Summary
Extreme myopia presents a notable clinical challenge in management, with existing approaches having limitations. Repeated low-level red-light therapy (RLRL) has shown potential for myopia control. This multi-center prospective study included 59 children (101 eyes, aged 3–16.3 years) with extreme (SER ≤ −10D) or high myopia (-6D to −9.75D), who underwent twice-daily RLRL for 12 months 55 children (94 eyes) completed the follow-up. After treatment, significant improvements were observed: spherical equivalent error increased by 0.549D (95% confidence interval [CI]: 0.430 to 0.668, p < 0.001), axial length shortened by 0.056 mm (95% CI: −0.087 to −0.025, p = 0.001), best-corrected visual acuity improved by 0.110 LogMAR, and choroidal thickness increased by 34.84 μm, with no adverse events. Subgroup analysis found comparable efficacy between the extreme and high myopia groups. These results indicate RLRL is a safe, non-invasive option for managing extreme myopia in children, with meaningful clinical value.
Subject areas: Ophthalmology, Pediatrics, Therapeutics
Graphical abstract

Highlights
-
•
RLRL improves spherical equivalent error, axial length, and visual acuity
-
•
RLRL shows comparable efficacy in extreme myopia and high myopia
-
•
This non-invasive therapy provides a safe approach for extreme myopia
Ophthalmology; Pediatrics; Therapeutics
Introduction
Extreme myopia, also referred to as extreme high myopia1 or ultra-high myopia,2 and defined as a spherical equivalent error (SER) ≤-10D, is characterized by excessive axial elongation.3 Compared to low-to-moderate and high myopia, it significantly raises the lifetime risk of vision-threatening complications, such as choroidal neovascularization, retinal detachment.4,5 Epidemiological data indicate that the prevalence of extreme myopia varies across different regions, ranging from 0.3% to 1.06%,6,7 and has shown an increasing trend in recent years.8,9 Considering the current world population,10 it can be inferred that the number of individuals with extreme myopia may have reached hundreds of millions.
The treatment of extreme myopia remains a significant clinical challenge, as existing intervention methods mainly face four major dilemmas. The first is the limitation of the scope of application: for instance, low-dose atropine,11 orthokeratology,12,13 and refractive surgery,14 which are commonly used for low-to-moderate myopia, are rarely used for extreme myopia. Although the implantable collamer lens (ICL) is applicable for high myopia and extreme myopia, it has age restrictions.15,16 The second is the lack of technical maturity: take multifocal contact lenses17 and scleral collagen cross-linking18,19,20 as examples. They lack long-term, large-sample evaluations, which limits their wider application in the clinical setting. The third is the clinical controversy, a typical example is the posterior scleral reinforcement (PSR) surgery, as an invasive procedure, its safety raises concerns.21,22 Fourth, currently, the treatment of extreme myopia is often symptom-oriented and fails to eliminate the root cause, which is to inhibit the growth of the axial length (AL). These constraints underscore the urgent need for safe, effective, and preferably non-invasive treatments for extreme myopia.
Repeated low-level red-light therapy (RLRL) has emerged as a promising approach for myopia control. Clinical trials have demonstrated its effectiveness in slowing AL elongation in low-to-moderate myopia,23,24,25,26 with a favorable safety profile.27 Preclinical evidence also indicates its potential for myopia prevention.28,29,30,31 Recent work by Xu et al.32 has extended these findings to the filed of high myopia, showing an adjusted one-year mean AL change of −0.06 mm in children with SER ranging from -4D to −10D (mean: −5.88D). This effect of RLRL in inhibiting the growth of the AL is believed to have a biological basis. It has been reported that a possible mechanism is that RLRL may counteract scleral hypoxia by increasing choroidal blood flow33,34 and regulate retinal dopamine signaling via D2 receptors to inhibit AL growth.35 However, the safety of RLRL must also be taken seriously, and it should be used under appropriate conditions. Ostrin and Schill36 evaluated two RLRL therapy devices and found that 3 min of continuous irradiation approached or exceeded the Maximum Permissible Exposure of the international safety standard.36 This may put the retina at risk of photochemical and thermal damage. Besides, a case report mentioned that a 12-year-old girl developed retinal outer segment damage and visual acuity decline after receiving RLRL therapy for 5 months,37 suggesting that some individuals may be more sensitive to the phototoxicity of red light.
To date, no studies have evaluated the effect of RLRL for extreme myopia. Our investigation aims to determine whether RLRL could expand the treatment options for managing extreme myopia.
