Abstract
Polybrominated diphenyl ether (PBDE) congeners are constituents of flame retardants, and there is growing concern regarding their persistence, bioaccumulation, and toxicity. We collected breast milk samples between late 2001 and early 2003 from 54 U.K.-resident mothers. Of these, 27 originated from southeast England (London), and the other 27 originated from northwest England (Lancaster). Analysis of milk-fat extracts by gas chromatography–mass spectrometry was performed to determine the levels of 15 PBDE congeners, 15 polychlorinated biphenyl (PCB) congeners, and other selected chlorinated compounds. PCB and organochlorine (OC) levels in southeast samples were consistently higher, and significant differences (p < 0.05) were observed. ∑PBDE levels ranged from 0.3 to 69 ng/g lipid (geometric mean, 6.6 ng/g), and PBDE-47 was the most abundant congener. ∑PCB levels ranged from 26 to 530 ng/g lipid (geometric mean, 150 ng/g) and were composed mainly of PCB-153 (26%), PCB-138 (20%), and PCB-180 (13%). OC levels for 1,1,1-trichloro-2,2-bis(p-chlorophenyl)ethane (p,p′-DDT) and its metabolites (∑DDX) ranged from 24 to 2,300 ng/g lipid (geometric mean, 160 ng/g); hexachlorobenzene ranged from nondetectable levels to 180 ng/g lipid (geometric mean, 17 ng/g); and ∑hexachlorocyclohexane levels ranged from 1.2 to 1,500 ng/g lipid (geometric mean, 16 ng/g). Using nuclear magnetic resonance–based metabonomics, samples (n = 7) containing the highest contaminant levels were compared with samples (n = 7) containing the lowest levels. Excellent separation along the first principal component implied that the chemical constituents of the two groups were significantly different. Although reasons for such differences remain obscure, lifestyle factors associated with a more heterogeneous London cohort could be responsible. Identifying primary routes of contaminant exposures and their biologic effects is of great importance.
Keywords: breast milk, flame retardants, gas chromatography–mass spectrometry, milk-fat extracts, organochlorines, PBDE-47, persistent contaminants, polybrominated diphenyl ethers, polychlorinated biphenyls, United Kingdom
Persistent organochlorine (OC) contaminants were first detected in breast milk in the early 1950s (Laug et al. 1951). Although many OCs were restricted in North America and Europe during the 1970s, specific requirements such as the control of malaria with 1,1,1-trichloro-2,2-bis(p-chlorophenyl)ethane (p,p′-DDT) or hexachlorocyclohexanes (HCHs) remain in some parts of the world. Although polychlorinated biphenyls (PCBs), predominantly industrial chemicals, were also banned in many countries in the 1970s, these are still present in the environment, and uncertainties exist regarding their current distribution (Breivik et al. 2004). Human tissue levels of PCBs and p,p’-DDT peaked in the 1970s but, probably because of restrictions on use, are now decreasing (Norén and Meironyté 2000). In contrast, polybrominated diphenyl ether (PBDE) levels, first detected in breast milk in 1972, have been increasing throughout the 1980s and 1990s (Meironyté et al. 1999; Norén and Meironyté 2000; Solomon and Weiss 2002).
Brominated diphenyl ethers (BDEs) are commercially available as pentabromodiphenyl ether (pentaBDE), octabromodiphenyl ether (octaBDE), and decabromodiphenyl ether (decaBDE), all of which have different PBDE profiles. DecaBDE is used in high-impact polystyrene for electronic enclosures and as a flame retardant in upholstery textiles (Hardy 2002a). OctaBDE is used in business equipment housings made of acrylonitrile–butadiene–styrene (ABS) resins (Hardy 2002b). PentaBDE is used as a flame retardant for flexible polyurethane foam (Hardy 2002b). As additive flame retardants, PBDEs are used in consumer items such as polyurethane foams, television sets, computers, radios, textiles, paints, and plastics. These brominated compounds act in the gas phase of the fire by reacting with free radicals generated during combustion, thus terminating the reaction (Rahman et al. 2001). PBDEs are dissolved in materials, not covalently bonded. This has led to suggestions that they leach out or volatilize into the environment (McDonald 2002). PBDEs investigated in this study are predominantly indicative of the pentaBDE commercial product.
PBDEs, PCBs, and OCs are hydrophobic, persistent chemicals that may bioaccumulate through food chains. The main route of exposure to PCBs or OCs in the general human population is via the diet (Duarte-Davidson and Jones 1994). In the case of PBDEs, the relative importance of different exposure routes remains obscure. It has been suggested that exposure may occur mainly through the consumption of PBDE-contaminated fish (Meironyté et al. 1999). However, PBDEs do appear to contaminate indoor human environments (Jakobsson et al. 2002), giving rise to the possibility of transfer via inhalation of vapor-phase chemicals and chemicals attached to indoor air/dust.
The human toxicity of PCBs and OCs is well documented [World Health Organization (WHO) 1993]. The toxic effects of PBDEs in humans remain to be properly ascertained (Hardy 2002b). Despite apparent similarities in chemical structure between PBDEs and PCBs, there are important three-dimensional differences due to the ether linkage found in the former (Hardy 2002a). Although currently found to be lower than PCB levels in human tissues, PBDE levels have been increasing (Hites 2004; Norén and Meironyté 2000). PBDEs, particularly congeners present in the pentaBDE product, may possess toxic properties, including endocrine-disrupting activity (Hallgren and Darnerud 2002) and interference with brain development (Branchi et al. 2003), and there is also a suggested association with incidence of non-Hodgkin lymphoma (Darnerud et al. 2001). Some evidence exists of PBDE carcinogenicity in animal bioassays, but the results of these studies remain at best unequivocal (Hardy 2002b; McDonald 2002).
A current profile of PBDE congeners in U.K. breast milk (as measured in extracts of milk fat) in relation to other contaminants remains to be established (Kalantzi et al. 2003). In the present study, our primary objective was to report in detail current U.K. PBDE levels (associated with the pentaBDE product) and, in the same samples, to relate these to current and temporal PCB and OC levels of the United Kingdom. The samples from which milk-fat extracts were obtained originated from London in the southeast and Lancaster in the northwest. We also investigated whether nuclear magnetic resonance (NMR)–based metabonomics (Nicholson et al. 1999), combining high-resolution NMR spectroscopy with pattern recognition, might be a potential platform to study the chemical constituents of milk-fat extracts shown to contain different contaminant profiles. This methodology allows the identification, quantification, and cataloguing of time-related changes in integrated biologic systems and thus may be employed to screen for profiles in tissue extracts (Lindon et al. 2003; Nicholson et al. 1999; Viant et al. 2003). Finally, we consider whether the quantification of such contaminants in biologic matrices remains sufficient, or whether it is more appropriate to analyze their biologic effects, either individually or as complex mixtures.
Materials and Methods
Sample collection.
Individual breast milk samples from U.K.-resident women, donated anonymously and without criteria (i.e., no prior selection or bias), were obtained with appropriate ethical approval. A total of 54 samples were collected between late 2001 and January 2003 from the maternity units of hospitals based in the Lancaster (northwest England; n = 27, via a neonatal unit) and London (southeast England; n = 27, via a milk bank) regions. Donor ages ranged from 24 to 34 years, and milk samples (≈100 mL from a single expression; in a small number of cases, samples expressed on different days were donated) were collected by manual expression into sterile collection bottles. Samples were immediately frozen and stored at −20°C before analysis.
