Abstract
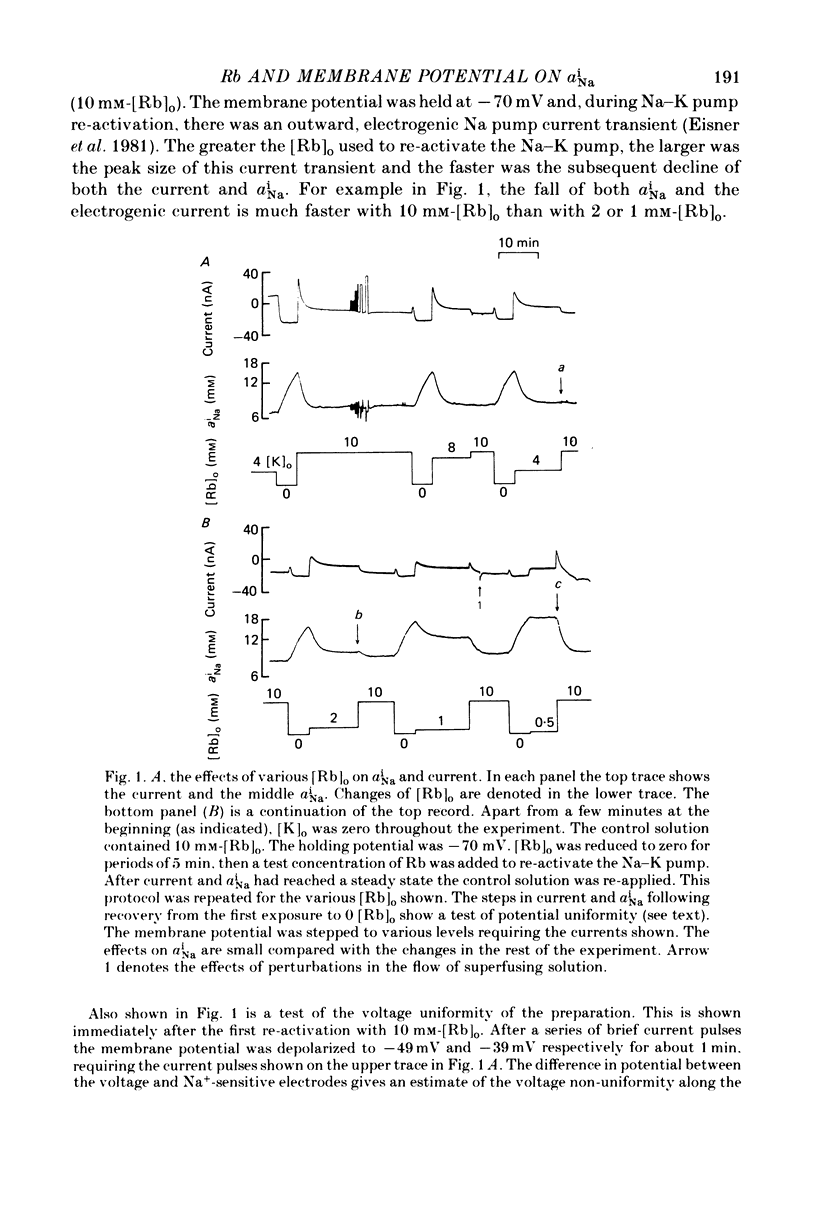
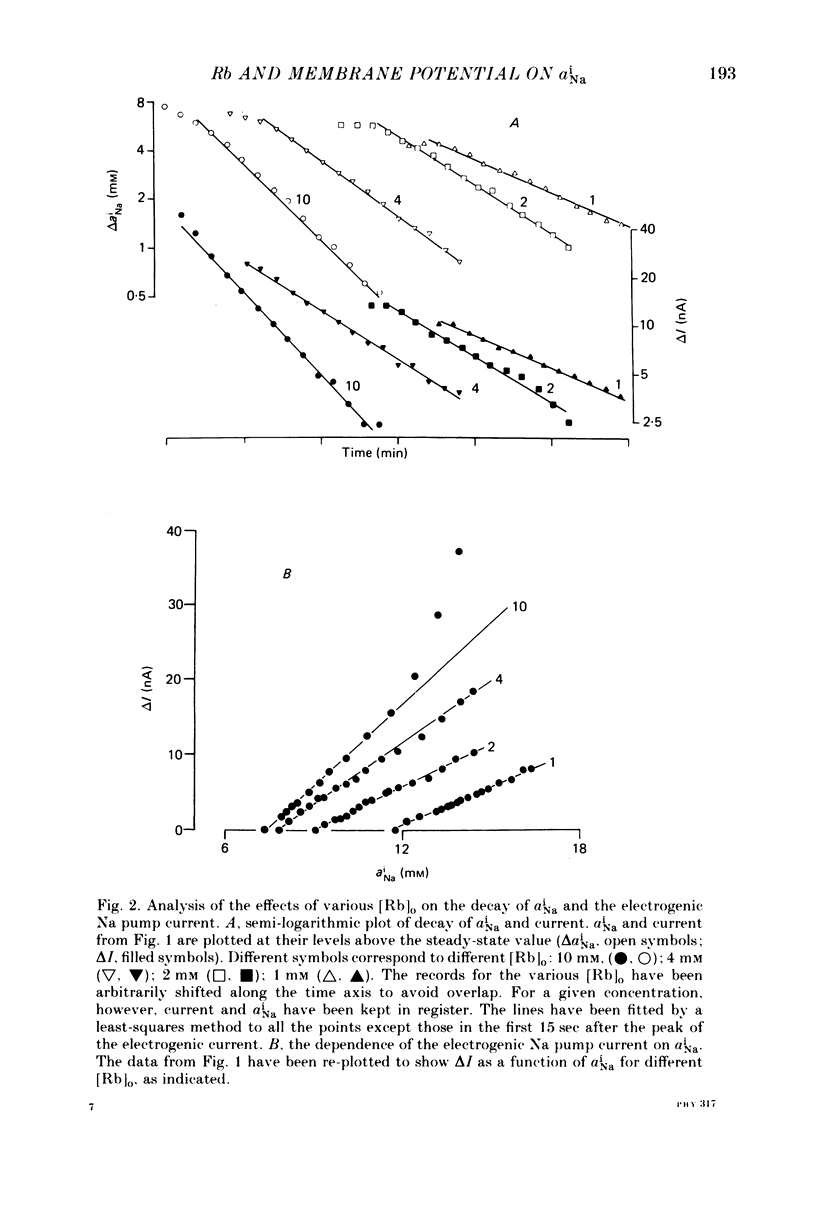
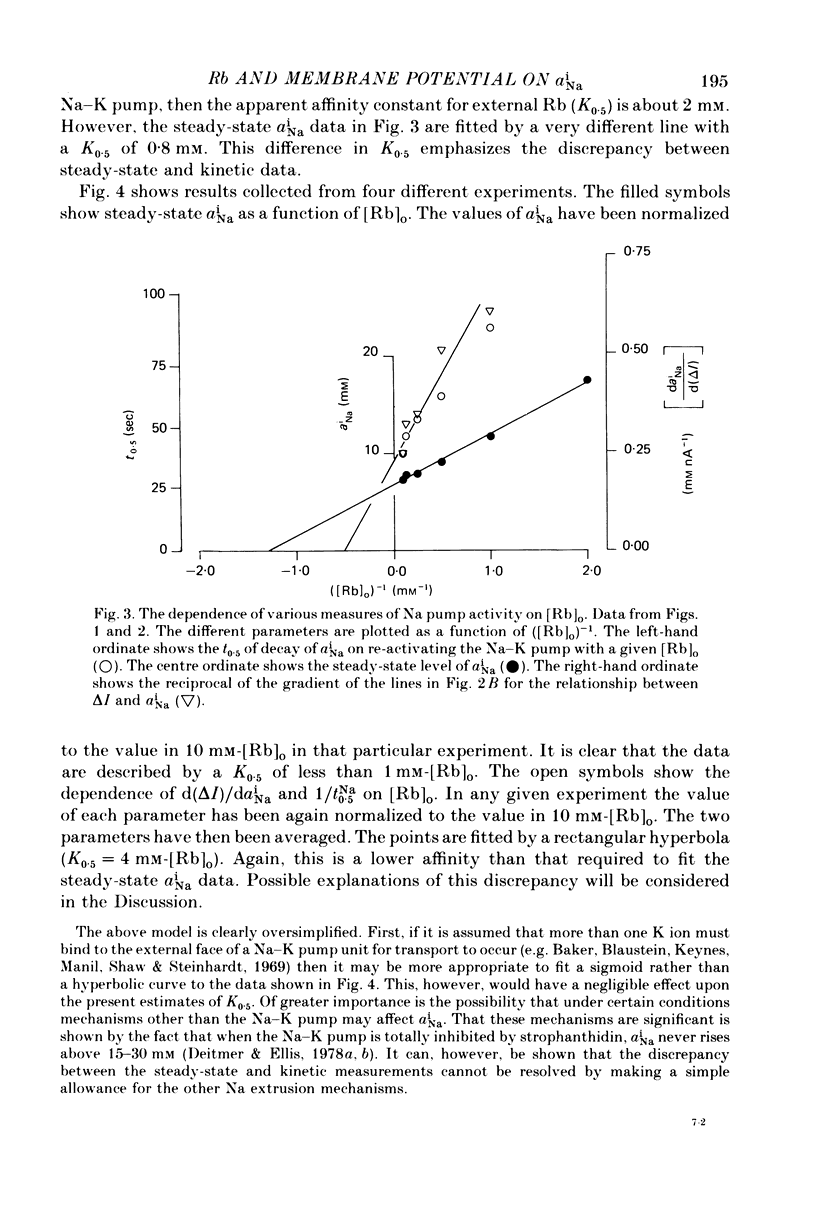
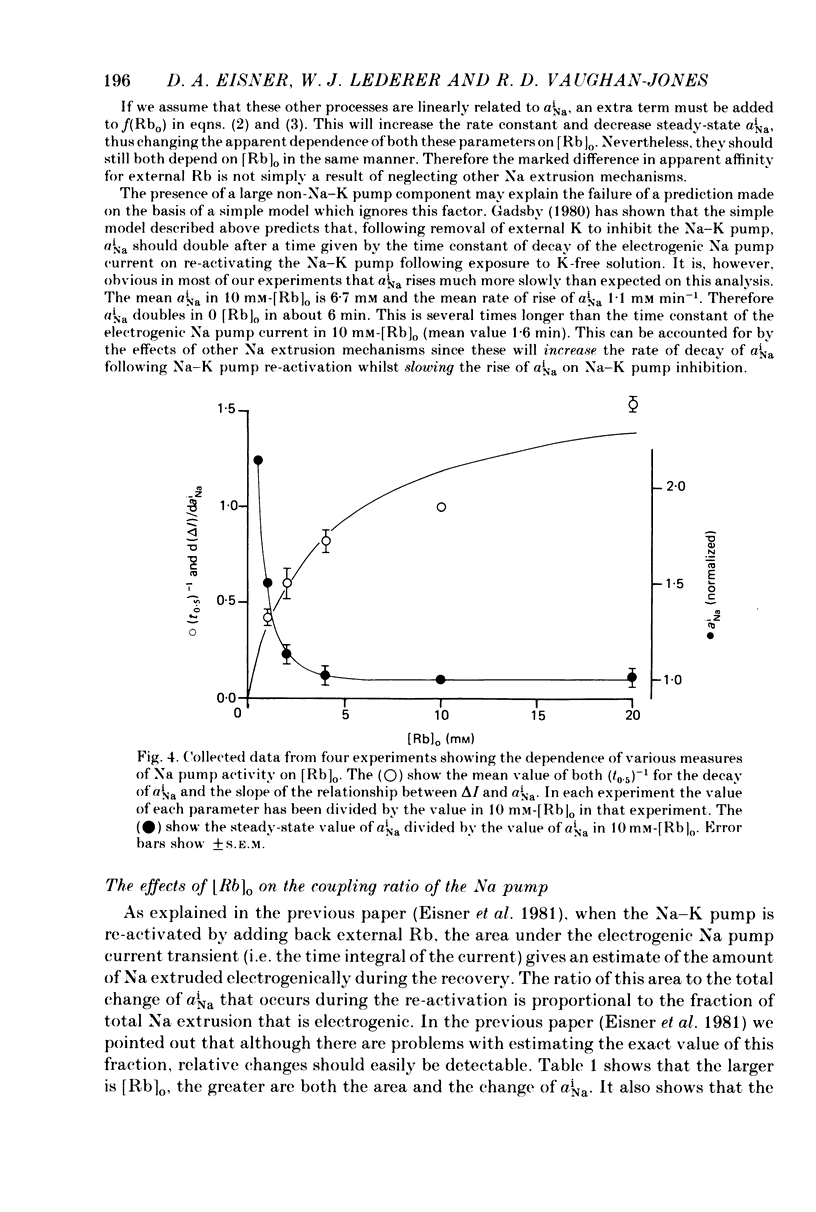
1. Intracellular Na activity, aiNa, was measured in voltage-clamped sheep cardiac Purkinke fibres. 2. Increasing [Rb]0 from 0 to 4 mM in K-free solutions (at a fixed membrane potential) decreased aiNa. Further increases of [Rb]0 (up to 20 mM) had little or no effect. 3. Following exposure to Rb-free, K-free solution, the addition of a test concentration of Rb produced an exponential decrease of aiNa. The rate constant of decay of aiNa increased with increasing [Rb]0 over the measured range (0-20 mM). 4. The accompanying electrogenic Na pump current transient decayed with the same rate constant as aiNa over the range of [Rb]0 examined. During this decay the electrogenic Na pump current was a linear