Results
Participants selection
Initially, 133 children were screened over a 6-month recruitment period, and 101 children (165 eyes) met the inclusion criteria. Exclusions at baseline included: 11 children (13 eyes) with macular holes; 6 children (6 eyes) with rhegmatogenous retinal detachment; 4 children (6 eyes) with choroidal neovascularization; 1 child (1 eye) with glaucoma; 9 children (18 eyes) with prior red-light therapy exposure; and 4 children (8 eyes) who lived at school and whose guardians could not accompany them during device use. Additionally, 12 eyes were excluded due to incomplete measurements (SER: 1 child, 2 eyes; BCVA: 2 children, 3 eyes; ChT: 4 children, 7 eyes), leaving 59 children (101 eyes). During the 12-month follow-up, 3 children (5 eyes) were excluded for missing AL data, and 1 child (2 eyes) was excluded for missing ChT data. Ultimately, 55 children (94 eyes) completed the study and were included in the final analysis (Figure 1).
Figure 1.
Flow chart depicts the selection process of participants
Baseline characteristics of the included children
Prior to treatment, 7 children with high myopia used Orthokeratology Lens, other children wear single-vision spectacle lenses they received no other treatment. The average age was 9.65 ± 3.77 years (Table1), with a range of 3–16.3 years. Of the children, 56.4% (31/55) were boys. The mean SER was −11.34 ± 3.88D, the mean AL was 27.52 ± 1.78 mm. Of all included eyes, 59.6% (56/94) had a SER ≤ −10D, 82.98% (78/94) had an AL of 26 mm or greater, and 59.6% (56/94) had an AL of 27 mm or greater. The mean BCVA (LogMAR) was 0.216 ± 0.360. The mean ChT was 157.42 ± 66.59 μm. The mean duration of follow-up was 358.47 days (Range: 252 to 399 days).
Table 1.
Baseline characteristics of included patients
| Characteristics | ALL N = 94 |
Extreme myopia N = 56 |
High myopia N = 38 |
p |
|---|---|---|---|---|
| Gender (N, %) | ||||
| Boys | 31 (56.4%) | 18 (56.25%) | 13 (56.52%) | 1.000 |
| Age (years) | ||||
| Mean ± SD | 9.65 ± 3.77 | 10.14 ± 3.94 | 8.93 ± 3.44 | 0.117 |
| Range | 3 to 16.3 | 3 to 16.3 | 3.4 to 15.6 | |
| SER (D) | ||||
| Mean ± SD | −11.34 ± 3.88 | −13.72 ± 3.19 | −7.83 ± 1.23 | <0.001 |
| Range | −23.5 to −6 | −23.5 to −10.125 | −9.875 to −6 | |
| AL (mm) | ||||
| Mean ± SD | 27.52 ± 1.78 | 28.40 ± 1.63 | 26.23 ± 1.07 | <0.001 |
| Range | 24.04 to 32.95 | 25.63 to 32.95 | 24.04 to 28.28 | |
| BCVA (LogMAR) | ||||
| Mean ± SD | 0.216 ± 0.360 | 0.210 ± 0.308 | 0.224 ± 0.430 | 0.867 |
| Range | 0 to 2.4 | 0 to 1.4 | 0 to 2.4 | |
| ChT (μm) | ||||
| Mean ± SD | 157.42 ± 66.59 | 135.34 ± 63.48 | 189.95 ± 57.71 | <0.001 |
| Range | 57 to 337 | 57 to 337 | 75 to 337 | |
| Follow-up duration (days) | ||||
| Mean ± SD | 358.47 ± 32.04 | 358.71 ± 33.94 | 358.11 ± 29.46 | 0.927 |
| Range | 252 to 399 | 252 to 399 | 273 to 398 | |
SD, standard deviation; SER, spherical equivalent error; AL, axial length; BCVA, best-corrected visual acuity; ChT, choroidal thickness.
The distribution of age and gender was balanced between the extreme myopia group and the high myopia group, and there was no statistically significant difference in follow-up duration between the two groups (Table1).
Mean changes of outcomes in children and comparisons between two subgroups
The mean compliance was 81.9% for all participants, and was 83.8% and 79.1% for extreme myopia children and high myopia children.
Mean changes in spherical equivalent error
All children showed a significant mean SER change of 0.549D (95% CI: 0.430 to 0.668, p < 0.001), Table 2. The extreme myopia subgroup had a mean change of 0.607D (95% CI: 0.446 to 0.768, p < 0.001), while the high myopia subgroup had a mean change of 0.464D (95% CI: 0.284 to 0.644, p < 0.001). No significant difference was found between the extreme and high myopia subgroups in mean changes (p = 0.235).
Table 2.
Mean changes of outcomes in children and subgroup comparisons (Extreme vs. High)
| Changes in outcomes | ALL |
Extreme myopia |
High myopia |
p |
|---|---|---|---|---|
| N = 94 | N = 56 | N = 38 | ||
| SER (D) | 0.549 (0.430–0.668)a | 0.607 (0.446–0.768)a | 0.464 (0.284–0.644)a | 0.235 |
| AL (mm) | −0.056 (−0.087 to −0.025)a | −0.058 (−0.097 to −0.018)a | −0.054 (−0.107 to −0.001)a | 0.908 |
| BCVA (LogMAR) | −0.110 (−0.166 to −0.053)a | −0.077 (−0.122 to −0.032)a | −0.158 (−0.283 to −0.032)a | 0.230 |
| ChT (μm) | 34.84 (22.06–47.62)a | 27.84 (12.07–43.61)a | 45.16 (23.24–67.08)a | 0.199 |
Data are expressed with mean changes with a confidence interval.