Donors originating from Lancaster completed simple lifestyle questionnaires; such information was not available for London samples. Lancaster samples were donated within the first month of parturition, several being donated within the first 2–3 days. From the information obtained on Lancaster samples, it was noted that all but two donors were nonsmokers, and cumulative past lactation ranged from 0 to 21 months. All consumed meat, a healthy amount of fresh fruit, and low amounts of alcohol (five donors, 1–2 units/day; one unit is defined as 7.9 g ethanol). Lifestyle data thus collected were not found to correlate with the levels of contaminants measured (data not shown). In the absence of more details, it is difficult to comment on similarities or differences between the two cohorts originating from the London or Lancaster regions. However, experience would suggest that no marked differences in range of socioeconomic class, outlets through which food might be sourced, age, or parity would exist. Although the London cohort would be envisaged to be a more heterogeneous population with an input from foreign donors, this was not expected to have an overwhelming influence.
Extraction of milk for PBDE and PCB/OC analyses.
All solvents were of HPLC or glass-distilled grade. Silica gel (0.063–0.200 mm; Merck, Poole, U.K.) and Na2SO4 were heated at 450°C overnight and stored in sealed containers. Standards were purchased from Promochem (Welwyn Garden City, U.K.) and QMx (Thaxted, U.K.).
After thawing, milk samples that originated from a single expression were centrifuged at 3,000 rpm for 15 min. After separation of the milk-fat layer from the aqueous phase, a mixture of milk fat (0.5 g), Na2SO4 (5 g), and hexane (50 mL) was boiled for 10 min and allowed to cool before lipid determination. Evaporated to 5 mL, these mixtures were applied to 25-mm inner-diameter columns containing 15 g acidified silica gel (2:1 silica gel:acid by weight) and eluted with hexane. Eluted samples were evaporated to 1 mL under a gentle stream of nitrogen and applied to gel permeation chromatography columns packed with Biobeads S-X3 (Biorad Laboratories, Hercules, CA, USA) and eluted with hexane: dichloromethane (1:1 by volume). 13C12-labeled PCB and dioxin recovery standards (added at the beginning of the procedure) and internal standards (added at the end of the procedure) in dodecane were incorporated when subsequent gas chromatography–mass spectrometry (GC-MS) analysis was carried out on whole milk-fat extracts but were excluded when extracts were generated for subsequent 1H-NMR spectroscopy.
Gas chromatography for PBDE analysis was performed on a Finnigan Trace GC2000 series gas chromatograph equipped with a 30-m DB-5MS 0.25-mm inner-diameter capillary column (J&W Scientific, Stockport, U.K.) fitted with a retention gap (2 m long, 0.53 mm inner diameter). Sample aliquots (2 μL) were injected by a Thermoquest AS2000 auto-injector (Finnigan, Hemel Hempstead, U.K.), with the injection port at 270°C, in splitless mode, with 100 kPa pressure surge. The carrier gas was helium at a flow rate of 1 mL/min. The oven temperature program was as follows: 80°C for 2 min, 25°C/min to 200°C, 4°C/min to 315°C, and 315°C for 10 min. The quadrupole TRACE mass spectrometer (Finnigan) was set in selected ion recording mode, in negative ion chemical ionization (CI–) mode, using ammonia as the reagent gas, a source temperature of 200°C, interface temperature of 315°C, and electron energy of 70 eV. The following PBDE congeners, chosen because of their reported occurrence in environmental samples, were screened: PBDE congeners 17, 28, 32, 35, 37, 47, 49, 71, 75, 85, 99, 100, 119, 153, and 154. Of these, congeners 28, 47, 99, 100, 153, and 154 were regularly detected in the milk samples.
Gas chromatography for PCB and OC analysis was performed on a Fisons GC8000 series gas chromatograph (Fisons, Manchester, U.K.) equipped with a 50-m CPSil8 0.25-mm inner-diameter capillary column (Chrompak, Walton-on-Thames, U.K.), fitted with a retention gap as for PBDE analysis. Sample aliquots (2 μL) were injected, with the injection port at 250°C, in splitless mode. The carrier gas was helium with a flow rate of 1 mL/min. The oven temperature program was as follows: 100°C for 2 min, 20°C/min to 140°C, 4°C/min to 200°C, 200°C for 13 min, 4°C/min to 300°C, and 300°C for 10 min. The quadrupole MD800 mass spectrometer (Fisons) was set in selected ion recording mode, in electron impact positive ion ionization (EI+) mode, with a source temperature of 250°C, interface temperature of 300°C, and electron energy of 70 eV. A screen for the following PCB congeners and OC pesticides was carried out: PCB congeners 18, 22, 28, 31, 41/64, 44, 49, 52, 54, 60/56, 70, 74, 87, 90/101, 95, 99, 104, 105, 110, 114, 118, 123, 132, 138, 141, 149, 151, 153, 155, 156, 157, 158, 167, 170, 174, 180, 183, 187, 188, 189, 194, 199, 203; α-HCH, β-HCH, γ-HCH, δ-HCH, hexachlorobenzene (HCB), α-chlordane, γ-chlordane, o,p′-dichlorodiphenyldichloroethane (DDD), p,p′-DDD, o,p′-dichlorodiphenyldichloroethylene (DDE), p,p′-DDE, o,p′-DDT, and p,p′-DDT. The following were regularly detected in the milk samples: PCB congeners 41/64, 44, 49, 60/56, 70, 74, 87, 90/101, 95, 99, 105, 110, 114, 118, 138, 149, 151, 153, 156, 157, 158, 167, 170, 180, 183, 187, 189, 194, 203; α-HCH, β-HCH, γ-HCH, HCB, p,p′-DDD, o,p′-DDE, p,p′-DDE, and p,p′-DDT.
A seven-point calibration was used for quantifying all PBDEs, PCBs, and OCs. Detection limits were defined as three times the standard deviation of the levels found in the analytical blanks or the instrument detection limit in the absence of detectable levels in the blanks.
Quality assurance/quality control.
Milk samples were analyzed in batches of six. Along with each batch a blank sample was included, and with every third batch an in-house reference material (a butter sample) was also extracted. This reference material had been analyzed on 12 separate occasions before these analyses and was considered a useful determinant of the reproducibility and rigorous nature of the analytical procedure employed.
A control chart was employed to identify the mean and warning limits (defined as plus two times the standard deviation of the average concentration found in the 12 reference material samples previously analyzed) of the reference materials analyzed throughout the study. The comparison quantity was defined as the sum of the levels of PBDE-47, PBDE-99, PBDE-100, PBDE-153, and PBDE-154 in the reference butter sample. Seven reference material samples were analyzed during the course of this study, and the value of the sum of the five PBDEs never exceeded the warning limits.
Recoveries ranged from 50 to 119%, with an average of 90%. Extracted milk fat ranged from 29 to 85% of centrifuged milk fat fresh weight (mean, 55%). Centrifuged milk fat fresh weight ranged from 0.7 to 5.7% (mean, 2%) of whole milk fresh weight.
1H-NMR spectroscopy.
