Abstract
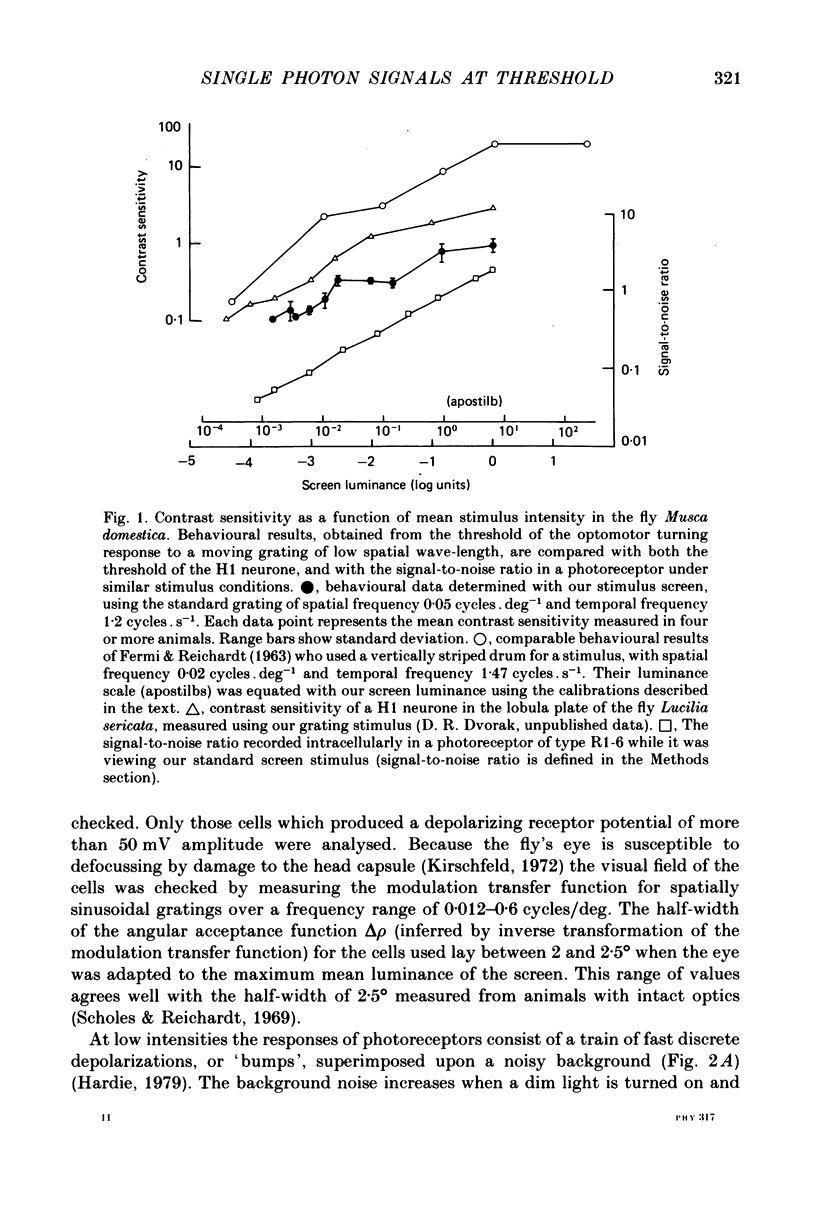
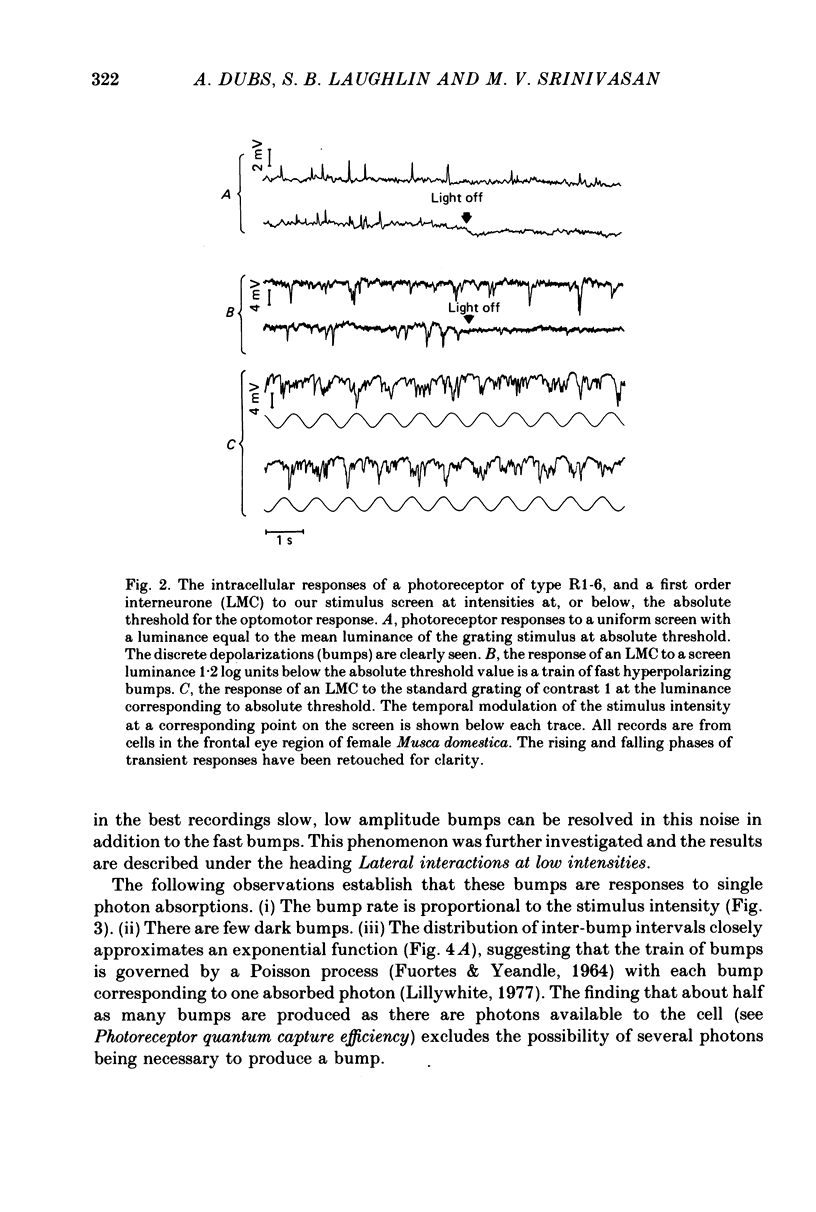
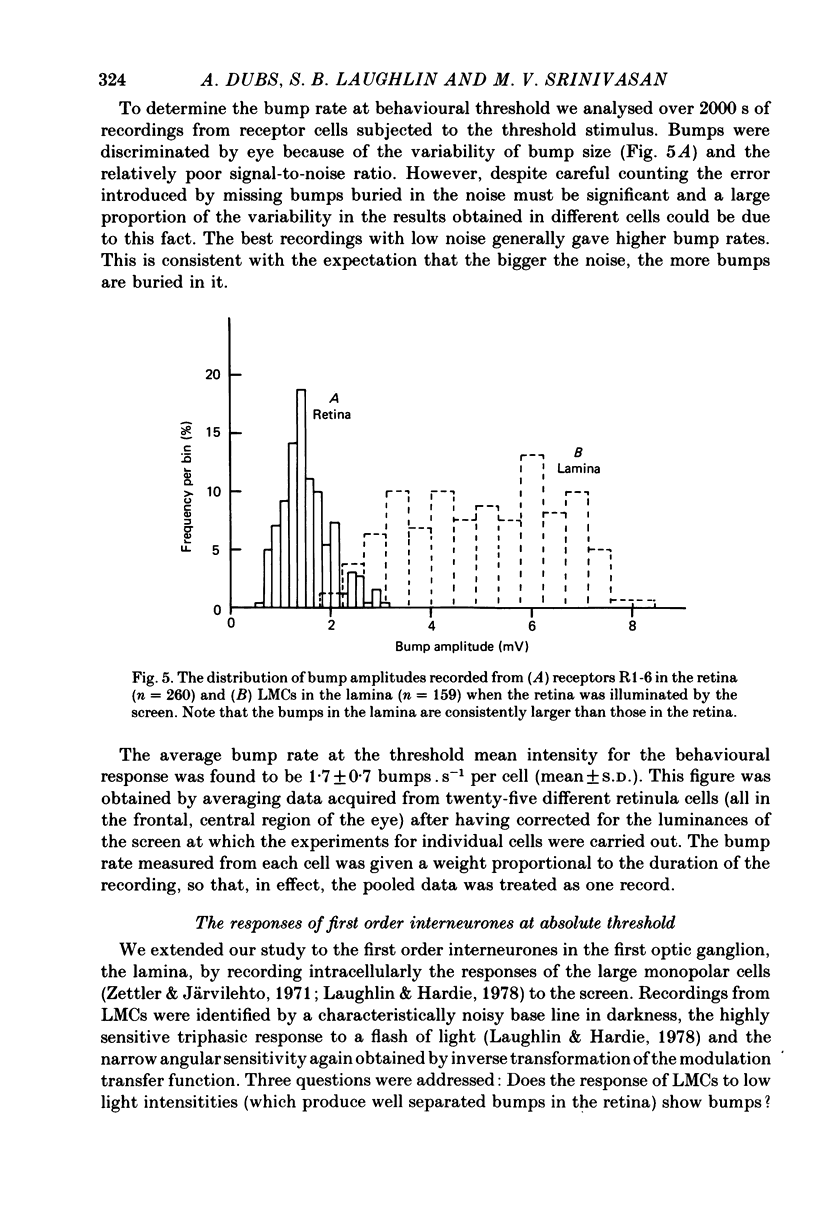
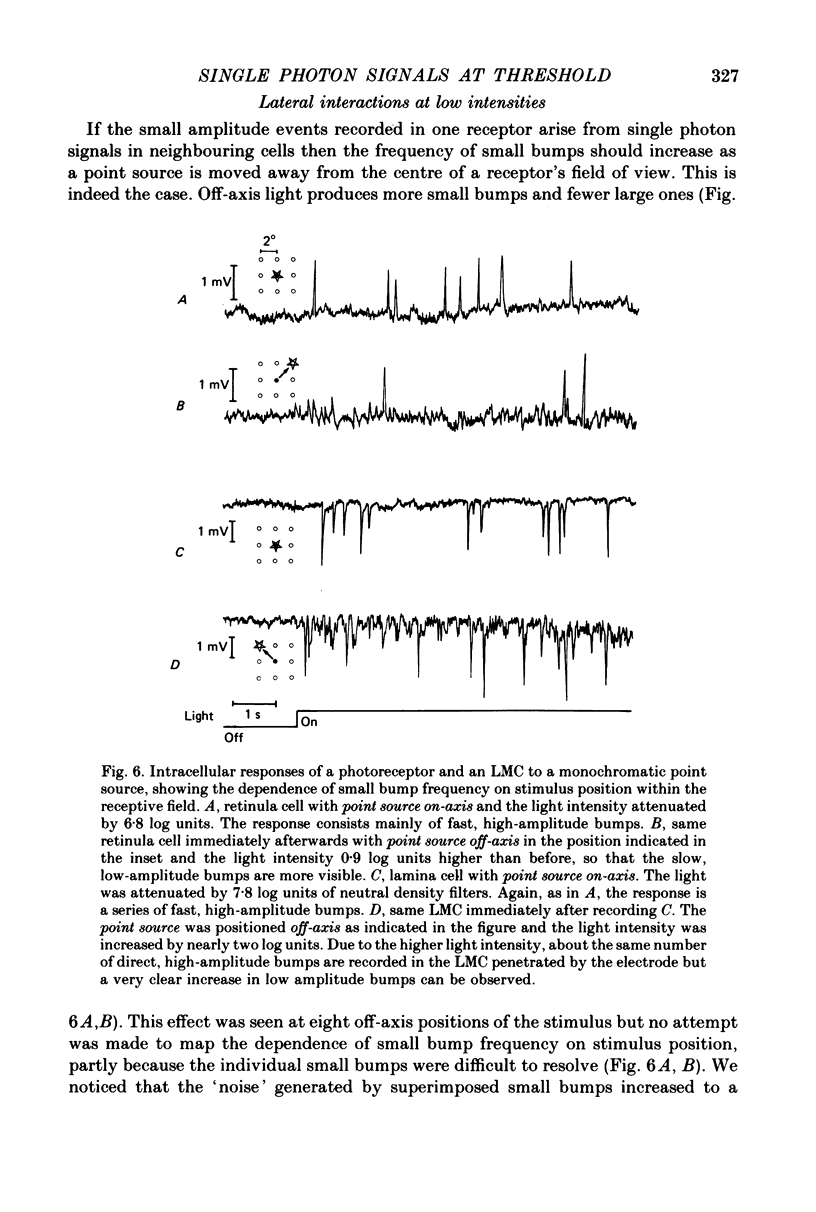
1. The contrast sensitivity of the optomotor response of the fly Musca domestica was measured using a moving sinusoidal grating as the stimulus. In parallel experiments intracellular recordings were made from photoreceptors and first order visual interneurones to to determine their responses to the same threshold stimuli. Measurements of the spatial modulation transfer function for photoreceptors confirm that the optics of the eye were intact during recordings. 2. At the lowest intensity at which one can obtain an optomotor response, the photoreceptor signal is a train of discrete depolarizations, or bumps. With constant intensity stimuli, the temporal distribution of bumps