SER, spherical equivalent error; AL, axial length; BCVA, best-corrected visual acuity; ChT, choroidal thickness.
Means significant difference in changes (significant difference between baseline and follow-up).
Mean changes in axial length
All children showed a significant mean AL reduction of −0.056 mm (95% CI: −0.087 to −0.025, p = 0.001). The extreme myopia subgroup had a mean reduction of −0.058 mm (95% CI: −0.097 to −0.018, p = 0.005), while the high myopia subgroup had a mean reduction of −0.054 mm (95% CI: −0.107 to −0.001, p = 0.048). No significant difference was observed between subgroups in mean AL changes (p = 0.908).
Mean changes in best-corrected visual acuity
All children showed a significant mean BCVA improvement of −0.110 LogMAR (95% CI: −0.166 to −0.053, p < 0.001). The extreme myopia subgroup had a mean improvement of −0.077 LogMAR (95% CI: −0.122 to −0.032, p = 0.001), while the high myopia subgroup had a mean improvement of −0.158 LogMAR (95% CI: −0.283 to −0.032, p = 0.015). No significant difference was found between subgroups in mean BCVA changes (p = 0.230).
Mean changes in choroid thickness
All children showed a significant mean ChT increase of 34.84 μm (95% CI: 22.06 to 47.62, p < 0.001). The extreme myopia subgroup had a mean increase of 27.84 μm (95% CI: 12.07 to 43.61, p = 0.001), while the high myopia subgroup had a mean increase of 45.16 μm (95% CI: 23.24 to 67.08, p < 0.001). No significant difference was found between subgroups in mean ChT changes (p = 0.199).
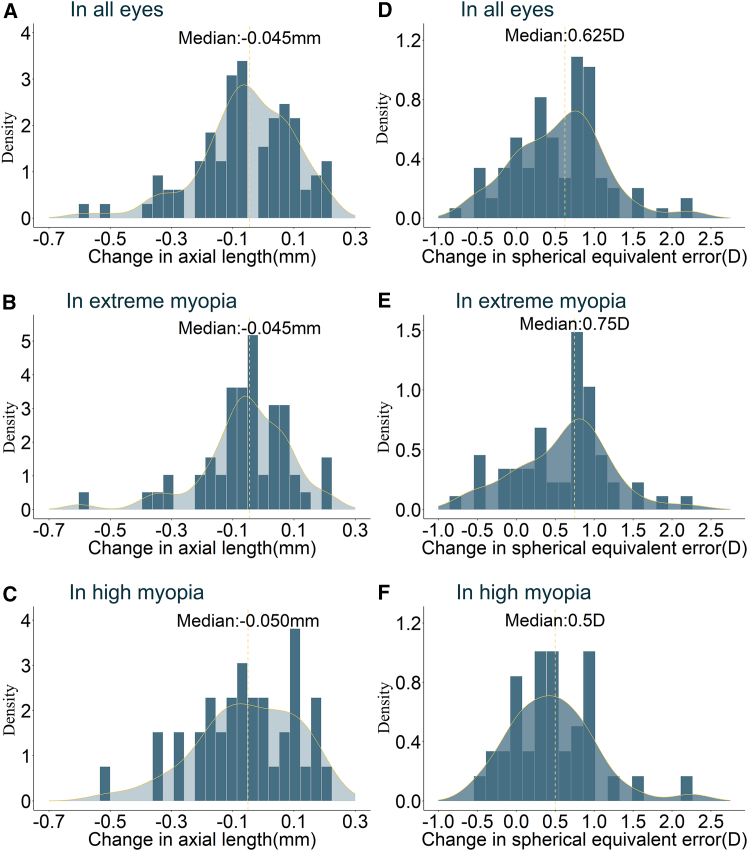
We generated histograms illustrating the distribution (with median values) of changes in SER and AL for all children and for each of the two subgroups, to better present the data (Figure 2).
Figure 2.
Histogram illustrates the changes in primary outcomes within all participants and extreme myopia subgroups
(A) Histogram of changes in axial length in all eyes. (B) Histogram of changes in axial length in eyes with extreme myopia. (C) Histogram of changes in axial length in eyes with high myopia. (D) Histogram of changes in spherical equivalent error in all eyes. (E) Histogram of changes in spherical equivalent error in eyes with extreme myopia. (F) Histogram of changes in spherical equivalent error in eyes with high myopia).