Extracts in hexane were transported to Imperial College (London) for analysis. Extracts of milk samples containing the lowest contaminant levels (n = 7, all from Lancaster) were compared with extracts of milk samples containing the highest levels (n = 7, all from London). These two sets of samples were chosen on the basis of the sum of all contaminants. To these, an equal volume of CDCl3 (99.9% deuterium; Sigma, Poole, U.K.) was added, and samples were purged with nitrogen gas. To ensure the removal of hexane, this purge process was repeated twice. Then samples were reconstituted in 500 μL of CDCl3 and transferred into 5-mm NMR tubes. 1H-NMR spectra were recorded on a Bruker DRX600 NMR spectrometer, at 300 kelvin, with a 5-mm inverse probe using a standard one-dimensional pulse sequence [RD-90°-t1-90°-tm-90°-acquisition]. The recycle delay (RD) was 3 sec; mixing time, tm, 150 msec; t1, 3 μsec. The 90° pulse length was adjusted to approximately 10 μsec. Transients (128) were collected into 32,768 data points for each spectrum with the spectral width of 12 kHz.
Data reduction and principal components analysis.
All free induction decays were multiplied by an exponential function equivalent to a 1 Hz line-broadening factor before Fourier transformation. NMR spectra were then phase and baseline corrected before being divided into 245 buckets, over the chemical shift range 0.2–10 ppm, using AMIX (Bruker Analytik, Rheinstetten, Germany). Each bucket was 0.04 ppm wide, and the area in each region was integrated. The regions δ6.8–δ7.5 ppm and 1.5–2.5 ppm containing residual solvents (chloroform and water, respectively) were discarded. The value of each bucket was normalized relative to the total sum of the spectral integral before principal components analysis (PCA), which was carried out with the software Simca-P 8.0 (Umetrics, Umeå, Sweden).
Results
PBDE congener levels in milk-fat extracts obtained from U.K. breast milk samples (n = 54) obtained between late 2001 and January 2003 are shown in Table 1. ∑PBDE levels ranged from 0.1 to 69 ng/g lipid, with a geometric mean of 6.6 ng/g lipid (median, 6.3 ng/g lipid). PBDE-47 (geometric mean, 3 ng/g lipid; median, 2.7 ng/g lipid; 45% of ∑PBDE content) was always the most abundant congener present in breast milk. As a percentage of ∑PBDE content, other congeners were found in the following order: PBDE-153 (21%) > PBDE-99 (14%) > PBDE-100 (9.0%) > PBDE-154 (7%), respectively. Making up much smaller proportions of ∑PBDE levels were PBDE congeners 28, 32, 35, 17, and 71.
Table 1.
PBDE congener levels in milk-fat extracts obtained from U.K. breast milk (n = 54), 2001–2003.
| PBDE congener | Geometric mean (ng/g lipid) | Median (ng/g lipid) | Minimum (ng/g lipid) | Maximum (ng/g lipid) | Percent samples positive |
|---|---|---|---|---|---|
| 17 | 0.1 | ND | ND | 1 | 32 |
| 28 | 0.3 | 0.2 | ND | 2.1 | 89 |
| 32 | 0.1 | ND | ND | 0.3 | 38 |
| 35 | 0.2 | ND | ND | 0.6 | 28 |
| 37 | 0.4 | ND | ND | 0.5 | 9 |
| 47 | 3 | 2.7 | 0.1 | 37 | 100 |
| 49 | 0.2 | ND | ND | 0.9 | 11 |
| 71 | 0.2 | ND | ND | 0.5 | 11 |
| 75 | 0.5 | ND | ND | 0.6 | 4 |
| 85 | 0.5 | ND | ND | 1.4 | 9 |
| 99 | 0.9 | 0.8 | ND | 13 | 92 |
| 100 | 0.6 | 0.5 | ND | 7 | 94 |
| 119 | 0.2 | ND | ND | 0.4 | 11 |
| 153 | 1.4 | 1.3 | ND | 4.9 | 89 |
| 154 | 0.5 | 0.4 | ND | 2.5 | 81 |
| ∑PBDE | 6.6 | 6.3 | 0.3 | 69 | — |
Abbreviations: —, not applicable; ND, not detected. For raw data, see Supplemental Material online (http://ehp.niehs.nih.gov/members/2004/6991/supplemental.pdf).
Published levels of ∑PBDE congeners in breast milk are shown in Table 2. Selected congeners have been included in the ∑PBDE values presented in Table 2 to facilitate comparisons with the results of different studies. Compared with European and Japanese milk samples, markedly higher (~10-fold) levels appear to occur in U.S. milk samples. Current ∑PBDE levels in U.K. milk samples, although not as high as those found in U.S. milk samples, are still much higher than in milk samples from other European countries and Japan. As a consequence of stringent fire regulations since 1988, the United Kingdom has seen the use of larger quantities of the pentaBDE mixture than many of her European counterparts. The United Kingdom is currently the fourth largest PBDE producer in the world, with an output of ~25,000 metric tons annually (Alaee et al. 2003).
Table 2.
A comparison of ∑PBDE levels in breast milk samples from different countries.
| Country | No. of samples analyzed | Year of sampling | ∑PBDE [ng/g, mean (range)] | PBDE congeners included in ∑PBDE | Reference |
|---|---|---|---|---|---|
| Canada | 10 | 1992 | 5.7 (0.7–28)a | 28, 47, 99, 100, 153, 154 | Ryan and Patry 2000 |
| Finland | 11 | 1994–1998 | 2.3 (0.9–5.9) | 28, 47, 99, 153 | Strandman et al. 2000 |
| Japan | 12 | NA | 0.7–2.8 | 28, 47, 99, 100, 153, 154 | Ohta et al. 2000 |
| Sweden | 40 | 1997 | 4 | 28, 47, 66, 100, 99, 85, 153, 154 | Norén and Meironyté 2000 |
| United Kingdom | 54 | 2001–2003 | 8.9 (0.1–63)a,b | 47, 99, 100, 153, 154 | This study |
| United States | 47 | 2001 | 73.9 (6.2–418.8) | 17, 28, 47, 66, 77, 85, 99, 100, 138, 153, 154, 183, 209 | Schecter et al. 2003 |
NA, data not available.
Sum includes PBDE congeners listed only to facilitate interstudy comparisons.
Arithmetic mean and concentration range in ng/g lipid.
PCB levels in milk-fat extracts obtained from U.K. breast milk are shown in Table 3. ∑PCB levels ranged from 26 to 530 ng/g lipid, with a geometric mean of 150 ng/g lipid (median, 180 ng/g lipid). The most commonly occurring PCB congeners were found to be present in the following order: PCB-153 > PCB-138 > PCB-180 > PCB-170 > PCB-118 > PCB-187 > PCB-99 > PCB-156. PCB-153 constituted some 26%, PCB-138 some 20%, and PCB-180 some 13% of ∑PCB levels. A comparison of the results from this study with previously reported U.K. levels (Table 4) suggests that ∑PCB levels are currently decreasing, probably as a consequence of bans imposed on the use of these contaminants.
Table 3.
