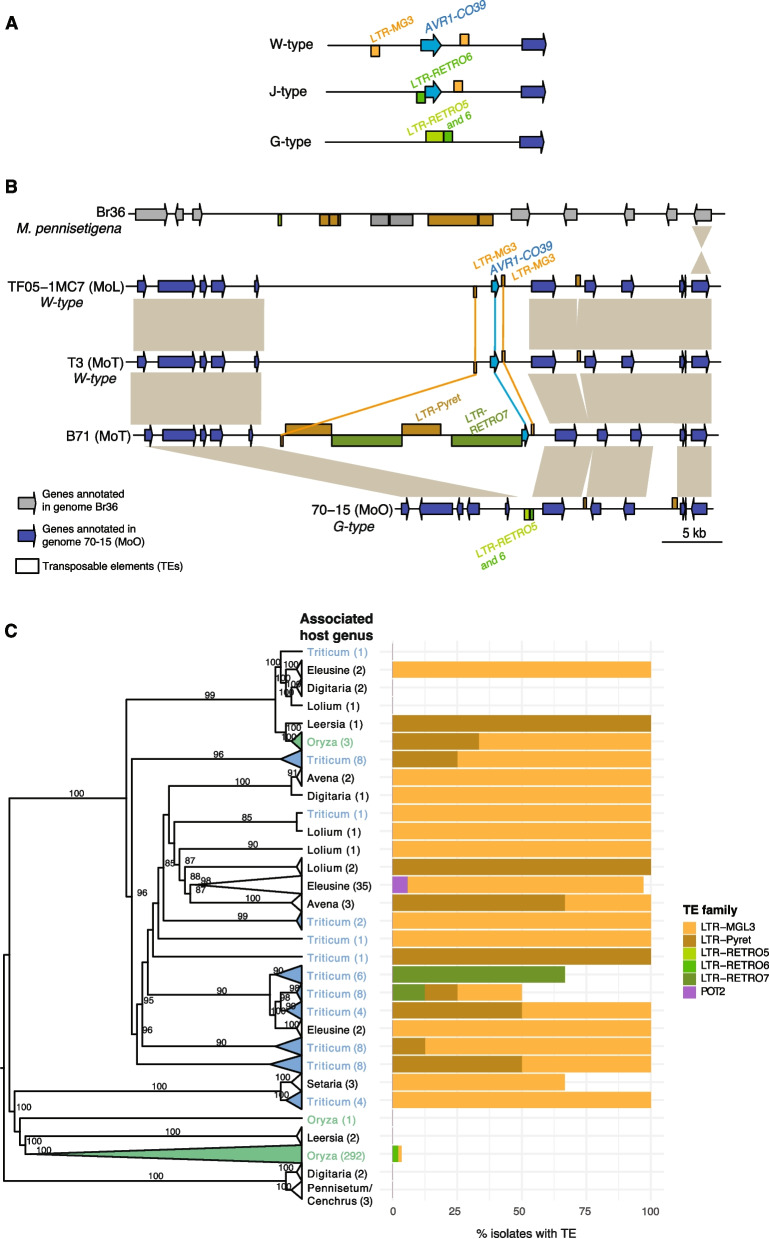
AVR1-CO39 locus dynamics
AVR1-CO39 was the only effector at low frequency in isolates of the pathotype (MoO) in which the effector function was originally described and at high frequency in all the other pathotypes (Fig. 3). AVR1-CO39 was previously characterized for a sequence rearrangement at the origin of the host switch to rice [67–69]. The two variants were described as the G- and J-type in MoO lacking a functional AVR and an alternative W-type associated with an intact AVR1-CO39 in isolates infecting weeping love grass [69]. The most frequent type in MoO (G-type) is characterized by a complete loss of the coding sequence through a deletion and TE replacement. The J-type consists of a loss-of-function version caused by a repetitive element called REP1 [69]. REP1 corresponds to LTR-RETRO6 in more recent TE annotations (Fig. 5A). We analyzed the evolution of the AVR1-CO39 locus organization across pathotypes by inspecting first the reference genomes. We used gene models annotated in the MoO genome 70–15 to obtain gene annotations in the additional M. oryzae reference genomes included in the comparison (Fig. 5B). The W-type was represented by the MoL and MoT reference genomes (Fig. 5B). The W-type carries genes adjacent to AVR1-CO39, which are absent in 70–15 (empty chromosomal region of the W-type; Fig. 5B). We identified no locus synteny with the outgroup reference genome (Br36) (Fig. 5B). As previously reported for the MoO isolate Guy11 [69], the 70–15 MoO reference genome shows a substantial contraction of the region adjacent to AVR1-CO39 compared to the MoL and MoT genomes (Fig. 5B).
Fig. 5.
Chromosomal synteny analyses of the AVR1-CO39 locus. A Sketch of AVR1-CO39 locus types as reported in the literature [69]. B Synteny plot of the AVR1-CO39 region on chromosome 2 of MoO, MoL and MoT reference genomes and M. pennisetigena. Grey blocks between chromosomes indicate homologous regions. C Comparison of TE types upstream of AVR1-CO39 among Magnaporthe lineages. The phylogenomic tree is based on 300 single-copy orthologs. Bootstrap confidence values > 80% are displayed
The contracted region in 70–15 exhibits different TEs compared to the W-type, with LTR-RETRO5 and LTR-RETRO6 occupying the contracted region. The LTR-RETRO5 has been previously identified in the G-type and the adjacent element was called REP1, which we identified here as LTR-RETRO6. The MoT isolate B71 exhibits yet distinct pattern with the AVR1-CO39 upstream region fully occupied by TEs (Fig. 5B). Two of these TEs were intact LTR-RETRO7, suggesting a recent insertion. One of the LTR-RETRO7 insertion resulted in the truncation of AVR1-CO39 in B71, differing in size from the J-type rearrangement. However, both retained ORF3, which encodes the AVR1-CO39 domain interacting with the corresponding resistance gene [70]. The Zambia wheat blast outbreak isolate ZM1-2 (MoT) was reported to share a similar AVR1-CO39 locus structure as B71 [52]. This highlights that the AVR1-CO39 loss-of-function is not restricted to MoO isolates but is also occurring independently in MoT isolates through a different TE insertion. Since the different AVR1-CO39 types are characterized by distinct TE content upstream of the coding sequence, we expanded our investigations to all isolates of the collection carrying the effector. We find that the LTR-MGL3, characteristic of the W-type rearrangement, was the most frequent among Magnaporthe lineages (Fig. 5C). In contrast, the J-type was rare and found in only 10 MoO isolates (Fig. 5C). The LTR-RETRO7, not characteristic of any of the previously described AVR1-CO39 locus types (Fig. 5A), was found in the reference genome B71 and a small number of additional MoT isolates (Fig. 5C). We identified two further TEs, LTR-Pyret and POT2, which were also found directly upstream of AVR1-CO39 in our dataset. Our investigations of the locus indicate that the W-type is likely ancestral and has undergone multiple rearrangements followed by different TE insertions leading ultimately to the loss of the effector (i.e. G-type).