Degree of changes in primary outcomes in children and comparisons between subgroups
Degree of changes in spherical equivalent error
A total of 6 children (6.38%) improved by ≥ 1.5D (Table 3), including 4 (7.14%) in extreme myopia and 2 (5.26%) in high myopia. Sixteen (17.02%) improved by 1–1.5D (12 [21.43%] for extreme myopia subgroup vs. 4 [10.53%] for high myopia subgroup), 34 (36.17%) by 0.5–1D (20 [35.71%] vs. 14 [36.84%]), 17 (18.09%) by < 0.5D (9 [16.07%] vs. 8 [21.05%]), and 8 (8.51%) showed no change (3 [5.36%] vs. 5 [13.16%]). Seven (7.45%) progressed by < 0.5D (3 [5.36%] vs. 4 [10.53%]), and 6 (6.38%) progressed by ≥ 0.5D (5 [8.93%] vs. 1 [2.63%]). No significant difference was found in the distribution of changes between subgroups (p = 0.465).
Table 3.
Degree of changes in primary outcomes in children and subgroup comparisons (Extreme vs. High)
| Degree of changes | ALL |
Extreme myopia |
High myopia |
p |
|---|---|---|---|---|
| N = 94 | N = 56 | N = 38 | ||
| For changes in SER | 0.465 | |||
| Improved 1.5D or more | 6 (6.38%) | 4 (7.14%) | 2 (5.26%) | |
| Improved 1D to 1.5D | 16 (17.02%) | 12 (21.43%) | 4 (10.53%) | |
| Improved 0.5 D to 1D | 34 (36.17%) | 20 (35.71%) | 14 (36.84%) | |
| Improved <0.5D | 17 (18.09%) | 9 (16.07%) | 8 (21.05%) | |
| Unchanged | 8 (8.51%) | 3 (5.36%) | 5 (13.16%) | |
| Progressed <0.5D | 7 (7.45%) | 3 (5.36%) | 4 (10.53%) | |
| Progressed 0.5 D or more | 6 (6.38%) | 5 (8.93%) | 1 (2.63%) | |
| For changes in AL | 0.712 | |||
| Shortened 0.4 mm or more | 2 (2.13%) | 1 (1.79%) | 1 (2.63%) | |
| Shortened 0.2 mm–0.4 mm | 9 (9.57%) | 5 (8.93%) | 4 (10.53%) | |
| Shortened less than 0.2 mm | 47 (50.00%) | 30 (53.57%) | 17 (44.74%) | |
| Unchanged | 1 (1.06%) | 1 (1.79%) | 0 | |
| Increased less than 0.2 mm | 33 (35.11%) | 17 (30.36%) | 16 (42.11%) | |
| Increased 0.2 mm or more | 2 (2.13%) | 2 (3.57%) | 0 |
Data are expressed with frequency and percentage.
In this table, “Progressed” means myopic shift (e.g., from −6 D at baseline to −6.5 D at follow-up), while “improved” means the reversed process (e.g., from −10 D at baseline to −9.5 D at follow-up).
SER, spherical equivalent error; AL, axial length.
Degree of changes in axial length
Two children (2.13%) showed ≥0.4 mm shortening (1 [1.79%] in extreme myopia, 1 [2.63%] in high myopia). Nine (9.57%) showed 0.2–0.4 mm shortening (5 [8.93%] vs. 4 [10.53%]), 47 (50.00%) showed <0.2 mm shortening (30 [53.57%] vs. 17 [44.74%]), and 1 (1.06%) showed no change (1 [1.79%] vs. 0). Thirty-three (35.11%) showed <0.2 mm increase (17 [30.36%] vs. 16 [42.11%]), and 2 (2.13%) showed ≥0.2 mm increase (2 [3.57%] vs. 0). No significant difference in change distribution between subgroups (p = 0.712).
Other analysis
We carefully compared the baseline and follow-up fundus photographs and OCT images of each child. No abnormal changes were observed.
We explored the relationships between age and AL progression, as well as age and ChT progression (see Figure S1). We performed quantitative fitting using equations (conducted separately for the overall group and the two subgroups), and found that none of the equations were statistically significant, meaning that age had no impact on the intervention outcomes.
We also generated boxplots to illustrate the distribution of SER and AL measurements obtained at baseline and follow-up for all participants, as well as for the two subgroups (see Figure S2).
33 of them had measurements of AER and AL taken six months before enrollment (without any treatment). The analysis results showed that the average progression of AL within six months was 0.173 (95%CI: 0.093 to 0.253) mm, and the mean SER progression was −0.575 (95%CI:-0.832 to −0.318) D.
Discussion
The management of extreme myopia has long represented a critical frontier in ophthalmology. This study establishes repeated RLRL as a novel non-invasive therapy for extreme myopia treatment. Over a 12-month period, in children with extreme myopia, RLRL achieves a significant reduction in AL (−0.056 mm), improvement in SER (+0.549D), and improvement in BCVA (0.110 LogMAR), with no adverse events noted. These findings challenge the traditional paradigm of complication-focused extreme myopia management, offering an evidence-based non-invasive approach for active axial control in this vulnerable population.