PCB and OC levels in milk-fat extracts obtained from U.K. breast milk (n = 54), 2001–2003.
| Contaminant | Geometric mean (ng/g lipid) | Median (ng/g lipid) | Minimum (ng/g lipid) | Maximum (ng/g lipid) | Percent samples in which contaminant detected |
|---|---|---|---|---|---|
| PCB-28 | 2.1 | 2.0 | 0.6 | 10 | 100 |
| PCB-74 | 6.5 | 6.6 | 1.4 | 40 | 100 |
| PCB-99 | 5.3 | 5.4 | 1.5 | 21 | 100 |
| PCB-105 | 1.9 | 2.0 | 0.4 | 11 | 100 |
| PCB-118 | 9.6 | 10 | 2.4 | 43 | 100 |
| PCB-138 | 31 | 37 | 4.2 | 100 | 100 |
| PCB-153 | 39.5 | 49 | 4.3 | 130 | 100 |
| PCB-156 | 4.0 | 5.1 | 0.6 | 13 | 100 |
| PCB-167 | 1.3 | 1.5 | 0.2 | 4.1 | 100 |
| PCB-170 | 9.1 | 11 | 0.9 | 49 | 100 |
| PCB-180 | 20 | 25 | 1.8 | 120 | 100 |
| PCB-183 | 2.5 | 2.9 | 0.3 | 8.9 | 100 |
| PCB-187 | 6.0 | 7.0 | 0.6 | 39 | 100 |
| PCB-194 | 2.7 | 2.7 | ND | 27 | 94 |
| PCB-203 | 2.2 | 2.4 | ND | 15 | 98 |
| ∑PCB | 150 | 180 | 26 | 530 | — |
| α-HCH | 0.2 | ND | ND | 2 | 35 |
| β-HCH | 15 | 17 | 1.2 | 1,500 | 100 |
| γ-HCH | 0.8 | 0.6 | ND | 7.7 | 91 |
| ∑HCH | 16 | 18 | 1.2 | 1,500 | — |
| HCB | 17 | 18 | ND | 180 | 98 |
| α-Chlordane | 0.3 | ND | ND | 1.4 | 11 |
| o,p′-DDD | 0.3 | ND | ND | 0.9 | 4 |
| o,p′-DDT | 0.7 | 0.6 | ND | 55 | 80 |
| o,p′-DDE | 0.2 | 0.1 | ND | 5.8 | 69 |
| p,p′-DDD | 0.3 | 0.3 | ND | 11 | 82 |
| p,p′-DDE | 150 | 150 | 22 | 1,600 | 100 |
| p,p′-DDT | 6.2 | 6.2 | 1.1 | 760 | 100 |
| ∑DDX | 160 | 160 | 24 | 2,300 | — |
Abbreviations: —, not applicable; ND, not detected. For raw data, see Supplemental Material online (http://ehp.niehs. nih.gov/members/2004/6991/supplemental.pdf).
Table 4.
Temporal levels of selected compounds observed in U.K. breast milk and adipose fat samples.
| Period of sampling | Mean (ng/g lipid) | Range | No. of samples | Tissue source | Reference |
|---|---|---|---|---|---|
| ∑PCB | |||||
| 1976–1977 | 600 | 100–1,500 | 81 | Adipose fat | U.K. MAFF 1983 |
| 1979–1980 | 500 | 100–2,100 | 102 | Milk fat | U.K. MAFF 1983 |
| 1990–1991 | 522 | 140–1,697 | 32 | Milk fat | Duarte-Davidson et al. 1994 |
| 2001–2003 | 200 | 26–530 | 54 | Milk fat | This study |
| p,p′-DDE | |||||
| 1969–1971 | 1,600 | NA | 85 | Adipose fat | Abbott et al. 1972 |
| 1976–1977 | 2,100 | NA | 81 | Adipose fat | Abbott et al. 1981 |
| 1979–1980 | 1,600 | 10–7,300 | 102 | Milk fat | Collins et al. 1982 |
| 1982–1983 | 1,300 | 50–5,100 | 187 | Adipose fat | Abbott et al. 1985 |
| 1997–1998 | 430 | 60–4,000 | 168 | Milk fat | Harris et al. 1999 |
| 2001–2003 | 220 | 22–1,600 | 54 | Milk fat | This study |
| HCB | |||||
| 1969–1971 | 50 | 10–290 | 85 | Adipose fat | Abbott et al. 1972 |
| 1976–1977 | 220 | 20–3,200 | 81 | Adipose fat | Abbott et al. 1981 |
| 1979–1980 | 140 | 10–1,000 | 102 | Milk fat | Collins et al. 1982 |
| 1982–1983 | 110 | 30–320 | 187 | Adipose fat | Abbott et al. 1985 |
| 1997–1998 | 43 | 12–333 | 168 | Milk fat | Harris et al. 1999 |
| 2001–2003 | 20 | ND–180 | 54 | Milk fat | This study |
| β-HCH | |||||
| 1969–1971 | 260 | NA | 85 | Adipose fat | Abbott et al. 1972 |
| 1976–1977 | 330 | NA | 81 | Adipose fat | Abbott et al. 1981 |
| 1979–1980 | 220 | 10–4,400 | 102 | Milk fat | Collins et al. 1982 |
| 1982–1983 | 270 | 10–810 | 187 | Adipose fat | Abbott et al. 1985 |
| 1997–1998 | 68 | 8–750 | 168 | Milk fat | Harris et al. 1999 |
| 2001–2003 | 40 | 1.2–1,500 | 54 | Milk fat | This study |
Abbreviation: NA, data not available; ND, not detected.
Arithmetic mean in ng/g lipid.
Total HCB levels ranged from below the detection limit to 180 ng/g lipid (geometric mean, 17 ng/g lipid; median, 18 ng/g lipid), whereas ∑HCH (defined as the sum of α-, β-, and γ-HCH) levels ranged from 1.2 to 1,500 ng/g lipid (geometric mean, 16 ng/g lipid; median, 18 ng/g lipid; Table 3). ∑DDX (p,p′-DDT and its metabolites) levels ranged from 24 to 2,300 ng/g lipid, giving rise to a geometric mean of 160 ng/g lipid (median, 160 ng/g lipid). The most commonly occurring isomers were p,p′-DDE (geometric mean, 150 ng/g lipid; median, 150 ng/g lipid; range, 20–1,600 ng/g lipid) and p,p′-DDT (geometric mean, 6.2 ng/g lipid; median, 6.2 ng/g lipid; range, 1.1–760 ng/g lipid). Of two milk samples that exhibited exceptionally high ∑DDX levels, one also exhibited exceptionally high ∑HCH levels and the other exhibited exceptionally high ∑HCB levels (data not shown). Table 4 again demonstrates that levels in U.K. breast milk of p,p′-DDE, HCB, and β-HCH have been falling for some time.
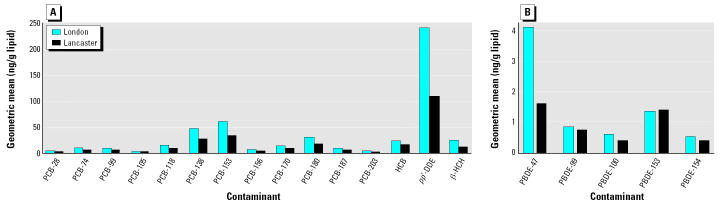
Geometric means, medians, and ranges of various contaminant concentrations in milk-fat extracts obtained from breast milk samples originating from the London region (n = 27) and the Lancaster region (n = 27) are compared in Table 5 and Figure 1A, B. The data were log-transformed, and a t-test was used to investigate differences in contaminant levels between the samples from London and those from the Lancaster region. Significantly higher (p < 0.0001) levels of PBDE-47 were found in London milk samples compared with those originating from Lancaster, but no differences were found in ∑PBDE and the levels of four other PBDE congeners (Table 5, Figure 1B). With regard to PCBs and OCs, significantly higher (p < 0.05) levels of PCB-138, PCB-153, PCB-180, ∑PCB, p,p′-DDE, ∑DDX, HCB, β-HCH, and ∑HCH were found in milk samples from London (Table 5, Figure 1A). Breast milk samples originating from London tended to exhibit higher geometric mean levels for all the contaminants analyzed in comparison with Lancaster levels (Figure 1A, B).
Table 5.