function of aiNa. Increasing [Rb]0 increased the steepness of the dependence of the electrogenic current on aiNa. 5. A constant fraction of the net Na efflux was electrogenic. This fraction was not affected by varying [Rb]0 over the range 0-20 mM. 6. Using a simple model, it is shown that the dependence of steady-state aiNa on [Rb]0 is half-saturated by less than 1 mM-[Rb]0. The rate constant of decay of aiNa and the slope of the relationship between electrogenic Na pump current and aiNa are, however, better fitted with a lower affinity for Rb (K0.5 = 4 mM-[Rb]0). 7. Depolarizing the membrane potential with the voltage clamp decreased aiNa; hyperpolarization increased it. These effects persisted in the presence of 10(-5) M-strophanthidin. An effect of membrane potential on the net passive Na influx can account for the observations. 8. The effects of membrane potential on the net passive Na influx were examined by measuring the maximum rate of rise of aiNa at different holding potentials after inhibiting the Na-K pump in a K-free, Rb-free solution. Depolarization decreased the Na influx. 9. Using the constant field equation, the net passive Na influx was used to estimate the apparent Na permeability coefficient, PNa. This was between 0.8 x 10(-8) and 1.5 x 10(-8) cm sec-1.
Full text
PDF



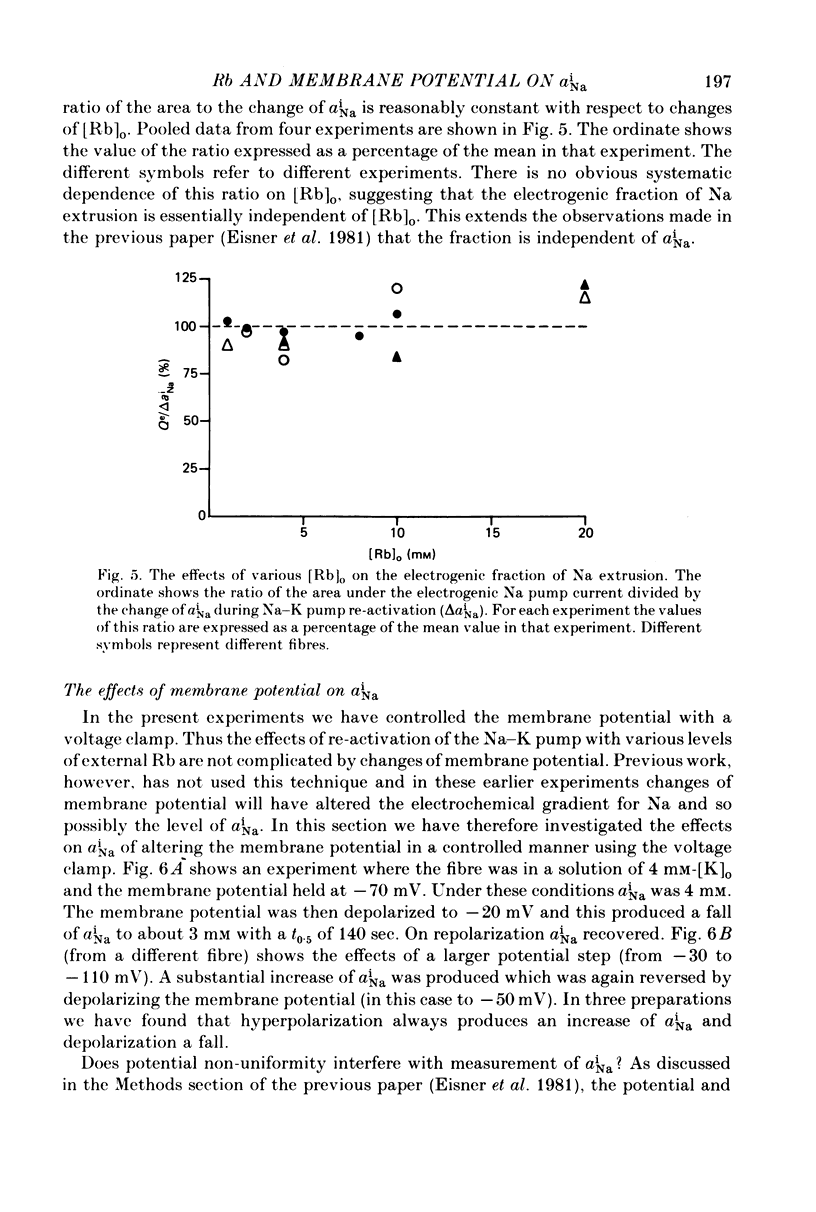
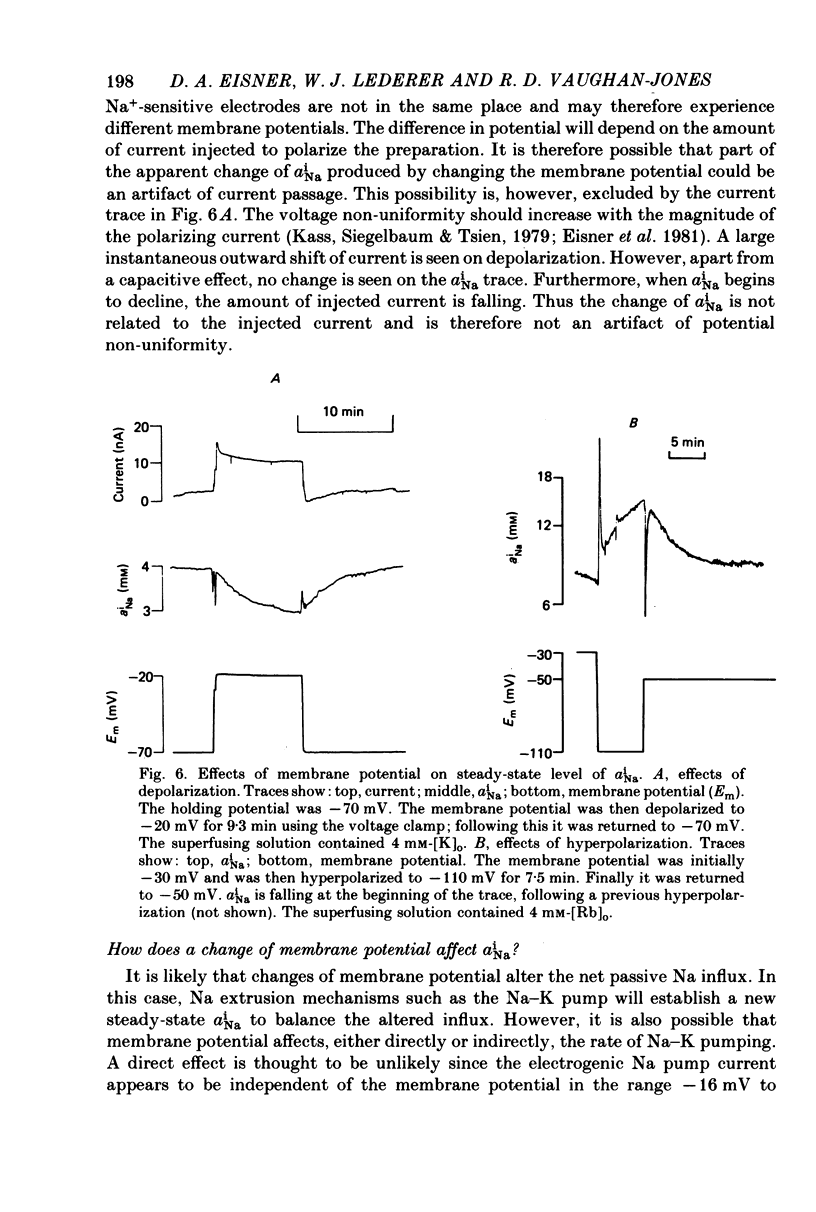
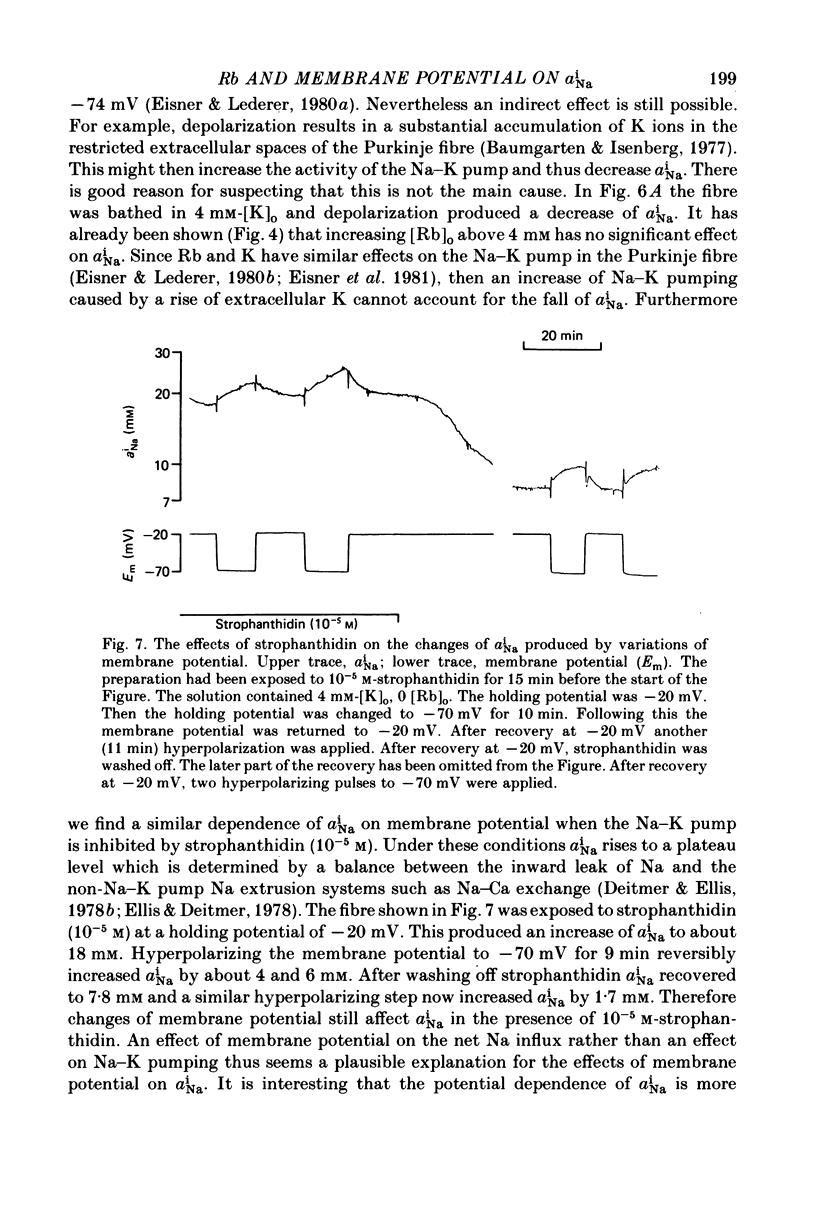
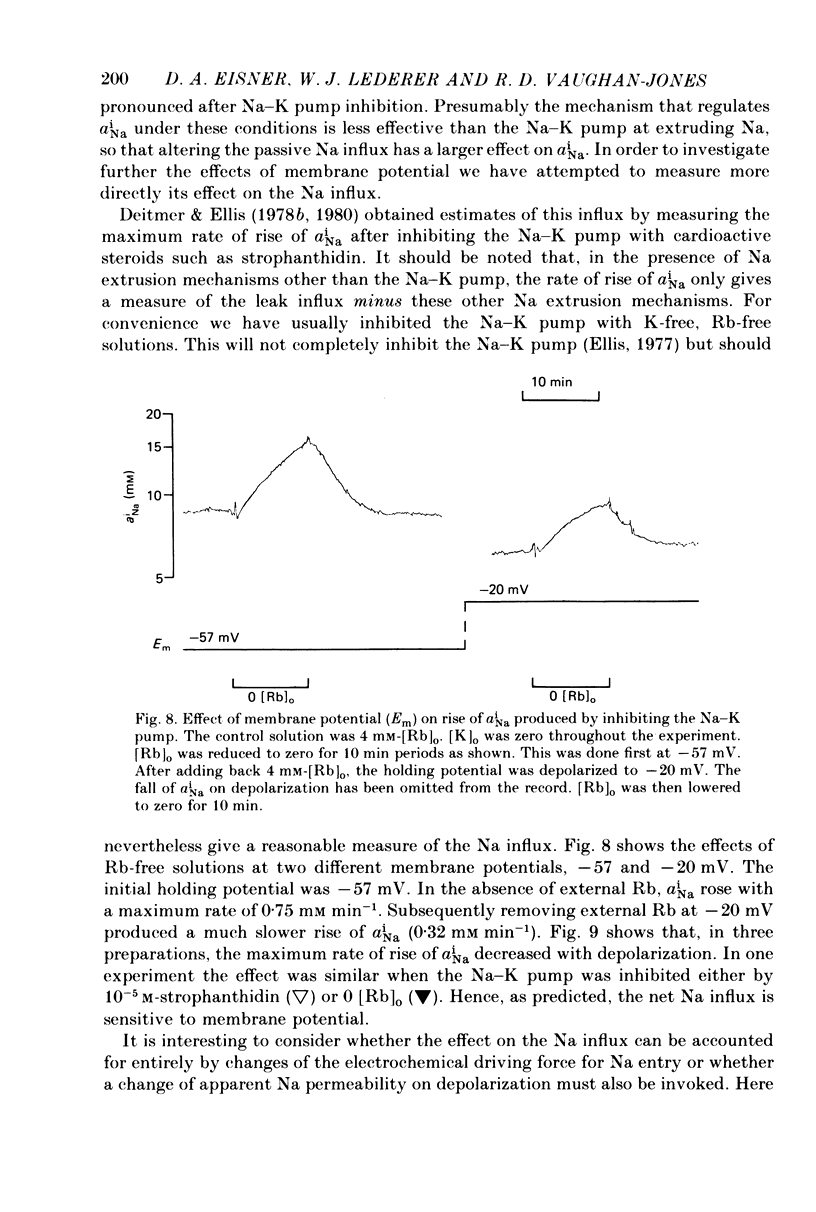




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abercrombie R. F., de Weer P. Electric current generated by squid giant axon sodium pump: external K and internal ADP effects. Am J Physiol. 1978 Jul;235(1):C63–C68. doi: 10.1152/ajpcell.1978.235.1.C63. [DOI] [PubMed] [Google Scholar]
