Abstract
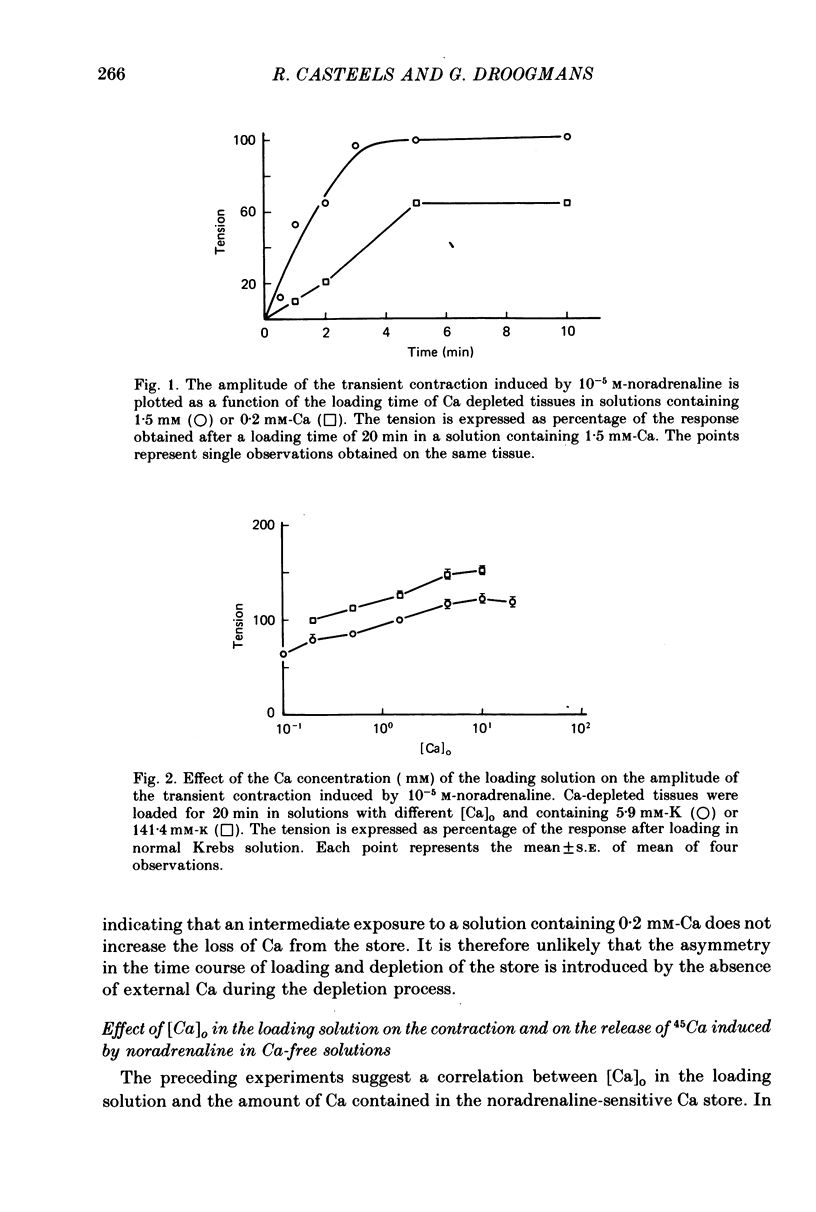
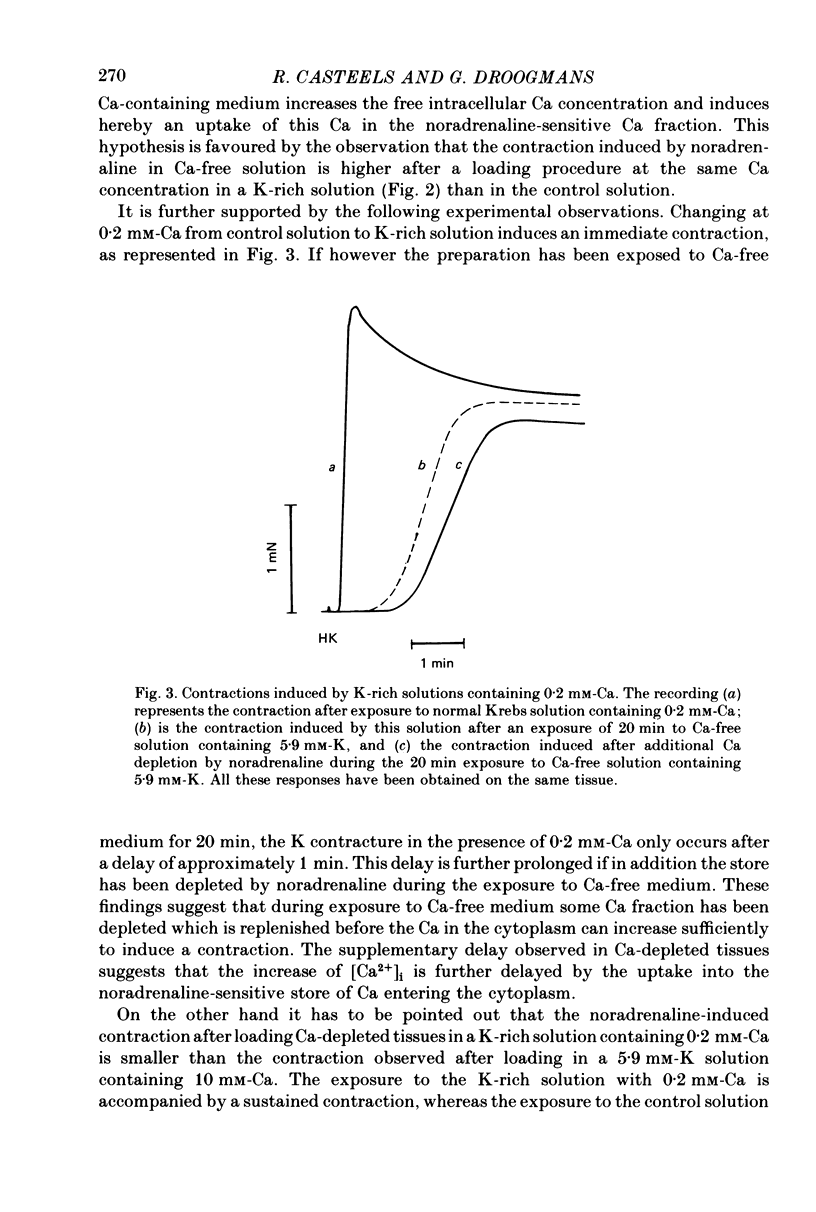
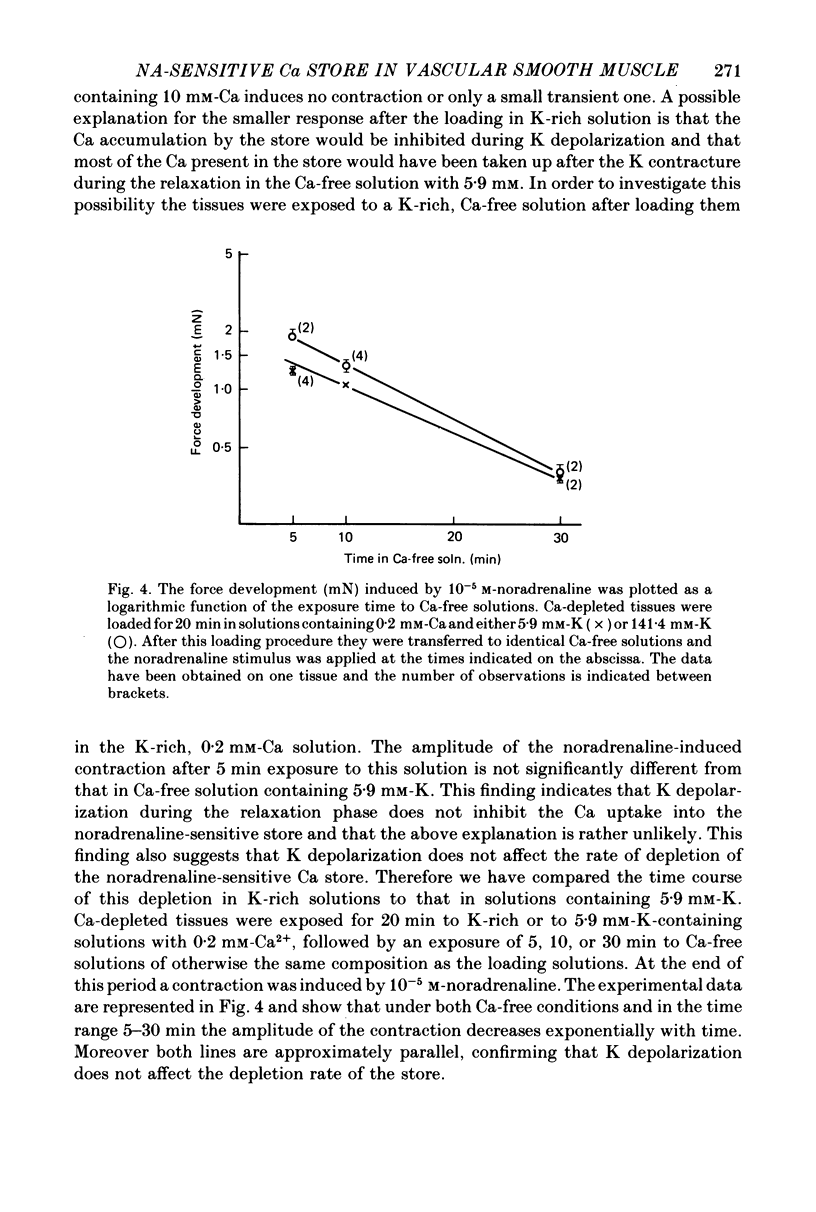
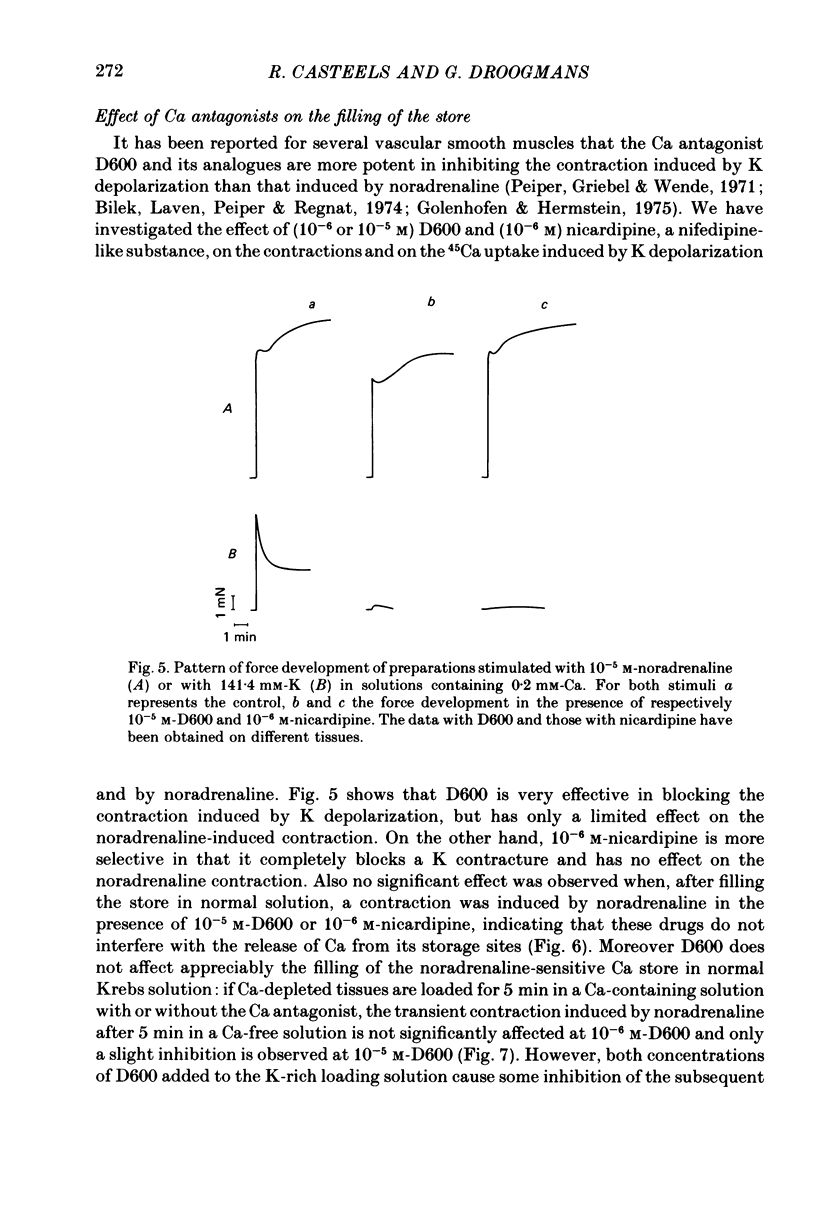
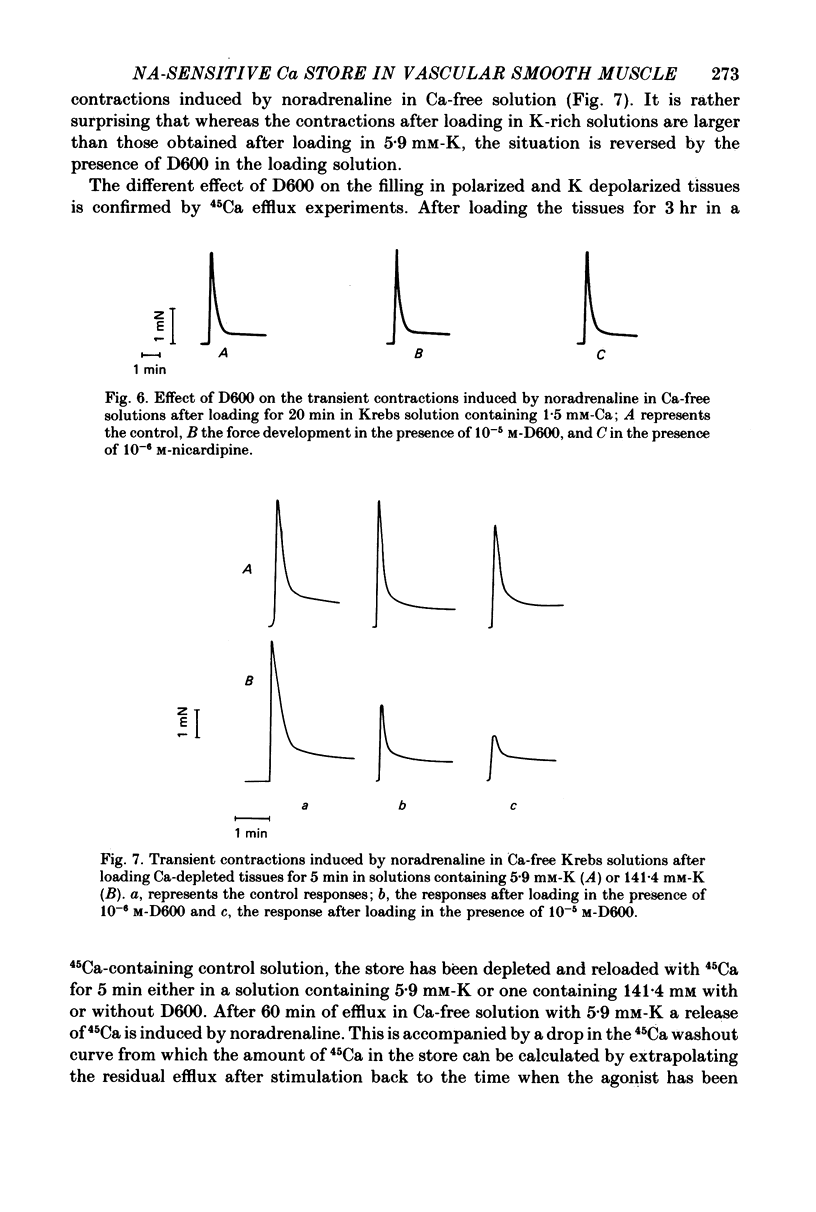
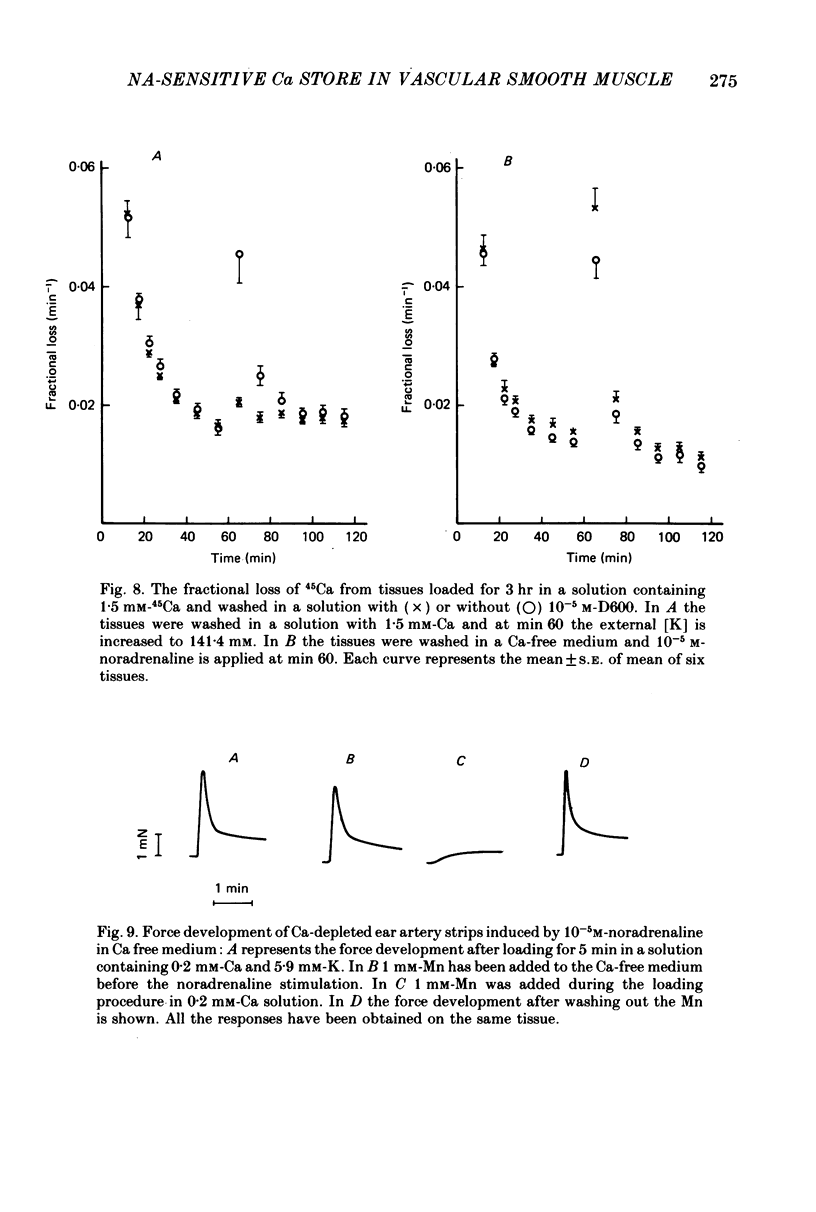
1. The amplitude of the noradrenaline-sensitive Ca stores has been estimated by measuring the amplitude of the transient contraction induced by the agonist in Ca-free solution. or by measuring the amount of 45Ca released under these conditions. 2. The rate of filling of this store after depletion is much faster than the rate of depletion in Ca-free solution, and depends on [Ca]o in the bathing solution. The degree of filling also depends on [Ca]o. 3. At the same [Ca]o the degree of filling is higher in K-depolarized tissues than in control tissues. However at 10 mM-[Ca]o and 5.9 mM-K the amount of Ca taken up by the store is larger than that after loading in 0.2 mM-Ca and 141.4 mM-K, although the tissues remain relaxed during