Comparison of geometric means [median; range (ng/g lipid)] of PBDE and PCB congeners, and OCs in breast milk from London and Lancaster regions of the United Kingdom after extraction of milk fat.
| Contaminant | London | Lancaster | Significancea (p-value) |
|---|---|---|---|
| PBDE-47 | 3.9 (4.6; 1.0–36) | 1.8 (2.2; 0.1–17) | Yes (0.000) |
| PBDE-99 | 0.9 (1.0; ND–12) | 0.8 (0.6; ND–6.8) | No (0.636) |
| PBDE-100 | 0.6 (0.5; 0.1–7.0) | 0.5 (0.4; ND–4.5) | No (0.186) |
| PBDE-153 | 1.4 (1.2; ND-4.9) | 1.4 (1.4; ND–3.5) | No (0.833) |
| PBDE-154 | 0.5 (0.5; ND-2.1) | 0.4 (0.3; ND–2.4) | No (0.173) |
| ∑PBDE | 7.8 (8.1; 3.1–69) | 4.6 (5.0; 0.3–34) | Yes (0.006) |
| PCB-138 | 41 (45; 6.4–100) | 22 (29; 4.2–73) | Yes (0.001) |
| PCB-153 | 53 (58; 5.6–130) | 29 (37; 4.3–100) | Yes (0.005) |
| PCB-180 | 27 (30; 1.9–120) | 15 (20; 1.8–54) | Yes (0.024) |
| ∑PCB | 204 (210; 47–530) | 110 (140; 26–370) | Yes (0.000) |
| p,p′-DDE | 230 (180; 76–1,600) | 82 (90; 22–260) | Yes (0.000) |
| ∑DDX | 240 (190; 77–2,300) | 87 (98; 24–270) | Yes (0.000) |
| HCB | 20 (21; 7.6–180) | 12 (14; ND–30) | Yes (0.003) |
| β-HCH | 20 (19; 5.6–1,500) | 10 (13; 1.2–33) | Yes (0.002) |
| ∑HCH | 22 (20; 8.6–1,500) | 11 (14; 1.2–36) | Yes (0.001) |
ND, Not detected. For raw data, see Supplemental Material online (http://ehp.niehs.nih.gov/members/2004/6991/supplemental.pdf).
As determined by a t-test after log transformation of the data.
Figure 1. A comparison (London vs. Lancaster) of the geometric mean levels of (A) PCBs and OCs and (B) PBDEs in breast milk samples obtained from U.K.-resident women.
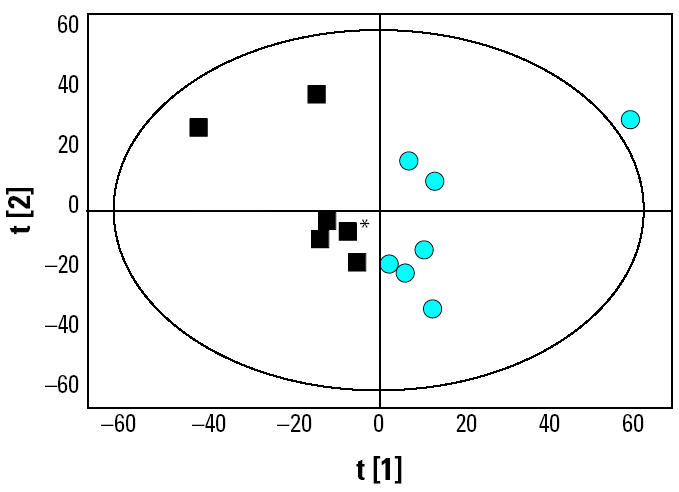
1H-NMR spectra showed that the major chemical constituents of extracts analyzed in this study were lipids (data not shown). Taking into account the remaining, unidentified NMR signals, seven of the milk-fat extracts shown to contain the lowest contaminant levels (all from the Lancaster region) were compared with seven extracts shown to contain the highest levels (all from the London region). NMR with pattern recognition methods gave rise to a multivariate PCA score plot (Figure 2) in which each point represents an individual extract. The observation that clustering occurred based on contaminant levels highlights the differences in the chemical composition of these two groups of milk-fat extracts. Excellent separation for the two groups of extracts was clearly evident along the first principal component, thus implying that the chemical constituents of both groups are significantly different.
Figure 2. PCA score plots of milk-extract samples; t[1] and t[2] are first and second principal components, respectively. Black squares represent sample extracts containing the lowest contaminant levels (n = 7, all from Lancaster). Blue circles represent sample extracts containing the highest contaminant levels (n = 7, all from London). *Two sample extracts closely aligned together.
Discussion
The occurrence of PBDE-47 in breast milk (Meironyté et al. 1999), human blood plasma (Klasson-Wehler et al. 1997), and human adipose tissue (She et al. 2000) has been reported. In Sweden, a PBDE congener pattern consisting of PBDE-47 > PBDE-99 > PBDE-153 > PBDE-100 > PBDE-154 in terms of relative content has been noted, whereas others have reported the presence of equal levels of PBDE-99 and PBDE-153 (Darnerud et al. 1998; Meironyté et al. 1999). Other studies from Japan, Sweden, the United States, and now the United Kingdom (present study) have found PBDE-153 to be present at higher levels than PBDE-99 (Meironyté et al. 2003; Ohta et al. 2000; She et al. 2002).
Estimates of market demand for major brominated flame retardants suggest that some 58% of world market volume occurs in Asia, followed by the Americas (26%) and then Europe (14%) [Bromine Science and Environmental Forum (BSEF) 2001]. Of three commercial PBDE mixtures—pentaBDE, octaBDE and decaBDE—the latter is the most commonly found in Europe in recent years with approximately 7,600 metric tons of decaBDE in current use (BSEF 2001; Hardy 2002b). PBDE congeners 47, 99, 100, and 153 are all components of pentaBDE. It has been tentatively suggested that since 1970 a cumulative sum of 3,000 metric tons of pentaBDE may have been manufactured in the United Kingdom (Alcock et al. 2003).
That lower brominated PBDEs are still present in U.K. milk samples (Table 1) suggests historical use of the pentaBDE product still plays a role in determining current congener patterns. It has been hypothesized that in sand, soil, or sediment decaBDE photode-grades and may to a minor degree give rise to lower brominated congeners (Sellström et al. 1998; Söderström et al. 2004). Diet may be an important route of exposure to PBDEs, particularly through the consumption of fish (Lind et al. 2002). Whether diet is the dominant route of uptake remains to be ascertained.
As PBDEs are used in indoor environments, the potential for transfer via inhalation of indoor dusts is a possibility. Foam from furniture and cars in the United Kingdom is likely to have been treated with the technical pentaBDE mixture profile in the past (Wilford et al. 2003), and the possibility of PBDEs volatilizing from foam has been previously demonstrated (Wilford et al. 2003). The pattern of PBDE congeners in U.K. breast milk suggests higher PBDE-47, PBDE-100, PBDE-153, and PBDE-154 levels but lower PBDE-99 levels (Table 1). Indoor dust samples from two U.K. parliamentary buildings (Santillo et al. 2001) and from houses in the United Kingdom (Santillo et al. 2003) exhibited similar patterns. PBDE patterns in fish from the U.K. North Sea (Thomas et al. 2003) also gave rise to a closely matching profile. U.S. data suggest a similar profile for foam (Hale et al. 2002) but a different indoor dust pattern (Rudel et al. 2003).