Treatment of extreme myopia: Limitations and challenges of current clinical strategies
To better understand the groundbreaking value of repeated RLRL, a comprehensive evaluation of existing myopia interventions is essential. Current clinical strategies can be categorized into four types: pharmacological agents (e.g., atropine), optical corrections (e.g., orthokeratology, multifocal soft contact lenses), and surgical procedures (e.g., PSR, ICL), and scleral collagen cross-linking. However, all faced dilemma in managing extreme myopia, as outlined in the introduction. This kind of dilemma is systemic. Not only is the treatment effect unsatisfactory, but many treatment measures also have limitations, such as inconvenience in use, limited application scope, the ability to only relieve symptoms, and even safety issues. For instance, while low-dose atropine is commonly used for low-to-moderate myopia, its application in high myopia, let alone extreme myopia, is limited. This is clearly reflected by the mean baseline SER in atropine studies, which ranges from −1.10D to −4.89D.11 Indeed, high-concentration atropine has been reported to show a strong effect on myopia control. For example, Pineles S L et al. reported that the myopic progression in the treatment group (1% atropine) was −0.28 ± 0.92 D over 2 years, compared with −1.20 ± 0.69 D in the control group (placebo),38 indicating a significant therapeutic effect. And high-concentration atropine works for no matter Mendelian myopia cases or non-Mendelian myopia cases.39 However, the side effects were severe. 100% of the children experienced photophobia, and 17% withdrew due to side effects. Orthokeratology40 and refractive surgery41 face similar challenges. Although ICL is applicable to extreme myopia, it is restricted to patients over 18 years old with stable refraction,15,16,42 thus excluding the pediatric population. While PSR was once considered the only method to inhibit AL elongation, its efficacy remains limited with substantial room for improvement.21 Dong X et al. reported that the average annual increase in AL in high-myopia children treated with PSR was 0.29 mm,43 while that in the control group was 0.82 mm per year. Similarly, Wang YH et al. reported that in a group of children with extreme myopia, the rate of AL elongation decreased from 0.505 mm per year to 0.382 mm per year before and after PSR surgery.44 Moreover, due to significant safety risks,45 PSR has sparked intense controversy and has not been widely implemented in large-scale clinical practice. The same is true for scleral collagen cross-linking. Due to the irreversible nature of the treatment process,18,46 many people are concerned about its safety. Currently, relevant clinical research primarily focuses on the treatment of keratoconus and thin corneas,20,47 while explorations in myopia control are still mostly at the animal-experiment stage.19,48,49
Repeated low-level red-light therapy: A breakthrough in solving the axial elongation dilemma of extreme myopia
There are some uncommon but confirmed cases where AL shortening occurs following certain myopia control interventions.50 However, in principle, aside from PSR, which can shorten the AL by generating mechanical force with the aid of reinforcement materials, other measures—whether it is creating myopic defocus51 (a typical example being multifocal soft contact lenses), changing the refractive power of the cornea (e.g., orthokeratology, refractive surgery), or altering the refractive power of the lens (e.g., ICL)—were originally designed to correct refraction. For most therapies, they can merely slow down the growth rate of the AL rather than shorten it. The crux of the problem of extreme myopia lies precisely in the excessive elongation of the AL. It is not merely about the extremely high degree of myopia. As the AL becomes overly long, a series of fundus complications are likely to occur, ranging from posterior staphyloma to myopic maculopathy52; and from myopic choroidal neovascularization to myopic macular retinoschisis. In our study, 4 cases of myopic choroidal neovascularization were excluded. These patients were already undergoing intravitreal anti-vascular endothelial growth factor injections, and the long-term prognosis may not be favorable.53 This is because these treatments are merely symptomatic, and the fundamental issue of excessive AL remains unresolved. It is difficult to even alleviate the symptoms, which was one of the dilemmas for extreme myopia treatment in the past. This has imposed a huge economic and health burden on patients. The advent of RLRL has offered great promise for surmounting this dilemma. In the present study, RLRL induced significant axial shortening (−0.056 mm) with no device-related adverse events, demonstrating superior risk-benefit profiles.
Unique biological mechanisms of repeated low-level red-light therapy in myopia regulation and its action differences
RLRL may regulate myopia through different biological mechanisms. Notably, RLRL treatment significantly induced choroidal thickening (+34.84 μm), and the magnitude was consistent with previous studies on low-to-moderate myopia.23,24,25,26 This structural change may reverse scleral hypoxia by improving choroidal blood flow. Scleral hypoxia has been proven to drive extracellular matrix remodeling by upregulating matrix metalloproteinases during myopia progression.54,55 Different from other interventions, such as the neurotransmitter regulation of atropine, RLRL directly acts on mitochondrial function through non-invasive photobiomodulation. RLRL activates cytochrome c oxidase, promoting the synthesis of adenosine triphosphate to support collagen reorganization in scleral fibroblasts, while inhibiting reactive oxygen species-mediated oxidative stress.56,57 This unique dual bioenergetic mechanism may explain why the efficacy of RLRL is independent of age (in this study, there was no significant correlation between age and AL change, p > 0.05).