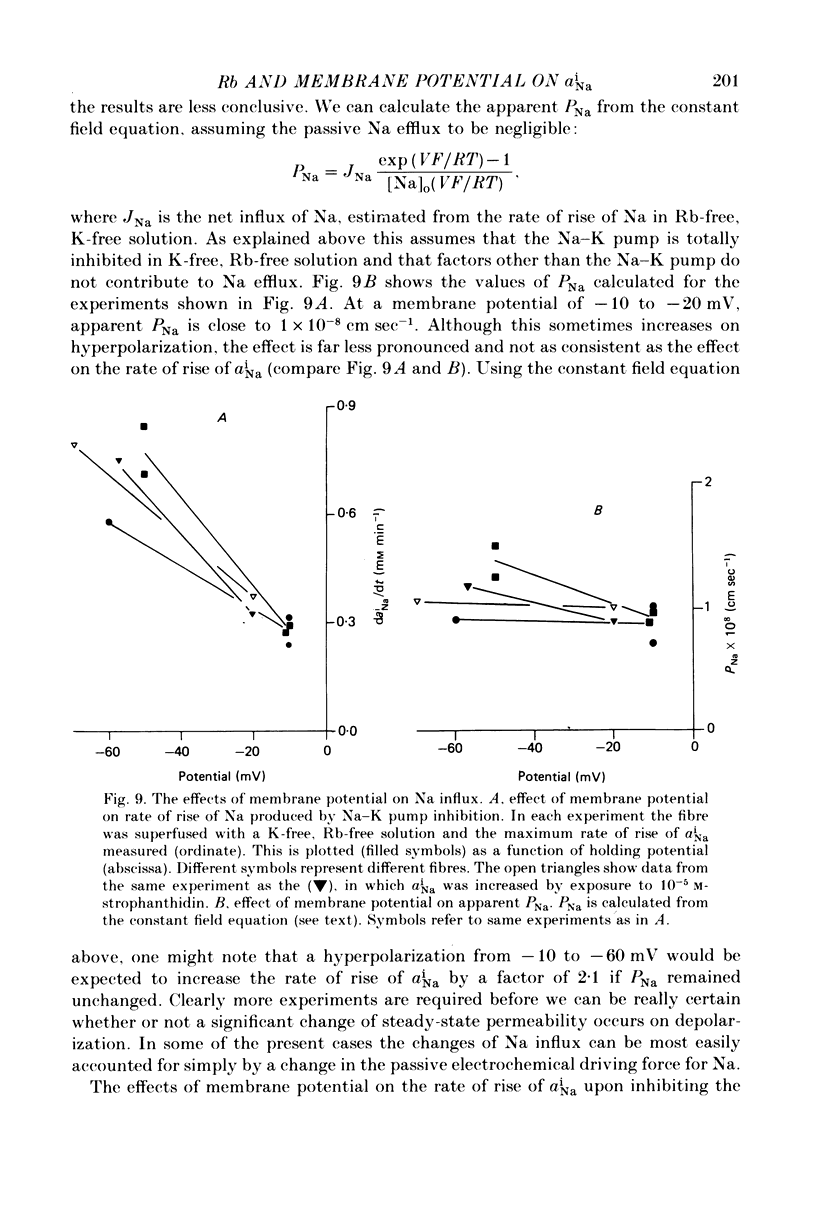
- Attwell D., Cohen I., Eisner D., Ohba M., Ojeda C. The steady state TTX-sensitive ("window") sodium current in cardiac Purkinje fibres. Pflugers Arch. 1979 Mar 16;379(2):137–142. doi: 10.1007/BF00586939. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Blaustein M. P., Keynes R. D., Manil J., Shaw T. I., Steinhardt R. A. The ouabain-sensitive fluxes of sodium and potassium in squid giant axons. J Physiol. 1969 Feb;200(2):459–496. doi: 10.1113/jphysiol.1969.sp008703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgarten C. M., Isenberg G. Depletion and accumulation of potassium in the extracellular clefts of cardiac Purkinje fibers during voltage clamp hyperpolarization and depolarization. Pflugers Arch. 1977 Mar 11;368(1-2):19–31. doi: 10.1007/BF01063450. [DOI] [PubMed] [Google Scholar]