London (population > 10 million) contrasts markedly with Lancaster, a semirural town (population ~100,000). Pearson correlations of the logged ∑PCB, ∑PBDE, ∑HCH, ∑DDX, and HCB levels were examined. ∑PCB significantly correlated with ∑PBDE (p < 0.01), ∑HCH (p < 0.05), ∑DDX (p < 0.01), and HCB (p < 0.01). ∑DDX also significantly correlated with ∑PBDE (p < 0.01), ∑HCH (p < 0.01), and HCB (p < 0.01), whereas ∑PBDE correlated well with ∑HCH (p < 0.05) and HCB (p < 0.05). PBDE congeners significantly correlated (p < 0.01) as follows: PBDE-47 with PBDE-99, PBDE-100, PBDE-153, and PBDE-153; PBDE-99 with PBDE-100, PBDE-153, and PBDE-154; and PBDE-153 with PBDE-154. Such correlation may indicate that humans are exposed to PBDEs predominantly via diet, as with PCBs. Higher breast milk PCB levels are associated with urban regions (Vartiainen et al. 1998). Contaminant levels absorbed in food may reflect ambient contamination (Kalantzi et al. 2001). The fact that 1H-NMR spectroscopy further demonstrates these regional differences suggests that this method may have future potential as a chemical fingerprinting methodology of biologic fluids such as breast milk.
We ascertained current contaminant levels in milk-fat extracts obtained from U.K. breast milk samples obtained from two sources: a milk bank in the southeast and a neonatal unit in the northwest. Breast milk has been hypothesized to be a surrogate tissue to monitor in vivo exposures to breast epithelial cells (Martin et al. 2001). Epidemiologic studies (Stellman et al. 2000) and experimental studies in rodents (Schiestl et al. 1997) suggest a role for OCs and PCBs in cancer causation. Others suggest a lack of association between exposure to OCs or PCBs and breast cancer risk (Gammon et al. 2002; Zheng et al. 2000), whereas individual dioxin-like PCBs may increase risk (Demers et al. 2002). Breast cancer incidence in these areas of the United Kingdom is 68 per 100,000 in the southeast versus 64 per 100,000 in the northwest (Great Britain Department of Health 1998). However, any such associations with breast cancer incidence or, indeed, any other disease is difficult in the context of such a small sample size (n = 54) and in the absence of prospective follow-up.
Lifetime exposures to OCs, PCBs, or PBDEs will vary considerably (Angulo et al. 1999), and the effects of fluctuating levels of endocrine modulators remain to be ascertained (Kalantzi et al. 2004; Yared et al. 2002). Rather than inducing DNA damage themselves, such agents may enhance the susceptibility of target cells to other xenobiotics (Davis et al. 2002). Endocrine disruptors may bind with the aryl hydrocarbon receptor, increasing transcription of CYP1A1 (Behnisch et al. 2001). Such differential regulation by xenoestrogen mixtures could dramatically enhance toxicity (Coumoul et al. 2001) and may also modulate cell cycle events (Dohr and Abel 1997).
Despite the presence of contaminants in U.K. breast milk, it must be emphasized that breast-feeding is protective to the neonate (Dundaroz et al. 2002; Oddy 2001) and also appears to confer a protective effect against breast cancer in the mother (Grover and Martin 2002). The value of this resource is that it potentially allows us to noninvasively analyze ongoing exposures. This work shows for the first time the presence of PBDEs in U.K. breast milk obtained from two different locations and also points to significant regional differences in contaminants in this biologic fluid. Future work is required to ascertain the underlying reasons for these regional differences and also the biologic effects of such exposures.
Correction
Values in the tables and in the corresponding text in the original manuscript published online were incorrect and have been corrected here.
Footnotes
Supplemental material is available online (http://ehp.niehs.nih.gov/members/2004/6991/supplemental.pdf).
References
- Abbott DC, Collins GB, Goulding R. Organochlorine pesticide residues in human fat in the United Kingdom 1969–71. Br Med J. 1972;2:553–556. doi: 10.1136/bmj.2.5813.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott DC, Collins GB, Goulding R, Hoodless RA. Organochlorine pesticide residues in human fat in the United Kingdom 1976–7. Br Med J. 1981;283:1425–1428. doi: 10.1136/bmj.283.6304.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott DC, Goulding R, Holmes DC, Hoodless RA. Organochlorine pesticide residues in human fat in the United Kingdom 1982–1983. Hum Toxicol. 1985;4:435–445. doi: 10.1177/096032718500400410. [DOI] [PubMed] [Google Scholar]
- Alaee M, Arias P, Sjodin A, Bergman A. An overview of commercially used brominated flame retardants, their applications, their use patterns in different countries/regions and possible modes of release. Environ Int. 2003;29:683–689. doi: 10.1016/S0160-4120(03)00121-1. [DOI] [PubMed] [Google Scholar]
- Alcock RE, Sweetman AJ, Prevedouros K, Jones KC. Understanding levels and trends of BDE-47 in the UK and North America: an assessment of principal reservoirs and source inputs. Environ Int. 2003;29:691–698. doi: 10.1016/S0160-4120(03)00120-X. [DOI] [PubMed] [Google Scholar]
- Angulo R, Martinez P, Jodral ML. PCB congeners transferred by human milk, with an estimate of their daily intake. Food Chem Toxicol. 1999;37:1081–1088. doi: 10.1016/s0278-6915(99)00101-5. [DOI] [PubMed] [Google Scholar]
- Behnisch PA, Hosoe K, Sakai S. Bioanalytical screening methods for dioxins and dioxin-like compounds: a review of bioassay/biomarker technology. Environ Int. 2001;27:413–439. doi: 10.1016/s0160-4120(01)00028-9. [DOI] [PubMed] [Google Scholar]
- Branchi I, Capone F, Alleva E, Costa LG. Polybrominated diphenyl ethers: neurobehavioral effects following developmental exposure. Neurotoxicology. 2003;24:449–462. doi: 10.1016/S0161-813X(03)00020-2. [DOI] [PubMed] [Google Scholar]
- Breivik K, Alcock R, Li YF, Bailey RE, Fielder H, Pacyna JM. Primary sources of selected POPs: regional and global scale emission inventories. Environ Int. 2004;128:3–16. doi: 10.1016/j.envpol.2003.08.031. [DOI] [PubMed] [Google Scholar]
- BSEF 2001. Major Brominated Flame Retardants Volume Estimates. Total Market Demand by Region in 2001. Brussels:Bromine Science and Environmental Forum. Available: http://www.bsef-site.com/docs/BFR_vols_2001.doc [accessed 12 April 2004].