RLRL can also stimulate the secretion of retinal dopamine in a dose-dependent manner.58,59,60 This neuromodulatory effect synergizes with its bioenergetic action via cyclic adenosine monophosphate (cAMP) pathway modulation: Dopamine binding to Gi/o-coupled D2-like receptors (D2/D3/D4) suppresses intracellular cAMP production, thereby reducing cAMP-dependent signaling. This mechanism antagonizes Gs protein-mediated adenylate cyclase activation, a biochemical cascade implicated in AL elongation.35 Crucially, the similar therapeutic effects observed in the high myopia and extreme myopia subgroups suggest that RLRL mainly targets the basic pathways of myopia development rather than refractive correction. The significant improvement in BCVA (an increase of 0.110 LogMAR) further confirms this pattern. This most likely reflects the enhancement of retinal neural processing through contrast sensitivity modulation,26,61 rather than the traditional optical correction mechanism. The improvement in visual acuity may be related to the enhancement of retinal neural processing through contrast sensitivity modulation. RLRL may affect the function of retinal neurons, improving their ability to process visual information. For example, Zhu M et al. observed through multifocal electroretinogram that after RLRL treatment, the response density and amplitude of the P1 wave in the first ring of the retina significantly increased,26 indicating enhanced retinal neuronal function. Besides, RLRL enhances the activity of mitochondrial cytochrome c oxidase through photobiomodulation, improves ATP production, and reduces oxidative stress and inflammation, thereby potentially improving the function of photoreceptor cells and the efficiency of retinal signal transmission.56,57
Comparison with Xu et al.'s high myopia cohort: Expansion of repeated low-level red-light therapy’s efficacy
Clinical evidence supporting RLRL for high/extreme myopia remains sparse. Previously, only Xu et al. have demonstrated a 0.06 mm AL reduction in high myopia (mean SER: −5.88D) children following 12-month RLRL therapy.32 Our study now extends these findings to previously excluded populations, achieving comparable AL shortening in extreme myopia (mean SER: −13.72D) and high myopia (mean SER: −7.83D) cohorts. This bridges a critical evidence gap for RLRL’s efficacy across the myopia severity spectrum. Nevertheless, due to the scarcity of existing data, large-sample studies are still needed for verification.
Advantages of repeated low-level red-light therapy in clinical application: Feasibility and broad treatment time window
From the perspective of clinical applicability, RLRL demonstrates a broader treatment time window than existing therapies. In this study, the youngest subject was only 3 years old, and the oldest was close to adulthood. This broad treatment time window allows extreme myopic children to initiate intervention at an earlier stage. Since the risks of fundus lesions (such as posterior staphyloma and macular schisis) are directly related to the progressive growth of the AL, early treatment can significantly reduce the incidence of irreversible complications.
In terms of operational feasibility, RLRL treatment does not rely on a hospital setting and can be carried out at home in a standardized manner after simple training. The standard protocol is twice a day, 3 min each time, and can be flexibly implemented for 5 or 7 days a week.29 The long-term compliance rate as high as 81.9% (12-month follow-up data in this study) further validates its sustainability as a routine prevention and control measure, which is closely related to its non-invasive nature and convenient usage mode. However, it should also be noted that there is a clear association between the level of compliance and the severity of myopia in patients. The extreme myopia group showed higher compliance compared with the high myopia group (83.8% vs. 79.1%).
It is worth emphasizing that RLRL has shown good efficacy at different power levels,62 providing more options for patients.
Safety of repeated low-level red-light therapy treatment and considerations for clinical application
The safety of RLRL treatment and its related considerations in clinical application are important prerequisites for its promotion as a new therapy. First, throughout the 12-month follow-up, no adverse events were observed in any participants. Detailed evaluations of fundus photographs and OCT images before and after treatment revealed no structural abnormalities in the retina, confirming the absence of photochemical or thermal damage. Second, consistent with previous studies (e.g., Zhu et al.26; Chen et al.27), our data support the favorable safety profile of RLRL. The device used in this study (YF020A) is a Class II medical device certified by Chinese regulatory authorities, with a Class I laser light source, which adheres to international safety standards for controlled light exposure. Third, practical safety considerations for clinical application: despite rare reports of retinal sensitivity to red light in individual cases (Liu et al.37), our high compliance rate (81.9%) and standardized protocol (3 min twice daily, under guardian supervision) demonstrate that RLRL can be safely implemented in pediatric populations with proper training and monitoring.
Conclusion
In this prospective study of pediatric extreme myopia, daily 650-nm RLRL therapy demonstrated significant refractive improvement, AL shortening, BCVA improvement, and choroidal thickening over 12 months, with comparable efficacy across high and extreme myopia subgroups. The treatment exhibited a satisfying safety profile, showing no structural abnormalities on advanced imaging. These findings position RLRL as a viable non-invasive option for extreme myopia management.
Strengths
This study’s strengths include its prospective design, objective compliance monitoring via networked devices, and standardized imaging protocols.