followed the Poisson distribution with a mean rate of proportional to luminance. The mean bump rate at the threshold intensity for a behavioural response is 1.7 +/- 0.7 s-1 (mean +/- S.D., n = 25). 3. Calibrations and the statistical properties of the bump train indicate that a bump represents one effective photon, implying that the bump : photon ratios are quantum capture efficiencies. 4. At low intensities the first order interneurones (the large monopolar cells or LMCs) show hyperpolarizing bumps each triggered by a receptor bump. Using a point source stimulus, centred in the field of view, the LMC bump rate is six times that in a single receptor viewing the same stimulus, as expected from the known projection of six receptor axons to each LMC. When using an extended stimulus (the grating), the bump rate is 18-20 times that in receptors. Comparison with earlier work suggests that this increased lateral summation of receptor inputs to LMCs only occurs at very low intensities. 5. In both receptor and LMCs the amplitudes and wave forms of bumps depend upon the position of a point source stimulus within the field of view. With the light in the periphery of the field the bumps are smaller and slower than when the light is in the centre. This difference in response suggests that spatial stimulation is brought about by lateral interactions, possibly between receptors. 6. At higher mean intensities the signal-to-noise ratios in receptors responding to the appropriate threshold stimuli increase with intensity. This is suggestive of a decrease in the extent of spatial and/or temporal summation in the optomotor pathway.
Full text
PDF






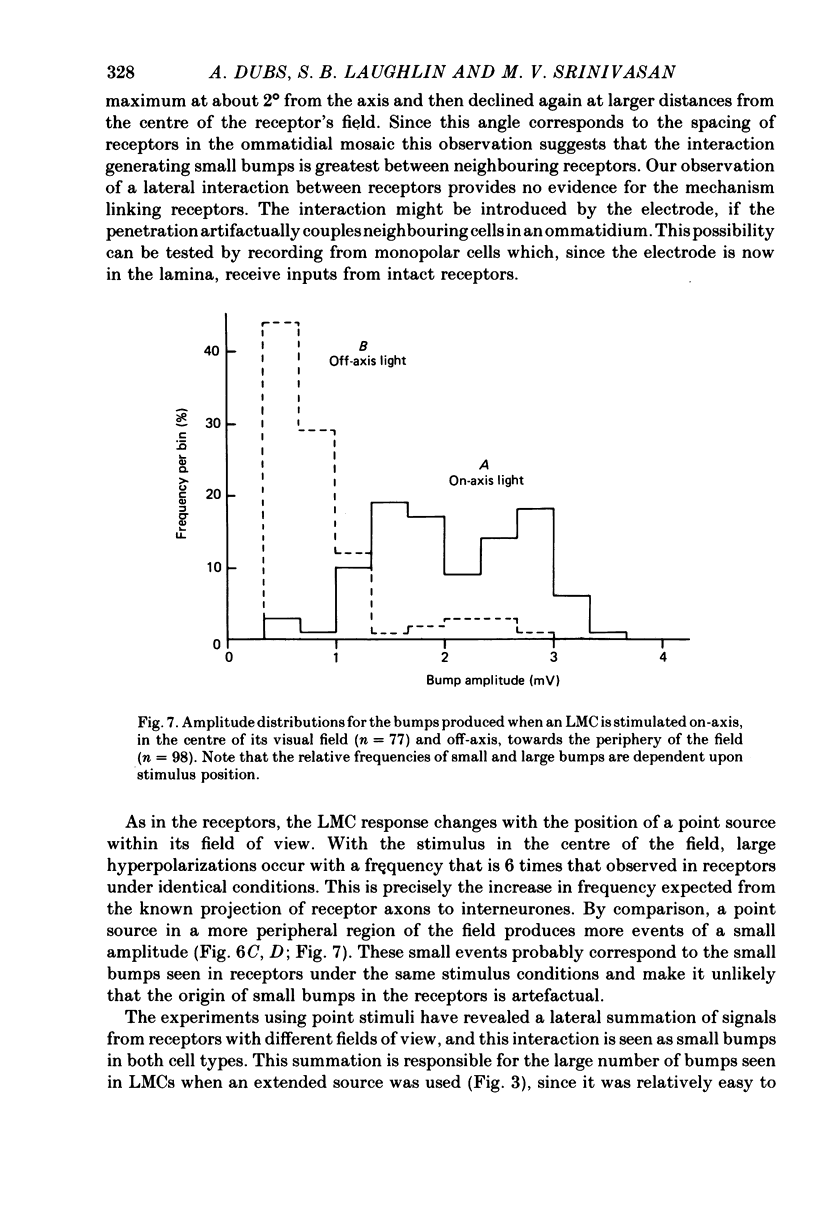