- Collins GB, Holmes DC, Hoodless RA. Organochlorine pesticide residues in human milk in Great Britain, 1979–1980. Hum Toxicol. 1982;1:425–431. doi: 10.1177/096032718200100409. [DOI] [PubMed] [Google Scholar]
- Coumoul X, Diry M, Robillot C, Barouki R. Differential regulation of cytochrome P450 1A1 and 1B1 by a combination of dioxin and pesticides in the breast tumor cell line MCF-7. Cancer Res. 2001;61:3942–3948. [PubMed] [Google Scholar]
- Darnerud PO, Atuma S, Aune M, Cnattingius S, Wernroth M-L, Wicklund-Glynn A. Polybrominated diphenyl ethers (PBDEs) in breast milk from primiparous women in Uppsala country, Sweden. Organohalogen Compounds. 1998;35:411–414. [Google Scholar]
- Darnerud PO, Eriksen GS, Johannesson T, Larsen PB, Viluksela M. Polybrominated diphenyl ethers: occurrence, dietary exposure, and toxicology. Environ Health Perspect. 2001;109(suppl 1):49–68. doi: 10.1289/ehp.01109s149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C, Bhana S, Shorrocks AJ, Martin FL. Oestrogens induce G1 arrest in benzo[a]pyrene-treated MCF-7 breast cells whilst enhancing genotoxicity and clonogenic survival. Mutagenesis. 2002;17:431–438. doi: 10.1093/mutage/17.5.431. [DOI] [PubMed] [Google Scholar]
- Demers A, Ayotte P, Brisson J, Dodin S, Robert J, Dewailly É. Plasma concentrations of polychlorinated biphenyls and the risk of breast cancer: a congener-specific analysis. Am J Epidemiol. 2002;155:629–635. doi: 10.1093/aje/155.7.629. [DOI] [PubMed] [Google Scholar]
- Dohr O, Abel J. Transforming growth factor-beta1 coregulates mRNA expression of aryl hydrocarbon receptor and cell-cycle-regulating genes in human cancer cell lines. Biochem Biophys Res Commun. 1997;241:86–91. doi: 10.1006/bbrc.1997.7773. [DOI] [PubMed] [Google Scholar]
- Duarte-Davidson R, Jones KC. Polychlorinated biphenyls (PCBs) in the UK population: estimated intake, exposure and body burden. Sci Total Environ. 1994;151:131–152. doi: 10.1016/0048-9697(94)90170-8. [DOI] [PubMed] [Google Scholar]
- Duarte-Davidson R, Wilson SC, Jones KC. PCBs and other organochlorines in human tissue samples from the Welsh population: II—milk. Environ Pollut. 1994;84:79–87. doi: 10.1016/0269-7491(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Dundaroz R, Aydin HI, Ulucan H, Baltaci V, Denli M, Gokcay E. Preliminary study on DNA damage in non breast-fed infants. Pediatr Int. 2002;44:127–130. doi: 10.1046/j.1328-8067.2001.01525.x. [DOI] [PubMed] [Google Scholar]
- Gammon MD, Wolff MS, Neugut AI, Eng SM, Teitelbaum SL, Britton JA, et al. Environmental toxins and breast cancer on Long Island. II. Organochlorine compound levels in blood. Cancer Epidemiol Biomarkers Prev. 2002;11:686–697. [PubMed] [Google Scholar]
- Great Britain Department of Health 1998. Nutritional Aspects of the Development of Cancer. Report on Health and Social Subjects No. 48. London:Her Majesty’s Stationery Office.
- Grover PL, Martin FL. The initiation of breast and prostate cancer. Carcinogenesis. 2002;23:1095–1102. doi: 10.1093/carcin/23.7.1095. [DOI] [PubMed] [Google Scholar]
- Hale RC, La Guardia MJ, Harvey E, Mainor TM. The potential role of fire retardant-treated polyurethane foam as a source of brominated diphenyl ethers to the US environment. Chemosphere. 2002;46:729–735. doi: 10.1016/s0045-6535(01)00237-5. [DOI] [PubMed] [Google Scholar]
- Hallgren S, Darnerud PO. Polybrominated diphenyl ethers (PBDEs), polychlorinated biphenyls (PCBs) and chlorinated paraffins (CPs) in rats-testing interactions and mechanisms for thyroid hormone effects. Toxicology. 2002;177:227–243. doi: 10.1016/s0300-483x(02)00222-6. [DOI] [PubMed] [Google Scholar]
- Hardy ML. A comparison of the properties of the major commercial PBDPO/PBDE product to those of major PBB and PCB products. Chemosphere. 2000a;46:717–728. doi: 10.1016/s0045-6535(01)00236-3. [DOI] [PubMed] [Google Scholar]
- Hardy ML. The toxicology of the three commercial polybrominated diphenyl oxide (ether) flame retardants. Chemosphere. 2002b;46:757–777. doi: 10.1016/s0045-6535(01)00240-5. [DOI] [PubMed] [Google Scholar]
- Harris CA, O’Hagan S, Merson GHJ. Organochlorine pesticide residues in human milk in the United Kingdom 1997–8. Hum Exp Toxicol. 1999;18:602–606. doi: 10.1191/096032799678839392. [DOI] [PubMed] [Google Scholar]
- Hites RA.2004Polybrominated diphenyl ethers in the environment and in people: a meta-analysis of concentrations Environ Sci Technol 38945–956.doi: 10.1021/es035082g. [DOI] [PubMed] [Google Scholar]
- Jakobsson K, Thuresson K, Rylander L, Sjodin A, Hagmar L, Bergman A. Exposure to polybrominated diphenyl ethers and tetrabromobisphenol A among computer technicians. Chemosphere. 2002;46:709–716. doi: 10.1016/s0045-6535(01)00235-1. [DOI] [PubMed] [Google Scholar]
- Kalantzi OI, Alcock RE, Johnston PA, Santillo D, Stringer RL, Thomas GO, et al. The global distribution of PCBs and organochlorine pesticides in butter. Environ Sci Technol. 2001;35:1013–1018. doi: 10.1021/es0002464. [DOI] [PubMed] [Google Scholar]
- Kalantzi OI, Alcock RE, Martin FL, Thomas GO, Jones KC. Polybrominated diphenyl ethers (PBDEs) and selected organochlorines in human breast milk samples from the United Kingdom. Organohalogen Compounds. 2003;61:9–12. [Google Scholar]
- Kalantzi OI, Hewitt R, Ford KJ, Cooper L, Alcock RE, Thomas GO, et al. 2004Low-dose induction of micronuclei by lindane Carcinogenesis 25613–622.doi: 10.1093/carcin/bgh047 [Online 19 December 2003]. [DOI] [PubMed] [Google Scholar]
- Klasson-Wehler E, Hovander L, Bergman A. New organohalogens in human plasma—identification and quantification. Organohalogen Compounds. 1997;33:420–425. [Google Scholar]
- Laug EP, Kunze FM, Pitchett CS. Occurrence of DDT in human fat and milk. Arch Ind Hyg. 1951;3:245–246. [PubMed] [Google Scholar]
- Lind Y, Aune M, Atuma S, Becker W, Bjerselius R, Glynn A, et al. Food intake of the brominated flame retardants PBDEs and HBCD in Sweden. Organohalogen Compounds. 2002;58:181–184. [Google Scholar]
- Lindon JC, Holmes E, Nicholson JK. So what’s the deal with metabonomics? Anal Chem. 2003;75:384A–391A. doi: 10.1021/ac031386+. [DOI] [PubMed] [Google Scholar]
- Martin FL, Cole KJ, Weaver G, Hong GS, Lam BC, Balaram P, et al. Genotoxicity of human breast milk from different countries. Mutagenesis. 2001;16:401–406. doi: 10.1093/mutage/16.5.401. [DOI] [PubMed] [Google Scholar]
- McDonald TA. A perspective on the potential health risks of PBDEs. Chemosphere. 2002;46:745–755. doi: 10.1016/s0045-6535(01)00239-9. [DOI] [PubMed] [Google Scholar]