Discussion
AVR effectors of M. oryzae have undergone rapid evolution to circumvent the matching plant receptors and increase pathogen virulence [71]. Point mutations, insertions and deletions are widely described mechanisms responsible for the loss of avirulence function of AVR genes [9, 43, 44, 47]. Abundance of TE sequences near effector regions can act as a mutagen or epigenetic regulator for accelerated effector evolution [72–74]. In M. oryzae, TE content correlates with host identity and contributes to genetic differentiation among lineages [49, 50]. Here, we show that effectors evolved well differentiated presence/absence patterns across Magnaporthe pathotypes, which at least in part reflects the diversity in recognition mechanisms by the different hosts. Across pathotypes of M. oryzae, we show that TE insertion dynamics most likely underpin the observed effector rearrangements. In contrast, conserved AVRs show no recent TE activity in the surrounding regions.
Variable AVR frequencies among M. oryzae host-specific pathotypes may be driven by adaptation to new hosts or the deployment of a new resistance gene. Here, we show that multiple MoO effectors were lost in different M. oryzae pathotypes including rice blast. This is consistent with the high rates of gene losses reported for rice-infecting M. oryzae compared to Triticum or Avena sp. infecting isolates [28]. How closely the AVR frequencies among hosts reflects pathogenicity and recognition capabilities remains to be determined. Effector loss may be sufficient to escape host recognition and therefore contributing to host range expansion. This has been reported for instance for PWT3, with its loss coinciding with the widespread deployment of the complementary R gene RWT3 [26]. In contrast, the more recently described MAX effectors do not necessarily reflect host specificity [75]. This is consistent with the absence of certain effectors, which may not reflect avoidance of host recognition. Well-conserved effectors across multiple pathotypes and an origin outside of M. oryzae may reflect a conserved function in virulence maintained by purifying selection. However, contributions to virulence may still vary among pathotypes, as observed for the ACE1 effector [31, 76].
TEs represent approximately 10% of the M. oryzae genome [72, 77] and can be inserted in or around AVR genes altering their virulence spectrum through transcriptional silencing, loss-of-function or loss of avirulence [11, 44, 47, 78, 79]. TE-mediated disruptions can result in the permanent loss of AVR genes, being a possible explanation for the observed deletion patterns. In AVR-Pib, a POT3 transposon insertion mediates effector loss-of-function in Philippine MoO isolates [80]. We found a striking association of the TE POT3 in the neighboring regions of AVR-Pib in MoO, suggesting that the TE plays a functional role. TE insertions can also promote the emergence of new virulent effector variants, as observed for the MGL retrotransposon insertion into the ACE1 gene [81] and POT3 insertion in AVR-Pita1 [11, 82] and AVR-Piz-t [32] ensuring the maintenance of such effectors. In our collection, all the four MoO isolates exhibiting TEs in the ACE1 surrounding regions exhibited this same TE overlapping with the ACE1 coding sequence. The remaining 270 MoO isolates were devoid of TEs near the effector. TEs can also facilitate the translocation of AVR genes such as AVR-Pita [9]. Here, we focused on MoO reference-quality genomes to investigate translocations of ACE1 and AVR-Pik. However, we found no association between these translocations and specific TEs.
M. oryzae shows lineage-specific TE activity with LTR-retrotransposons expanded in certain lineages, rather than undergoing a single expansion followed by selective deletion [49]. TEs were often found near genes with presence/absence variation including effectors [83]. Here, we observed that well maintained AVRs had almost no TE activity in the surrounding area. This is possibly explained by their chromosomal location. In contrast to most of the M. oryzae AVR genes, usually located in telomeric or subtelomeric regions [9, 43, 58, 84, 85], highly conserved AVRs could have been selected to occupy euchromatic and repeat-poor regions. AVR-Pi9, a widely present effector among M. oryzae pathotypes, is located in a genomic region close to the chromosome 7 centromere, which is one of the most stable regions of the genome [46]. Similarly, AVR-Piz-t is found at high frequency among pathotypes and located 230 kb from AVR-Pi9 [32]. Effectors with highly variable presence/absence frequencies among pathotypes include AVR-Pia, AVR-Pii, AVR-Pita and PWL and are well-known to be located in unstable chromosomal regions such as subtelomeres prone to rearrangement [34, 35, 43, 58, 86]. Despite the broad spectrum in chromosomal locations, most of the effectors are expressed during the early stages of infection. AVR-Pi9 accumulates in the biotrophic interfacial complex structure and is translocated in the early stages of infection to the host cell [46]. A similar expression and translocation pattern was also observed for less conserved effectors such as AVR-Pia and AVR-Pita [87–89].
TE activity is typically high in telomeric and subtelomeric regions [8, 90]. Here, we show that AVRs with highly variable frequencies among pathotypes are indeed surrounded by TEs. The presence of TE can also affect epigenetic regulation through changes in heterochromatic structure [15–17]. These results reinforce the idea that in M. oryzae different TE environments impact effector evolution in distinct ways. Our findings also indicate that certain TE superfamilies may have more affinity for specific AVRs loci, reflected also in the fact that the TE presence is largely conserved among pathotypes. This suggests that despite differences in TE activity among fungal plant pathogens [91–93], including in M. oryzae [49, 50], the effector regions may have been selected for a variety of genomic niche features. The clearest evidence for TE insertions creating transitional states at effector loci was found for AVR1-CO39, with TEs likely contributing to the loss in MoO. Future studies will be able to significantly expand on AVR locus dynamics by shifting to a comprehensive set of long-read genomes. This will overcome the limitations of draft assemblies resolving only AVR-proximate sequence dynamics.