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
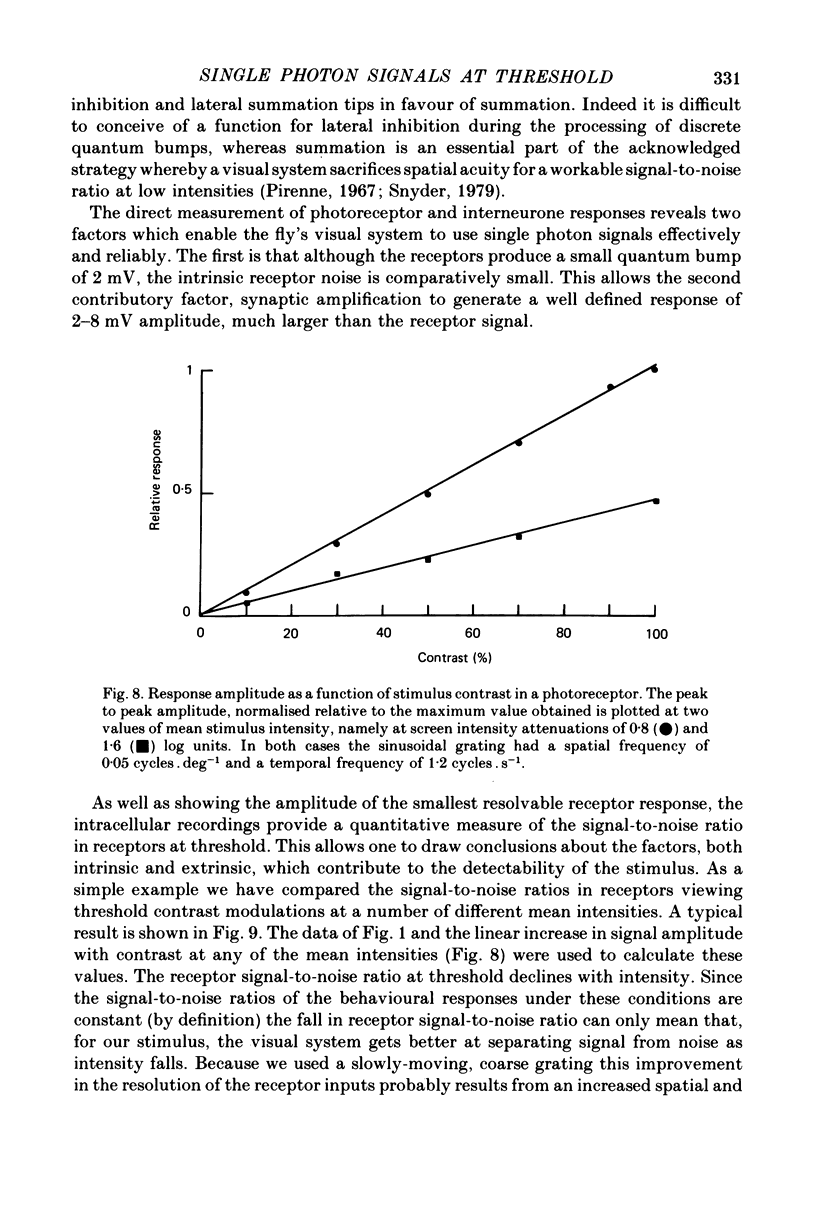
- DeVoe R. D., Ockleford E. M. Intracellular responses from cells of the medulla of the fly, Calliphora erythrocephala. Biol Cybern. 1976 Jun 18;23(1):13–24. doi: 10.1007/BF00344147. [DOI] [PubMed] [Google Scholar]
- Dvorak D., Snyder A. The relationship between visual acuity and illumination in the fly, lucilia sericata. Z Naturforsch C. 1978 Jan-Feb;33(1-2):139–143. doi: 10.1515/znc-1978-1-225. [DOI] [PubMed] [Google Scholar]
- Dvorak D., Srinivasan M. V., French A. S. The contrast sensitivity of fly movement-detecting neurons. Vision Res. 1980;20(5):397–407. doi: 10.1016/0042-6989(80)90030-9. [DOI] [PubMed] [Google Scholar]
- FERMI G., REICHARDT W. OPTOMOTORISCHE REAKTIONEN DER FLIEGE MUSCA DOMESTICA ABHAENGIGKEIT DER REAKTION VON DER WELLENLAENGE, DER GESCHWINDIGKEIT, DEM KONTRAST UND DER MITTLEREN LEUCHTDICHTE BEWEGTER PERIODISCHER MUSTER. Kybernetik. 