Limitations of the study
Limitations include the inclusion of both eyes per participant, though statistically adjusted, which may introduce inter-eye correlation bias. Besides, we did not screen for genetic syndromes associated with high myopia, nor did we ensure the inclusion of idiopathic progressive myopia. In addition, there is a lack of control group and randomization, which may lead to an overestimation of the treatment effect. Lastly, due to the limited sample size, we did not conduct further subgroup analyses stratified by male and female. Therefore, it remains unclear what impact gender may have on treatment efficacy.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Ying Jie (jie_yingcn@aliyun.com).
Materials availability
The data that support these findings of the study are available upon request from the corresponding authors.
Data and code availability
-
•
All original data will be made available from the lead contact upon request.
-
•
All original code will be made available from the lead contact upon request.
-
•
Any additional information required to analyze the data reported in this article is available from the lead contact upon request.
Acknowledgments
We thank for Beijing Meier Eye Hospital for helping recruiting participants.
The present study was funded by Capital Health Research and Development of Special (Ying Jie 2022-1-1081).
Author contributions
All authors read and approved the final article. Research concept and design (J.Y., and D.A.L.); data collection and performance of the research (T.L., C.K., and F.W.Y.); analysis and interpretation of data (T.L., C.K., F.W.Y., and M.D.L); writing of the article (T.L., C.K., and F.W.Y.); critical revision of the article (O.M.K., and D.A.L.); and supervision (J.Y., and D.A.L.).
Declaration of interests
The authors declare no competing interests.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological samples | ||
| Children with high or extreme myopia | Beijing Tongren Hospital, Beijing Meier Eye Hospital |
Not applicable |
| Software and algorithms | ||
| R Project for Statistical Computing, version 4.4.2 | R Foundation for Statistical Computing | https://www.r-project.org/ |
Experimental model and study participant details
Participants were children and adolescents aged 3 to 18 years, with no gender restrictions, recruited from the outpatient departments of Beijing Tongren Hospital and the Beijing Meier Eye Hospital, Beijing, China. Participants were recruited from February 28, 2022 to August 23, 2022, with a planned 12-month follow-up for each child. There were 55 children, they were all allocated to the treatment group as this is a single-arm study. All participants were Chinese, with 31 males and 24 female. The mean age was 9.65 ± 3.77 years.
This study adhered to the tenets of the Declaration of Helsinki, and was approved by the Ethics Committee of Beijing Tongren Hospital, Capital Medical University (NO. TRECKY2021-239). The present study was registered on ClinicalTrials.gov (registration number: NCT06738095).
Method details
Study design and setting
This was a 12-month, multi-center, prospective, single-arm study.
Inclusion and exclusion criteria for participants
Inclusion criteria
A. Age: 3 to 18 years old. B. SER: ≤-6D. C. Cooperation: Able to complete required ophthalmic examinations. D. Follow-up Compliance: Available for follow-up visits at specified times. E. Guardian Assistance: Guardian can assist with the use of the RLRL instrument.
Exclusion criteria
A. High Myopia Complications: Conditions such as choroidal neovascularization, macular hole, or rhegmatogenous retinal detachment. B. Other macular diseases: e.g., central serous retinopathy, glaucoma, cataract, uveitis. C. Systemic Conditions: Photosensitivity, psoriasis, albinism, hyperactivity disorder, nephrotic syndrome. D. Immune System Diseases: Systemic lupus erythematosus or other immune system diseases. E. Pregnancy. F. Prior RLRL Instrument Use: Use of similar RLRL instruments for treatment in the past six months. G. Guardian Non-Compliance: Guardian unable to assist with the use of the instrument. H. Inability to Cooperate: Unable to complete required ophthalmic examinations or have difficulty in attending follow-up appointments.
Intervention
The children received daily 650-nm RLRL treatment using a head-mounted device (Product Model: YF020A, Manufacturer: Hunan EnVan Technology Co., Ltd.) for 3 minutes twice a day over 12 months. The light source used is a Class I laser. The device has obtained a Class II medical device certification issued by Chinese regulatory authorities.
Intervention compliance
The networked device automatically recorded the frequency and duration of its use. Compliance is calculated as the ratio of actual usage time to expected usage time. The expected usage duration is determined by multiplying the follow-up period (in days) by 2 and then by 3 min minutes. For example, for a child followed up for 399 days, the expected usage duration is 2394 minutes (399×2×3). If the actual usage duration was 2250 minutes, compliance can be calculated as 93.98% (2250/2394).