Conclusions
Overall, we show that M. oryzae AVR locus evolution was characterized by parallel and well differentiation dynamics in TEs. Spanning the spectrum of effectors exhibiting rapid changes in frequencies across M. oryzae pathotypes to conserved effector loci retained at high frequency and devoid of TEs. This highlights that host-mediated selection plays not only a role in AVR frequencies but that the genomic niche of the effectors displays likely similar associated dynamics. The dynamics of TE insertions within plant pathogens as a response to host selection remains understudied. Our work demonstrates though that TE dynamics can be an integral component of genomic niche evolution. In-depth tracking of effector niches will likely augment our ability to predict the durability of host resistance.
Methods
Magnaporthe genomic datasets and genome assemblies
We performed analyses on a global collection of genomic datasets comprising 458 Magnaporthe isolates (Additional file 2: Tables S1 and S2). All genomes were accessed from public databases reported by previous studies [51–54]. The geographic origin of isolates and the host organism information was retrieved from metadata attributes and cross-checked with the associated literature. The isolates were from Asia (n = 256), South America (n = 96), Africa (n = 69), Europe (n = 11), North America (n = 8), and a small number without reported origin (n = 18). Out of the 458 isolates, 448 belonged to M. oryzae collected from 18 different host species, including cereals and grasses. We also included as outgroups two isolates of M. pennisetigena (collected on Pennisetum sp.) and eight isolates of M. grisea isolated from Cenchrus and Digitaria sp. Overall, 447 isolates were sequenced using Illumina paired-end whole-genome sequencing (WGS) (Additional file 2: Table S1). Illumina sequencing data was initially filtered using fastp v0.23.4 with default settings [94] to remove adapter sequence and low-quality reads. De novo draft assemblies were generated using the software SPAdes v3.15.5 [95] with the “careful” method and automated k-mer selection. All genomes were verified to have more than 95% completeness using BUSCO version 5.8.2 [96] searching the Ascomycota orthology database. We used QUAST to calculate assembly metrics [97]. We retained assemblies with N50 (Length of the shortest contig for which longer Length contigs cover at least 50% of the assembly) above 17,034 bp and a total assembly size above 36.72 Mbp. Eleven Magnaporthe reference-quality genome assemblies were also included in this study [77, 98–100]. These genomes included seven M. oryzae genomes (3 MoO, 3 MoT, 1 MoL) and four outgroup genomes (2 M. grisea and 2 M. pennisetigena) (Additional file 2: Table S2).
Phylogenetic analyses
Phylogenetic relationships were assessed separately for the set of Magnaporthe reference-quality genomes and for 405 Magnaporthe draft genomes associated with the most frequently sampled host species. For the phylogenomic tree, we used single-copy genes predicted by AUGUSTUS v3.5.0 [101] using the pretrained gene prediction database available for the M. grisea genome. Predicted protein sequences were used for orthology analyses performed with Orthofinder v2.5.5 [102]. Single-copy orthologs present in at Least 90% of the total number of isolates were kept. For the phylogenetic reconstruction of the large Magnaporthe worldwide collection, we retained 300 randomly selected single-copy orthologs to reduce computational load. Selected ortholog protein sequences were aligned as a supermatrix using the AMAS tool v1.0 [103]. Phylogenetic trees were built using RAxML v8 [104] to construct a maximum-likelihood phylogenetic tree with the parameters -m PROTGAMMAAUTO for protein sequences with 1000 bootstrap replicates. We used the hclust function in R to perform hierarchical clustering of AVRs.
Identification of effector homologues
We analyzed the 447 draft genomes produced by SPAdes and the 11 reference-quality genomes (including outgroups) to search for homologs of 16 M. oryzae effectors. We focused on cloned and well-characterized effectors identified in Oryza or Triticum pathotype isolates (Table 1). For effectors present in more than one pathotype, effector sequences with best hits in the reference-quality genomes were used as query for BLASTn analyses [105]. Hits were filtered for a maximum e-value of 10–5, followed by individual minimum length filtering for each effector based on visual inspection of alignment length distributions. The ORF finder tool (https://www.ncbi.nlm.nih.gov/orffinder/) was used to refine effector open reading frames detected by BLASTn hits.
Transposable element annotation and analysis
To detect TE insertions near effectors, all draft assemblies, reference-quality and outgroup genomes were annotated with the TE consensus sequences reported for Magnaporthe [50] (available from https://github.com/S-t-ing/mBio-data-availablility/blob/main/Mo.TE_Consensus.fasta). For this, we used RepeatMasker v4.0.9_p2 with “-no_is” and “-nolow” parameters to skip simple repeat and low complexity region annotations. Annotated TEs shorter than 50 bp were filtered out. The search for TEs near effector loci was restricted to a 1000 bp window up- and downstream the effector gene. Only MoO and MoT isolates clearly identified in large clades based on the phylogenetic tree were retained these analyses. Percentage of TE superfamilies per AVR in MoO and MoT was calculated per effector based on the total TE number per pathotype. Percentage of TE superfamilies per insertion site (bp) has been calculated per effector based on the total number of isolates containing at least one TE for this AVR. We used the aov function in R to perform an ANOVA testing the effects of the following factors: TE family, AVR identity, and AVR pathotype (MoO vs. MoT) with the response variable being TE counts near effectors. We assessed how much each factor, and interactions thereof, contributed to the overall variation by using the sum of squares from the ANOVA.
Analyses of the AVR1-CO39 locus
To assess the AVR1-CO39 locus organization in genomes of different pathotypes, we first analyzed reference-quality genomes. Synteny was plotted with genoplotR v0.8.11 [106] for the Magnaporthe reference genomes using gene and TE annotation [50]. For M. oryzae isolates, we mapped coding sequences from the MoO 70–15 (GCF_000002495.2) gene annotation [77], and for outgroup M. pennisetigena we mapped coding sequences from the Br36 (GCA_004337985.1) gene annotation [98]. The two annotations of 70–15 and Br36 provided complementary coverage of major haplotypes found at the locus. AUGUSTUS gene annotations as described above were used to infer coding sequences in non-MoO isolates. Magnaporthe genomes assembly having AVR1-CO39 were inspected for TE presence surrounding the effector gene.