loading at 5.9 mM-K and contracted at 141.4 mM-K. 4. The Ca antagonists D600 and nicardipine selectively block the contraction induced by K depolarization, but do not affect appreciably the noradrenaline-induced contraction. 5. The filling of the store is not significantly reduced by the presence of the Ca antagonists in solutions containing 5.9 mM-K. However these antagonists reduce the degree of filling in K-rich loading solution to a level which is lower than that observed in the control. 6. Mn blocks both the contraction induced by K-rich solution and the tonic component of the noradrenaline-induced contraction and its also inhibits filling of the store. 7. The results suggest that the filling of the store under physiological conditions occurs by a direct pathway between the store and the extracellular medium.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bilek I., Laven R., Peiper U., Regnat K. The effect of verapamil on the response to noradrenaline or to potassium-depolarization in isolated vascular strips. Microvasc Res. 1974 Mar;7(2):181–189. doi: 10.1016/0026-2862(74)90004-1. [DOI] [PubMed] [Google Scholar]
- Bolton T. B. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev. 1979 Jul;59(3):606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- Brading A. F., Burnett M., Sneddon P. The effect of sodium removal on the contractile response of the guinea-pig taenia coli to carbachol. J Physiol. 1980 Sep;306:411–429. doi: 10.1113/jphysiol.1980.sp013404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Kitamura K., Kuriyama H., Suzuki H. The membrane properties of the smooth muscle cells of the rabbit main pulmonary artery. J Physiol. 1977 Sep;271(1):41–61. doi: 10.1113/jphysiol.1977.sp011989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debbas G., Hoffman L., Landon E. J., Hurwitz L. Electron microscopic localization of calcium in vascular smooth muscle. Anat Rec. 1975 Aug;182(4):447–471. doi: 10.1002/ar.1091820405. [DOI] [PubMed] [Google Scholar]