- Meironyté D, Aronsson A, Ekma -, Ordeberg G, Bergman A, Norén K. Human prenatal and postnatal exposure to polybrominated diphenyl ethers, polychlorinated biphenyls, polychlorobiphenylols, and pentachlorophenol. Environ Health Perspect. 2003;111:1235–1241. doi: 10.1289/ehp.5946. [Online 21 January 2003]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meironyté D, Norén K, Bergman A. Analysis of polybrominated diphenyl ethers in Swedish human milk. A time-related trend study, 1972–1997. J Toxicol Environ Health. 1999;58:329–341. doi: 10.1080/009841099157197. [DOI] [PubMed] [Google Scholar]
- Nicholson JK, Lindon JC, Holmes E. “Metabonomics”: understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica. 1999;29:1181–1189. doi: 10.1080/004982599238047. [DOI] [PubMed] [Google Scholar]
- Norén K, Meironyté D. Certain organochlorine and organobromine contaminants in Swedish human milk in perspective of past 20–30 years. Chemosphere. 2000;40:1111–1123. doi: 10.1016/s0045-6535(99)00360-4. [DOI] [PubMed] [Google Scholar]
- Oddy WH. Breastfeeding protects against illness and infection in infants and children: a review of the evidence. Breastfeed Rev. 2001;9:11–18. [PubMed] [Google Scholar]
- Ohta S, Ishizuka D, Nishimura H, Nakao T, Aozasa O, Shimidzu Y, et al. Real situation of contamination by polybrominated diphenyl ethers as flame retardant in market fish and mother milk of Japan. Organohalogen Compounds. 2000;47:218–221. [Google Scholar]
- Rahman F, Langford KH, Scrimshaw MD, Lester JN. Polybrominated diphenyl ether (PBDE) flame retardants. Sci Total Environ. 2001;275:1–17. doi: 10.1016/s0048-9697(01)00852-x. [DOI] [PubMed] [Google Scholar]
- Rudel RA, Camann DE, Spengler JD, Korn LR, Brody JG.2003Phthalates, alkylphenols, pesticides, polybrominated diphenyl ethers, and other endocrine-disrupting compounds in indoor air and dust Environ Sci Technol 374543–4553.doi: 10.1021/es0264596 [Online 13 September 2003]. [DOI] [PubMed] [Google Scholar]
- Ryan JJ, Patry B. Determination of brominated diphenyl ethers (BDEs) and levels in Canadian human milks. Organohalogen Compounds. 2000;47:57–60. [Google Scholar]
- Santillo D, Johnston P, Brigden K. 2001. Presence of Brominated Flame Retardants and Organotin Compounds in Dusts Collected from Parliament Buildings in Eight Countries. Greenpeace Research Laboratories Technical Note 03/2001. Exeter, UK:Greenpeace Research Laboratories. Available: http://www.greenpeace.to/html/commreps.htm [accessed 12 April 2004].
- Santillo D, Labunska I, Davidson H, Johnston P, Strutt M, Knowles O. 2003. Consuming Chemicals: Hazardous Chemicals in House Dust as an Indicator of Chemical Exposure in the Home. Greenpeace Research Laboratories Technical Note 01/2003. Exeter, UK:Greenpeace Research Laboratories. Available: http://www.greenpeace.to/html/commreps.htm [accessed 12 April 2004].
- Schecter A, Pavuk M, Päpke O, Ryan JJ, Birnbaum L, Rosen R.2003Polybrominated diphenyl ethers (PBDEs) in U.S. mothers’ milk Environ Health Perspect 1111723–1729.doi:10.1289/ehp.6466 [Online 5 August 2003]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiestl RH, Aubrecht J, Yap WY, Kandikonda S, Sidhom S. Polychlorinated biphenyls and 2,3,7,8-tetrachlorodibenzo-p-dioxin induce intrachromosomal recombination in vitro and in vivo. Cancer Res. 1997;57:4378–4383. [PubMed] [Google Scholar]
- Sellström U, Söderström G, de Wit C, Tysklind M. Photolytic debromination of decabromodiphenyl ether (DeBDE) Organohalogen Compounds. 1998;35:447–450. doi: 10.1021/es034682c. [DOI] [PubMed] [Google Scholar]
- She J, Petreas M, Winkler J, Visita P, McKinney M, Kopec D. PBDEs in San Francisco Bay Area: measurements in harbor seal blubber and human breast adipose tissue. Chemosphere. 2002;46:697–707. doi: 10.1016/s0045-6535(01)00234-x. [DOI] [PubMed] [Google Scholar]
- She J, Winkler J, Visita P, McKinney M, Petreas M. Analysis of PBDEs in seal blubber and human breast adipose tissue samples. Organohalogen Compounds. 2000;47:53–56. [Google Scholar]
- Söderström G, Sellström U, de Wit CA, Tysklind M.2004Photolytic debromination of decabromodiphenyl ether (BDE 209) Environ Sci Technol 38127–132.doi: 10.1021/es034682c [Online 13 November 2003]. [DOI] [PubMed] [Google Scholar]
- Solomon GM, Weiss PM. Chemical contaminants in breast milk: time trends and regional variability. Environ Health Perspect. 2002;110:A339–A347. doi: 10.1289/ehp.021100339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellman SD, Djordjevic MV, Britton JA, Muscat JE, Citron ML, Kemeny M, et al. Breast cancer risk in relation to adipose concentrations of organochlorine pesticides and polychlorinated biphenyls in Long Island, New York. Cancer Epidemiol Biomarkers Prev. 2000;9:1241–1249. [PubMed] [Google Scholar]
- Strandman T, Koistinen J, Vartiainen T. Polybrominated diphenyl ethers (PBDEs) in placenta and human milk. Organohalogen Compounds. 2000;47:61–64. [Google Scholar]
- Thomas GO, Moss S, Hall AJ, Jones KC. Absorption of PBDEs and PCBs by grey seals (Halichoerus grypus) Organohalogen Compounds. 2003;62:232–235. [Google Scholar]
- U.K. MAFF (Ministry of Agriculture, Fisheries and Food) 1983. Polychlorinated Biphenyl (PCB) Residues in Food and Human Tissues. The 13th report of the Steering Group of Food Surveillance. London:Her Majesty’s Stationery Office.
- Vartiainen T, Jaakkola JJK, Saarikoski S, Tuomisto J. Birth weight and sex of children and the correlation to the body burden of PCDDs/PCDFs and PCBs of the mother. Environ Health Perspect. 1998;106:61–66. doi: 10.1289/ehp.9810661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viant MR, Rosenblum ES, Tieerdema RS.2003NMR-based metabolomics: a powerful approach for characterizing the effects of environmental stressors on organism health Environ Sci Technol 374982–4989.doi: 10.1021/es034281x [Online 19 September 2003]. [DOI] [PubMed] [Google Scholar]
- WHO 1993. Environmental Health Criteria 140, Polychlorinated Biphenyls and Terphenyls. Geneva:World Health Organization. Available: http://www.inchem.org/documents/ehc/ehc/ehc140.htm [accessed 12 April 2004].
- Wilford BH, Thomas GO, Alcock RE, Jones KC. Polyurethane foam as a source of PBDEs to the environment. Organohalogen Compounds. 2003;61:219–222. [Google Scholar]
- Yared E, McMillan TJ, Martin FL. Genotoxic effects of oestrogens in breast cells detected by the micronucleus assay and the comet assay. Mutagenesis. 2002;17:345–352. doi: 10.1093/mutage/17.4.345. [DOI] [PubMed] [Google Scholar]
- Zheng T, Holford TR, Tessari J, Mayne ST, Owens PH, Ward B, et al. Breast cancer risk associated with congeners of polychlorinated biphenyls. Am J Epidemiol. 2000;152:50–58. doi: 10.1093/aje/152.1.50. [DOI] [PubMed] [Google Scholar]