Supplementary Information
Additional file 1: Figures S1-S2. Figure S1: Correlation between total genome assembly size and N50 metrics for de novo assembled genomes. Figure S2: Presence of TEs in a +/- 1000 bp window surrounding AVR loci in Setaria (MoS), Eleusine (MoE), Avena (MoA), Lolium (MoL), Leersia (MoLe), Digitaria (MoD), Cenchrus (MoC), and Pennisetum (MoP) pathotype isolates.
Additional file 2: Tables S1-S5. Table S1: Analyzed Magnaporthe oryzae isolates sequenced by Illumina paired-end whole-genome sequencing. Table S2: Analyzed reference-quality Magnaporthe genomes. Table S3: Blast hit statistics to identify the different AVR genes in the Magnaporthe genomes (draft and reference genomes). Table S4: Transposable elements (TEs) detected near each AVR. Table S5: ANOVA of TE occupancy near AVRs.
Acknowledgements
We are grateful to group members for critical discussions.
Abbreviations
- TEs
Transposable elements
- AVR
Avirulence effector
- Mo
Magnaporthe oryzae
- MoO
Magnaporthe oryzae Oryza
- MoT
Magnaporthe oryzae Triticum
- MoL
Magnaporthe oryzae Lolium
- MoE
Magnaporthe oryzae Eleusine
- MoA
Magnaporthe oryzae Avena
- MoS
Magnaporthe oryzae Setaria
- PAMP
Pathogen-associated molecular pattern
- R proteins/genes
Resistance protein/genes
Authors’ contributions
AMS and DC conceived the study, wrote and revised the manuscript. AMS analyzed the data. All authors read and approved the final manuscript.
Funding
This study was supported by a Swiss National Science Foundation grant to DC (201149).
Data availability
The data analyzed in the frame of this study were retrieved from NCBI repositories as indicated in the Additional File 2. Draft genome assembly data, a Magnaporthe spp. full-size phylogenetic tree with AVR presence/absence screening is available from Zenodo (10.5281/zenodo.15875042) [107].
Declarations
Ethics approval and consent to participate
N/A.
Consent for publication
N/A.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sironi M, Cagliani R, Forni D, Clerici M. Evolutionary insights into host–pathogen interactions from mammalian sequence data. Nat Rev Genet. 2015;16:224–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Remick BC, Gaidt MM, Vance RE. Effector-Triggered Immunity. Annu Rev Immunol. 2023;41:453–81. [DOI] [PubMed] [Google Scholar]
- 3.Lo Presti L, Lanver D, Schweizer G, Tanaka S, Liang L, Tollot M, et al. Fungal Effectors and Plant Susceptibility. Annu Rev Plant Biol. 2015;66:513–45. [DOI] [PubMed] [Google Scholar]
- 4.Stukenbrock EH, McDonald BA. Population genetics of fungal and oomycete effectors involved in gene-for-gene interactions. Mol Plant Microbe Interact. 2009;22:371–80. [DOI] [PubMed] [Google Scholar]
- 5.Kanyuka K, Rudd JJ. Cell surface immune receptors: the guardians of the plant’s extracellular spaces. Curr Opin Plant Biol. 2019;50:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sánchez-Vallet A, Fouché S, Fudal I, Hartmann FE, Soyer JL, Tellier A, et al. The Genome Biology of Effector Gene Evolution in Filamentous Plant Pathogens. Annu Rev Phytopathol. 2018;56:21–40. [DOI] [PubMed] [Google Scholar]
- 7.Dong S, Raffaele S, Kamoun S. The two-speed genomes of filamentous pathogens: waltz with plants. Curr Opin Genet Dev. 2015;35:57–65. [DOI] [PubMed] [Google Scholar]
- 8.Faino L, Seidl MF, Shi-Kunne X, Pauper M, Van Den Berg GCM, Wittenberg AHJ, et al. Transposons passively and actively contribute to evolution of the two-speed genome of a fungal pathogen. Genome Res. 2016;26:1091–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chuma I, Isobe C, Hotta Y, Ibaragi K, Futamata N, Kusaba M, et al. Multiple Translocation of the AVR-Pita Effector Gene among Chromosomes of the Rice Blast Fungus Magnaporthe oryzae and Related Species. PLoS Pathog. 2011;7:e1002147. [DOI] [PMC free article] [PubMed]
- 10.Fouché S, Plissonneau C, Croll D. The birth and death of effectors in rapidly evolving filamentous pathogen genomes. Curr Opin Microbiol. 2018;46:34–42. [DOI] [PubMed] [Google Scholar]
- 11.Kang S, Lebrun MH, Farrall L, Valent B. Gain of virulence caused by insertion of a Pot3 transposon in a Magnaporthe grisea avirulence gene. Mol Plant Microbe Interact. 2001;14:671–4. [DOI] [PubMed] [Google Scholar]
- 12.Sampaio AM, Tralamazza SM, Mohamadi F, De Oliveira Y, Enjalbert J, Saintenac C, et al. Diversification, loss, and virulence gains of the major effector AvrStb6 during continental spread of the wheat pathogen Zymoseptoria tritici. PLoS Pathog. 2025;21: e1012983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seidl MF, Thomma BPHJ. Transposable Elements Direct The Coevolution between Plants and Microbes. Trends Genet. 2017;33:842–51. [DOI] [PubMed] [Google Scholar]
- 14.Whisson SC, Vetukuri RR, Avrova AO, Dixelius C. Can silencing of transposons contribute to variation in effector gene expression in Phytophthora infestans? Mob Genet Elements. 2012;2:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aparicio OM, Billington BL, Gottschling DE. Modifiers of position effect are shared between telomeric and silent mating-type loci in S. cerevisiae. Cell. 1991;66:1279–87. [DOI] [PubMed] [Google Scholar]