- Deitmer J. W., Ellis D. Changes in the intracellular sodium activity of sheep heart Purkinje fibres produced by calcium and other divalent cations. J Physiol. 1978 Apr;277:437–453. doi: 10.1113/jphysiol.1978.sp012283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitmer J. W., Ellis D. The intracellular sodium activity of cardiac Purkinje fibres during inhibition and re-activation of the Na-K pump. J Physiol. 1978 Nov;284:241–259. doi: 10.1113/jphysiol.1978.sp012539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitmer J. W., Ellis D. The intracellular sodium activity of sheep heart Purkinje fibres: effects of local anaesthetics and tetrodotoxin. J Physiol. 1980 Mar;300:269–282. doi: 10.1113/jphysiol.1980.sp013161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J. Characterization of the electrogenic sodium pump in cardiac Purkinje fibres. J Physiol. 1980 Jun;303:441–474. doi: 10.1113/jphysiol.1980.sp013298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J. The relationship between sodium pump activity and twitch tension in cardiac Purkinje fibres. J Physiol. 1980 Jun;303:475–494. doi: 10.1113/jphysiol.1980.sp013299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J., Vaughan-Jones R. D. The dependence of sodium pumping and tension on intracellular sodium activity in voltage-clamped sheep Purkinje fibres. J Physiol. 1981 Aug;317:163–187. doi: 10.1113/jphysiol.1981.sp013819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis D., Deitmer J. W. The relationship between the intra- and extracellular sodium activity of sheep heart Purkinje fibres during inhibition of the Na-K pump. Pflugers Arch. 1978 Nov 30;377(3):209–215. doi: 10.1007/BF00584274. [DOI] [PubMed] [Google Scholar]