1963 Sep;2:15–28. doi: 10.1007/BF00292106. [DOI] [PubMed] [Google Scholar]
- FUORTES M. G., YEANDLE S. PROBABILITY OF OCCURRENCE OF DISCRETE POTENTIAL WAVES IN THE EYE OF LIMULUS. J Gen Physiol. 1964 Jan;47:443–463. doi: 10.1085/jgp.47.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain G. L., Granda A. M., Maxwell J. M. Voltage signal of photoreceptors at visual threshold. Nature. 1977 Jan 13;265(5590):181–183. doi: 10.1038/265181a0. [DOI] [PubMed] [Google Scholar]
- Kirschfeld K. Das neurale Superpositionsauge. Fortschr Zool. 1973;21(2):229–257. [PubMed] [Google Scholar]
- Kirschfeld K., Franceschini N., Minke B. Evidence for a sensitising pigment in fly photoreceptors. Nature. 1977 Sep 29;269(5627):386–390. doi: 10.1038/269386a0. [DOI] [PubMed] [Google Scholar]
- Lillywhite P. G., Dvorak D. R. Responses to single photons in a fly optomotor neurone. Vision Res. 1981;21(2):279–290. doi: 10.1016/0042-6989(81)90122-x. [DOI] [PubMed] [Google Scholar]
- Reichardt W., Braitenberg V., Weidel G. Auslösung von Elementarprozessen durch einzelne Lichtquanten im Fliegenauge. Verhaltensexperimente an der Stubenfliege Musca. Kybernetik. 1968 Nov;5(4):148–169. doi: 10.1007/BF00271248. [DOI] [PubMed] [Google Scholar]
- SCHOLES J. H. DISCRETE SUBTHRESHOLD POTENTIALS FROM THE DIMLY LIT INSECT EYE. Nature. 1964 May 9;202:572–573. doi: 10.1038/202572a0. [DOI] [PubMed] [Google Scholar]
- Srinivasan M. V., Bernard G. D. The fly can discriminate movement at signal/noise ratios as low as one-eighth. Vision Res. 1977;17(5):609–616. doi: 10.1016/0042-6989(77)90136-5. [DOI] [PubMed] [Google Scholar]