- 16.De Las PA, Pan SJ, Castaño I, Alder J, Cregg R, Cormack BP. Virulence-related surface glycoproteins in the yeast pathogen Candida glabrata are encoded in subtelomeric clusters and subject to RAP1- and SIR-dependent transcriptional silencing. Genes Dev. 2003;17:2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan C, Zhang Y, Yu Y, Rounsley S, Long M, Wing RA. The subtelomere of Oryza sativa chromosome 3 short arm as a hot bed of new gene origination in rice. Mol Plant. 2008;1:839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi-Kunne X, Faino L, van den Berg GCM, Thomma BPHJ, Seidl MF. Evolution within the fungal genus Verticillium is characterized by chromosomal rearrangement and gene loss. Environ Microbiol. 2018;20:1362–73. [DOI] [PubMed] [Google Scholar]
- 19.De Jonge R, Bolton MD, Kombrink A, Van Den Berg GCM, Yadeta KA, Thomma BPHJ. Extensive chromosomal reshuffling drives evolution of virulence in an asexual pathogen. Genome Res. 2013;23:1271–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Q, Sun M, Zhang Y, Song Z, Zhang S, Zhang Q, et al. Extensive chromosomal rearrangements and rapid evolution of novel effector superfamilies contribute to host adaptation and speciation in the basal ascomycetous fungi. Mol Plant Pathol. 2020;21:330–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson PK, Cunningham AA, Patel NG, Morales FJ, Epstein PR, Daszak P. Emerging infectious diseases of plants: pathogen pollution, climate change and agrotechnology drivers. Trends Ecol Evol. 2004;19:535–44. [DOI] [PubMed] [Google Scholar]
- 22.Wilson RA, Talbot NJ. Under pressure: investigating the biology of plant infection by Magnaporthe oryzae. Nat Rev Microbiol. 2009;7:185–95. [DOI] [PubMed] [Google Scholar]
- 23.Choi J, Park SY, Kim BR, Roh JH, Oh IS, Han SS, et al. Comparative analysis of pathogenicity and phylogenetic relationship in Magnaporthe grisea species complex. PLoS ONE. 2013;8: e57196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim KT, Ko J, Song H, Choi G, Kim H, Jeon J, et al. Evolution of the Genes Encoding Effector Candidates Within Multiple Pathotypes of Magnaporthe oryzae. Front Microbiol. 2019;10: 491941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valent B. Rice Blast as a Model System for Plant Pathology. Phytopathology. 1990;80:33. [Google Scholar]
- 26.Inoue Y, Vy TTP, Yoshida K, Asano H, Mitsuoka C, Asuke S, et al. Evolution of the wheat blast fungus through functional losses in a host specificity determinant. Science. 2017;357:80–3. [DOI] [PubMed] [Google Scholar]
- 27.Couch BC, Fudal I, Lebrun MH, Tharreau D, Valent B, Van Kim P, et al. Origins of Host-Specific Populations of the Blast Pathogen Magnaporthe oryzae in Crop Domestication With Subsequent Expansion of Pandemic Clones on Rice and Weeds of Rice. Genetics. 2005;170:613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshida K, Saunders DGO, Mitsuoka C, Natsume S, Kosugi S, Saitoh H, et al. Host specialization of the blast fungus Magnaporthe oryzae is associated with dynamic gain and loss of genes linked to transposable elements. BMC Genomics. 2016;17:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Wit PJGM, Mehrabi R, Van Den Burg HA, Stergiopoulos I. Fungal effector proteins: past, present and future. Mol Plant Pathol. 2009;10:735–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Böhnert HU, Fudal I, Dioh W, Tharreau D, Notteghem JL, Lebrun MH. A putative polyketide synthase/peptide synthetase from Magnaporthe grisea signals pathogen attack to resistant rice. Plant Cell. 2004;16:2499–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Collemare J, Pianfetti M, Houlle AE, Morin D, Camborde L, Gagey MJ, et al. Magnaporthe grisea avirulence gene ACE1 belongs to an infection-specific gene cluster involved in secondary metabolism. New Phytol. 2008;179:196–208. [DOI] [PubMed] [Google Scholar]
- 32.Li W, Wang B, Wu J, Lu G, Hu Y, Zhang X, et al. The Magnaporthe oryzae Avirulence Gene The AvrPiz-t Encodes a Predicted Secreted Protein That Triggers the Immunity in Rice Mediated by the Blast Resistance Gene Piz-t. 2009;22:411–20. [DOI] [PubMed]
- 33.Park CH, Chen S, Shirsekar G, Zhou B, Khang CH, Songkumarn P, et al. The Magnaporthe oryzae Effector AvrPiz-t Targets the RING E3 Ubiquitin Ligase APIP6 to Suppress Pathogen-Associated Molecular Pattern-Triggered Immunity in Rice. Plant Cell. 2012;24:4748–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang S, Sweigard JA, Valent B. The PWL host specificity gene family in the blast fungus Magnaporthe grisea. Mol Plant Microbe Interact. 1995;8:939–48. [DOI] [PubMed] [Google Scholar]
- 35.Sweigard JA, Carroll AM, Kang S, Farrall L, Chumley FG, Valent B. Identification, cloning, and characterization of PWL2, a gene for host species specificity in the rice blast fungus. Plant Cell. 1995;7:1221–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Guillen K, Ortiz-Vallejo D, Gracy J, Fournier E, Kroj T, Padilla A. Structure Analysis Uncovers a Highly Diverse but Structurally Conserved Effector Family in Phytopathogenic Fungi. PLoS Pathog. 2015;11: e1005228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Varden FA, Saitoh H, Yoshino K, Franceschetti M, Kamoun S, Terauchi R, et al. Cross-reactivity of a rice NLR immune receptor to distinct effectors from the rice blast pathogen Magnaporthe oryzae provides partial disease resistance. J Biol Chem. 2019;294:13006–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farman ML. Telomeres in the rice blast fungus Magnaporthe oryzae: the world of the end as we know it. FEMS Microbiol Lett. 2007;273:125–32. [DOI] [PubMed] [Google Scholar]