- Devine C. E., Somlyo A. V., Somlyo A. P. Sarcoplasmic reticulum and excitation-contraction coupling in mammalian smooth muscles. J Cell Biol. 1972 Mar;52(3):690–718. doi: 10.1083/jcb.52.3.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droogmans G., Raeymaekers L., Casteels R. Electro- and pharmacomechanical coupling in the smooth muscle cells of the rabbit ear artery. J Gen Physiol. 1977 Aug;70(2):129–148. doi: 10.1085/jgp.70.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golenhofen K., Hermstein N. Differentiation of calcium activation mechanisms in vascular smooth muscle by selective suppression with verapamil and D 600-1. Blood Vessels. 1975;12(1):21–37. doi: 10.1159/000158036. [DOI] [PubMed] [Google Scholar]
- Karaki H., Kubota H., Urakawa N. Mobilization of stored calcium for phasic contraction induced by norepinephrine in rabbit aorta. Eur J Pharmacol. 1979 Jun 15;56(3):237–245. doi: 10.1016/0014-2999(79)90176-6. [DOI] [PubMed] [Google Scholar]
- Peiper U., Griebel L., Wende W. Activation of vascular smooth muscle of rat aorta by noradrenaline and depolarization: two different mechanisms. Pflugers Arch. 1971;330(1):74–89. doi: 10.1007/BF00588736. [DOI] [PubMed] [Google Scholar]
- Popescu L. M., Diculescu I. Calcium in smooth muscle sarcoplasmic reticulum in situ. Conventional and X-ray analytical electron microscopy. J Cell Biol. 1975 Dec;67(3):911–918. doi: 10.1083/jcb.67.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo A. P., Somlyo A. V., Shuman H. Electron probe analysis of vascular smooth muscle. Composition of mitochondria, nuclei, and cytoplasm. J Cell Biol. 1979 May;81(2):316–335. doi: 10.1083/jcb.81.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo A. V., Somlyo A. P. Strontium accumulation by sarcoplasmic reticulum and mitochondria in vascular smooth muscle. Science. 1971 Nov 26;174(4012):955–958. doi: 10.1126/science.174.4012.955. [DOI] [PubMed] [Google Scholar]
- Sugi H., Daimon T. Translocation of intracellularly stored calcium during the contraction-relaxation cycle in guinea pig taenia coli. Nature. 1977 Sep 29;269(5627):436–438. doi: 10.1038/269436a0. [DOI] [PubMed] [Google Scholar]
- Takenaka T., Usuda S., Nomura T., Maeno H., Sado T. Vasodilator profile of a new 1,4-dihydropyridine derivative, 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylic acid 3-[2-(N-benzyl-N-methylamino)]-ethyl ester 5-methyl ester hydrochloride (YC-93). Arzneimittelforschung. 1976;26(12):2172–2178. [PubMed] [Google Scholar]
- Van Breemen C., Farinas B. R., Gerba P., McNaughton E. D. Excitation-contraction coupling in rabbit aorta studied by the lanthanum method for measuring cellular calcium influx. Circ Res. 1972 Jan;30(1):44–54. doi: 10.1161/01.res.30.1.44. [DOI] [PubMed] [Google Scholar]
- Zucker R., Nolte J. Light-induced calcium release in a photosensitive vertebrate smooth muscle. Nature. 1978 Jul 6;274(5666):78–80. doi: 10.1038/274078a0. [DOI] [PubMed] [Google Scholar]