- Ellis D. The effects of external cations and ouabain on the intracellular sodium activity of sheep heart Purkinje fibres. J Physiol. 1977 Dec;273(1):211–240. doi: 10.1113/jphysiol.1977.sp012090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLYNN I. M. Sodium and potassium movements in human red cells. J Physiol. 1956 Nov 28;134(2):278–310. doi: 10.1113/jphysiol.1956.sp005643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glitsch H. G., Grabowski W., Thielen J. Activation of the electrogenic sodium pump in guinea-pig atria by external potassium ions. J Physiol. 1978 Mar;276:515–524. doi: 10.1113/jphysiol.1978.sp012250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass R. S., Siegelbaum S. A., Tsien R. W. Three-micro-electrode voltage clamp experiments in calf cardiac Purkinje fibres: is slow inward current adequately measured? J Physiol. 1979 May;290(2):201–225. doi: 10.1113/jphysiol.1979.sp012768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman E. M. Effect of external potassium on the coupled sodium: potassium transport ratio of axons. Pflugers Arch. 1979 Jan 31;378(3):243–249. doi: 10.1007/BF00592742. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. Intracellular sodium activity and the sodium pump in snail neurones. J Physiol. 1972 Jan;220(1):55–71. doi: 10.1113/jphysiol.1972.sp009694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdicombe J. H. Ouabain-sensitive ion fluxes in the smooth muscle of the guinea-pig's taenia coli. J Physiol. 1977 Apr;266(2):235–254. doi: 10.1113/jphysiol.1977.sp011766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hertog A. Some further observations on the electrogenic sodium pump in non-myelinated nerve fibres. J Physiol. 1973 Jun;231(3):493–509. doi: 10.1113/jphysiol.1973.sp010245. [DOI] [PMC free article] [PubMed] [Google Scholar]