- 39.Rehmeyer C, Li W, Kusaba M, Kim YS, Brown D, Staben C, et al. Organization of chromosome ends in the rice blast fungus. Magnaporthe oryzae Nucleic Acids Res. 2006;34:4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dai Y, Jia Y, Correll J, Wang X, Wang Y. Diversification and evolution of the avirulence gene AVR-Pita1 in field isolates of Magnaporthe oryzae. Fungal Genet Biol. 2010;47:973–80. [DOI] [PubMed] [Google Scholar]
- 41.Kanzaki H, Yoshida K, Saitoh H, Fujisaki K, Hirabuchi A, Alaux L, et al. Arms race co-evolution of Magnaporthe oryzae AVR-Pik and rice Pik genes driven by their physical interactions. Plant J. 2012;72:894–907. [DOI] [PubMed] [Google Scholar]
- 42.Longya A, Chaipanya C, Franceschetti M, Maidment JHR, Banfield MJ, Jantasuriyarat C. Gene duplication and mutation in the emergence of a novel aggressive allele of the AVR-PIK effector in the rice blast fungus. Mol Plant Microbe Interact. 2019;32:740–9. [DOI] [PubMed] [Google Scholar]
- 43.Orbach MJ, Farrall L, Sweigard JA, Chumley FG, Valent B. A Telomeric Avirulence Gene Determines Efficacy for the Rice Blast Resistance Gene Pi-ta. Plant Cell. 2000;12:2019–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang S, Wang L, Wu W, He L, Yang X, Pan Q. Function and evolution of Magnaporthe oryzae avirulence gene AvrPib responding to the rice blast resistance gene Pib. Sci Rep. 2015;5:11642. [DOI] [PMC free article] [PubMed]
- 45.Inoue Y, Vy TTP, Tani D, Tosa Y. Suppression of wheat blast resistance by an effector of Pyricularia oryzae is counteracted by a host specificity resistance gene in wheat. New Phytol. 2021;229:488–500. [DOI] [PubMed] [Google Scholar]
- 46.Wu J, Kou Y, Bao J, Li Y, Tang M, Zhu X, et al. Comparative genomics identifies the Magnaporthe oryzae avirulence effector AvrPi9 that triggers Pi9-mediated blast resistance in rice. New Phytol. 2015;206:1463–75. [DOI] [PubMed] [Google Scholar]
- 47.Hu ZJ, Huang YY, Lin XY, Feng H, Zhou SX, Xie Y, et al. Loss and Natural Variations of Blast Fungal Avirulence Genes Breakdown Rice Resistance Genes in the Sichuan Basin of China. Front Plant Sci. 2022;13: 788876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khang CH, Park SY, Lee YH, Valent B, Kang S. Genome organization and evolution of the AVR-Pita avirulence gene family in the Magnaporthe grisea species complex. Mol Plant Microbe Interact. 2008;21:658–70. [DOI] [PubMed] [Google Scholar]
- 49.Nakamoto AA, Joubert PM, Krasileva K V. Intraspecific Variation of Transposable Elements Reveals Differences in the Evolutionary History of Fungal Phytopathogen Pathotypes. Genome Biol Evol. 2023;15:evad206. [DOI] [PMC free article] [PubMed]
- 50.Lin L, Sun T, Guo J, Lin L, Chen M, Wang Z, et al. Transposable elements impact the population divergence of rice blast fungus Magnaporthe oryzae. mBio. 2024;15:e0008624. [DOI] [PMC free article] [PubMed]
- 51.Thierry M, Charriat F, Milazzo J, Adreit H, Ravel S, Cros-Arteil S, et al. Maintenance of divergent lineages of the Rice Blast Fungus Pyricularia oryzae through niche separation, loss of sex and post-mating genetic incompatibilities. PLoS Pathog. 2022;18:e1010687. [DOI] [PMC free article] [PubMed]
- 52.Latorre SM, Were VM, Foster AJ, Langner T, Malmgren A, Harant A, et al. Genomic surveillance uncovers a pandemic clonal lineage of the wheat blast fungus. PLoS Biol. 2023;21:e3002052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhong Z, Chen M, Lin L, Han Y, Bao J, Tang W, et al. Population genomic analysis of the rice blast fungus reveals specific events associated with expansion of three main clades. ISME J. 2018;12:1867–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gladieux P, Condon B, Ravel S, Soanes D, Maciel JLN, Nhani A, et al. Gene flow between divergent cereal- and grass-specific lineages of the rice blast fungus Magnaporthe oryzae. mBio. 2018;9:e01219-17. [DOI] [PMC free article] [PubMed]
- 55.Ray S, Singh PK, Gupta DK, Mahato AK, Sarkar C, Rathour R, et al. Analysis of Magnaporthe oryzae genome reveals a fungal effector, which is able to induce resistance response in transgenic rice line containing resistance gene, Pi54. Front Plant Sci. 2016;7: 210426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brabham HJ, De La Cruz DG, Were V, Shimizu M, Saitoh H, Hernández-Pinzón I, et al. Barley MLA3 recognizes the host-specificity effector Pwl2 from Magnaporthe oryzae. Plant Cell. 2024;36:447–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gómez D, Cruz D La, Zdrzałek R, Banfield MJ, Talbot NJ, Moscou MJ. Molecular mimicry of a pathogen virulence target by a plant immune receptor. bioRxiv. 2024;2024-07.26.605320.
- 58.Yoshida K, Saitoh H, Fujisawa S, Kanzaki H, Matsumura H, Yoshida K, et al. Association Genetics Reveals Three Novel Avirulence Genes from the Rice Blast Fungal Pathogen Magnaporthe oryzae. Plant Cell. 2009;21:1573–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xiao G, Laksanavilat N, Cesari S, Lambou K, Baudin M, Jalilian A, et al. The unconventional resistance protein PTR recognizes the Magnaporthe oryzae effector AVR-Pita in an allele-specific manner. Nat Plants. 2024;10:994–1004. [DOI] [PubMed] [Google Scholar]
- 60.Cesari S, Thilliez G, Ribot C, Chalvon V, Michel C, Jauneau A, et al. The Rice Resistance Protein Pair RGA4/RGA5 Recognizes the Magnaporthe oryzae Effectors AVR-Pia and AVR1-CO39 by Direct Binding. Plant Cell. 2013;25:1463–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Farman ML, Leong SA. Chromosome Walking to the AVR1-CO39 Avirulence Gene of Magnaporthe grisea: Discrepancy Between the Physical and Genetic Maps. Genetics. 1998;150:1049–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Anh VL, Anh NT, Tagle AG, Vy TTP, Inoue Y, Takumi S, et al. Rmg8, a new gene for resistance to Triticum isolates of Pyricularia oryzae in hexaploid wheat. Phytopathology. 2015;105:1568–72. [DOI] [PubMed] [Google Scholar]
- 63.Asuke S, Umehara Y, Inoue Y, Vy TTP, Iwakawa M, Matsuoka Y, et al. Origin and Dynamics of Rwt6, a Wheat Gene for Resistance to Nonadapted Pathotypes of Pyricularia oryzae. Phytopathology. 2021;111:2023–9. [DOI] [PubMed] [Google Scholar]
- 64.Jeon J, Choi J, Lee GW, Park SY, Huh A, Dean RA, et al. Genome-wide profiling of DNA methylation provides insights into epigenetic regulation of fungal development in a plant pathogenic fungus. Magnaporthe oryzae Sci Rep. 2015;5:8567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abraham LN, Oggenfuss U, Croll D. Population-level transposable element expression dynamics influence trait evolution in a fungal crop pathogen. mBio. 2024;15:e02840-23. [DOI] [PMC free article] [PubMed]
- 66.Le N-V, Charriat F, Gracy J, Cros-Arteil S, Ravel S, Veillet F, et al. Adaptive evolution in virulence effectors of the rice blast fungus Pyricularia oryzae. PLoS Pathog. 2023;19: e1011294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vy TTP, Inoue Y, Asuke S, Chuma I, Nakayashiki H, Tosa Y. The ACE1 secondary metabolite gene cluster is a pathogenicity factor of wheat blast fungus. Commun Biol. 2024;7:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dean RA, Talbot NJ, Ebbole DJ, Farman ML, Mitchell TK, Orbach MJ, et al. The genome sequence of the rice blast fungus Magnaporthe grisea. Nature. 2005;434:980–6. [DOI] [PubMed] [Google Scholar]
- 69.Li J, Lu L, Li C, Wang Q, Shi Z. Insertion of Transposable Elements in AVR-Pib of Magnaporthe oryzae Leading to LOSS of the Avirulent Function. Int J Mol Sci. 2023;24:15542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miki S, Matsui K, Kito H, Otsuka K, Ashizawa T, Yasuda N, et al. Molecular cloning and characterization of the AVR-Pia locus from a Japanese field isolate of Magnaporthe oryzae. Mol Plant Pathol. 2009;10:361–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Olukayode T, Quime B, Shen YC, Yanoria MJ, Zhang S, Yang J, et al. Dynamic insertion of POT3 in AvrPib prevailing in a field rice blast population in the Philippines led to the high virulence frequency against the resistance gene Pib in rice. Phytopathology. 2019;109:870–7. [DOI] [PubMed] [Google Scholar]
- 72.Fudal I, Böhnert HU, Tharreau D, Lebrun MH. Transposition of MINE, a composite retrotransposon, in the avirulence gene ACE1 of the rice blast fungus Magnaporthe grisea. Fungal Genet Biol. 2005;42:761–72. [DOI] [PubMed] [Google Scholar]
- 73.Zhou E, Jia Y, Singh P, Correll JC, Lee FN. Instability of the Magnaporthe oryzae avirulence gene AVR-Pita alters virulence. Fungal Genet Biol. 2007;44:1024–34. [DOI] [PubMed] [Google Scholar]
- 74.Joubert PM, Krasileva KV. Distinct genomic contexts predict gene presence–absence variation in different pathotypes of Magnaporthe oryzae. Genetics. 2024;226:iyae012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen QH, Wang YC, Li AN, Zhang ZG, Zheng XB. Molecular mapping of two cultivar-specific avirulence genes in the rice blast fungus Magnaporthe grisea. Mol Genet Genomics. 2007;277:139–48. [DOI] [PubMed] [Google Scholar]
- 76.Chuma I, Zhan SW, Asano S, Nga NTT, Vy TTP, Shirai M, et al. PWT1, an avirulence gene of Magnaporthe oryzae tightly linked to the rDNA Locus, is recognized by two staple crops, common wheat and barley. Phytopathology. 2010;100:436–43. [DOI] [PubMed] [Google Scholar]
- 77.Peyyala R, Farman ML. Magnaporthe oryzae isolates causing gray leaf spot of perennial ryegrass possess a functional copy of the AVR1-CO39 avirulence gene. Mol Plant Pathol. 2006;7:157–65. [DOI] [PubMed]
- 78.Yasuda N, Tsujimoto Noguchi M, Fujita Y. Partial mapping of avirulence genes AVR-Pii and AVR-Pia in the rice blast fungus Magnaporthe oryzae. Can J Plant Path. 2006;28:494–8. [Google Scholar]
- 79.Sornkom W, Miki S, Takeuchi S, Abe A, Asano K, Sone T. Fluorescent reporter analysis revealed the timing and localization of AVR-Pia expression, an avirulence effector of Magnaporthe oryzae. Mol Plant Pathol. 2017;18:1138–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Han J, Wang X, Wang F, Zhao Z, Li G, Zhu X, et al. The Fungal Effector Avr-Pita Suppresses Innate Immunity by Increasing COX Activity in Rice Mitochondria. Rice. 2021;14:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Khang CH, Berruyer R, Giraldo MC, Kankanala P, Park SY, Czymmek K, et al. Translocation of Magnaporthe oryzae Effectors into Rice Cells and Their Subsequent Cell-to-Cell Movement. Plant Cell. 2012;22:1388–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Croll D, McDonald BA. The Accessory Genome as a Cradle for Adaptive Evolution in Pathogens. PLoS Pathog. 2012;8: e1002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gourlie R, McDonald M, Hafez M, Ortega-Polo R, Low KE, Abbott DW, et al. The pangenome of the wheat pathogen Pyrenophora tritici-repentis reveals novel transposons associated with necrotrophic effectors ToxA and ToxB. BMC Biol. 2022;20:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Oggenfuss U, Badet T, Wicker T, Hartmann FE, Singh NK, Abraham L, et al. A population-level invasion by transposable elements triggers genome expansion in a fungal pathogen. Elife. 2021;10:e69249. [DOI] [PMC free article] [PubMed]
- 85.Shirke MD, Mahesh HB, Gowda M. Genome-Wide Comparison of Magnaporthe Species Reveals a Host-Specific Pattern of Secretory Proteins and Transposable Elements. PLoS ONE. 2016;11: e0162458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen S, Zhou Y, Chen Y, Gu J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34:i884–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19:455–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Simão FA, Waterhouse RM, Ioannidis P, Kriventseva EV, Zdobnov EM. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics. 2015;31:3210–2. [DOI] [PubMed] [Google Scholar]
- 89.Gurevich A, Saveliev V, Vyahhi N, Tesler G. QUAST: quality assessment tool for genome assemblies. Bioinformatics. 2013;29:1072–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gomez-Luciano LB, Tsai IJ, Chuma I, Tosa Y, Chen YH, Li JY, et al. Blast Fungal Genomes Show Frequent Chromosomal Changes, Gene Gains and Losses, and Effector Gene Turnover. Mol Biol Evol. 2019;36:1148–61. [DOI] [PubMed] [Google Scholar]
- 91.Peng Z, Oliveira-Garcia E, Lin G, Hu Y, Dalby M, Migeon P, et al. Effector gene reshuffling involves dispensable mini-chromosomes in the wheat blast fungus. PLoS Genet. 2019;15:e1008272. [DOI] [PMC free article] [PubMed]
- 92.Liu S, Lin G, Ramachandran SR, Daza LC, Cruppe G, Tembo B, et al. Rapid mini-chromosome divergence among fungal isolates causing wheat blast outbreaks in Bangladesh and Zambia. New Phytol. 2024;241:1266–76. [DOI] [PubMed] [Google Scholar]
- 93.Stanke M, Steinkamp R, Waack S, Morgenstern B. AUGUSTUS: a web server for gene finding in eukaryotes. Nucleic Acids Res. 2004;32:W309–W312. [DOI] [PMC free article] [PubMed]
- 94.Emms DM, Kelly S. OrthoFinder: Phylogenetic orthology inference for comparative genomics. Genome Biol. 2019;20:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Borowiec ML. AMAS: a fast tool for alignment manipulation and computing of summary statistics. PeerJ. 2016;4:e1660. [DOI] [PMC free article] [PubMed]
- 96.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, et al. BLAST+: Architecture and applications. BMC Bioinformatics. 2009;10:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Guy L, Kultima JR, Andersson SGE, Quackenbush J. genoPlotR: comparative gene and genome visualization in R. Bioinformatics. 2010;26:2334–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sampaio M, Croll D. Transposable elements create distinct genomic niches for effector evolution among Magnaporthe oryzae lineages. Zenodo. 2025. 10.5281/zenodo.15875042.
- 100.Sugihara Y, Abe Y, Takagi H, Abe A, Shimizu M, Ito K, et al. Disentangling the complex gene interaction networks between rice and the blast fungus identifies a new pathogen effector. PLoS Biol. 2023;21: e3001945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tosa Y, Osue J, Eto Y, Oh HS, Nakayashiki H, Mayama S, et al. Evolution of an avirulence gene, AVR1-CO39, concomitant with the evolution and differentiation of Magnaporthe oryzae. Mol Plant Microbe Interact. 2005;18:1148–60. [DOI] [PubMed]
- 102.Farman ML, Eto Y, Nakao T, Tosa Y, Nakayashiki H, Mayama S, et al. Analysis of the structure of the AVR1-CO39 avirulence locus in virulent rice-infecting isolates of Magnaporthe grisea. Mol Plant Microbe Interact. 2002;15:6–16. [DOI] [PubMed] [Google Scholar]
- 103.Ribot C, Césari S, Abidi I, Chalvon V, Bournaud C, Vallet J, et al. The Magnaporthe oryzae effector AVR1-CO39 is translocated into rice cells independently of a fungal-derived machinery. Plant J. 2013;74:1–12. [DOI] [PubMed] [Google Scholar]
- 104.Huang J, Si W, Deng Q, Li P, Yang S. Rapid evolution of avirulence genes in rice blast fungus Magnaporthe oryzae. BMC Genet. 2014;15:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bao J, Chen M, Zhong Z, Tang W, Lin L, Zhang X, et al. PacBio Sequencing Reveals Transposable Elements as a Key Contributor to Genomic Plasticity and Virulence Variation in Magnaporthe oryzae. Mol Plant. 2017;10:1465–8. [DOI] [PubMed] [Google Scholar]
- 106.Rahnama M, Condon B, Ascari JP, Dupuis JR, Del Ponte EM, Pedley KF, et al. Recent co-evolution of two pandemic plant diseases in a multi-hybrid swarm. Nat Ecol Evol. 2023;7(12):2055–66. [DOI] [PMC free article] [PubMed]
- 107.Onaga G, Suktrakul W, Wanjiku M, Lorenzo Quibod I, Baka J-4, Entfellner D, et al. Magnaporthe oryzae populations in Sub-Saharan Africa are diverse and show signs of local adaptation. bioRxiv. 2020;11:17.377325.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figures S1-S2. Figure S1: Correlation between total genome assembly size and N50 metrics for de novo assembled genomes. Figure S2: Presence of TEs in a +/- 1000 bp window surrounding AVR loci in Setaria (MoS), Eleusine (MoE), Avena (MoA), Lolium (MoL), Leersia (MoLe), Digitaria (MoD), Cenchrus (MoC), and Pennisetum (MoP) pathotype isolates.
Additional file 2: Tables S1-S5. Table S1: Analyzed Magnaporthe oryzae isolates sequenced by Illumina paired-end whole-genome sequencing. Table S2: Analyzed reference-quality Magnaporthe genomes. Table S3: Blast hit statistics to identify the different AVR genes in the Magnaporthe genomes (draft and reference genomes). Table S4: Transposable elements (TEs) detected near each AVR. Table S5: ANOVA of TE occupancy near AVRs.
Data Availability Statement
The data analyzed in the frame of this study were retrieved from NCBI repositories as indicated in the Additional File 2. Draft genome assembly data, a Magnaporthe spp. full-size phylogenetic tree with AVR presence/absence screening is available from Zenodo (10.5281/zenodo.15875042) [107].