Outcomes and measurements
Primary outcomes include changes in SER and changes in AL. SER, calculated from the dioptric powers of the sphere and half of the cylinder, was measured using an autorefractor (ARK-510A; Nidek Co. Ltd., Aichi, Japan) after pupil dilation with Mydrin-P eye drops. High Myopia and extreme myopia were defined as SER ≤ -6D, and ≤ -10D, respectively. Additional outcome measures encompassed best-corrected visual acuity (BCVA), which was quantified using a standardized logarithmic visual acuity chart. In order to evaluate safety, fundus photography and optical coherence tomography (OCT) imaging were also conducted. OCT images were obtained using the enhanced-depth imaging technique (Spectralis HRA+OCT; Heidelberg Engineering). The subfovea choroid thickness (ChT) was measured automatically using the built-in software of OCT. Fundus photography was performed with a Canon retinal fundus camera (CR-DGI; Canon, Inc.) after pupil dilation.
Quantification and statistical analysis
Calculation of sample size see protocol in supplemental information file. For continuous data, basic statistical descriptions were presented as the mean ± standard deviation (SD), along with the range. For categorical data, basic statistical descriptions were provided in the form of frequency and percentage. An independent t-test was employed to compare continuous outcomes (e.g., changes in SER, calculated by eye. In the present study, only age and gender were described by person) between the high myopia group and the extreme myopia group. The mean and 95% confidence interval (CI) were utilized to quantify the effect size. A chi-square test was used for comparing categorical outcomes across groups. A paired t-test was applied to compare measurements before and after treatment. The mean difference and 95% CI were used to quantify the effect size. All analyses were conducted using the open-source R program (https://www.r-project.org/, version 4.4.2). The significance level was set at 0.05, two-tailed.
Additional resources
The present study was registered on ClinicalTrials.gov (registration number: NCT06738095). https://clinicaltrials.gov/.
Core measures to ensure the safety of RLRL treatment
-
1.
Strict inclusion/exclusion criteria for vulnerable fundus conditions: To avoid exacerbating pre-existing fundus vulnerabilities, we explicitly excluded patients with high myopia complications (e.g., choroidal neovascularization, macular hole, rhegmatogenous retinal detachment) and other macular diseases (e.g., central serous retinopathy, uveitis) at baseline. This ensured that only patients with relatively stable fundus status were enrolled, reducing the risk of adverse events.
-
2.
Standardized and safe treatment protocols: The RLRL device used (YF020A) is a Class II medical device certified by Chinese regulatory authorities, with a Class I laser light source that adheres to international safety standards. The treatment regimen (3 minutes per session, twice daily) was designed to minimize retinal exposure time while maintaining efficacy, avoiding excessive light exposure that could compromise the delicate fundus.
-
3.
Comprehensive safety monitoring throughout the study: All participants underwent regular fundus examinations, including dilated fundus photography and enhanced-depth imaging OCT at baseline and follow-up. These imaging modalities allowed for detailed assessment of retinal structure, choroidal thickness, and macular integrity. No abnormal changes (e.g., retinal detachment, choroidal thinning, or macular edema) were detected in any patient, confirming that RLRL did not induce fundus damage.
-
4.
Supervision and compliance management: Given the pediatric population, treatment was administered under guardian supervision, ensuring adherence to the prescribed protocol. The networked device automatically recorded usage duration and frequency.
Published: August 26, 2025
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2025.113459.
Contributor Information
An-Li Duan, Email: drduananli@ccmu.edu.cn.
Ying Jie, Email: jie_yingcn@aliyun.com.
Supplemental information
References
- 1.Xia F., Qin B., Shang J., Chen Z., Zhou X., Zhao J., Wang X., Zhou X. Four-year outcomes of small incision lenticule extraction for extreme high myopia and myopic astigmatism. Front. Med. 2020;7 doi: 10.3389/fmed.2020.575779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao H.Y., Zhang J.S., Li M., Chen D.J., Yang X., Wan X.H. Tilt and decentration of the crystalline lens in ultra-high myopia with cataract and its influencing factors: a study based on casia2. Eur. J. Ophthalmol. 2025;35:524–530. doi: 10.1177/11206721241267028. [DOI] [PubMed] [Google Scholar]
- 3.Yuan J., Zhuang Y.Y., Liu X., Zhang Y., Li K., Chen Z.J., Li D., Chen H., Liang J., Yao Y., et al. Exome-wide association study identifies kdelr3 mutations in extreme myopia. Nat. Commun. 2024;15:6703. doi: 10.1038/s41467-024-50580-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang N.K., Chen Y.P., Lai C.C., Chen T.L., Yang K.J., Kuo Y.H., Chao A.N., Wu W.C., Chen K.J., Hwang Y.S., et al. Paediatric retinal detachment: comparison of high myopia and extreme myopia. Br. J. Ophthalmol. 2009;93:650–655. doi: 10.1136/bjo.2008.145920. [DOI] [PubMed] [Google Scholar]
- 5.Zhang X.J., Chen X.N., Tang F.Y., Szeto S., Ling X.T., Lin Z.X., Tham C.C., Pang C.P., Chen L.J., Yam J.C. Pathogenesis of myopic choroidal neovascularization: a systematic review and meta-analysis. Surv. Ophthalmol. 2023;68:1011–1026. doi: 10.1016/j.survophthal.2023.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Nakao S.Y., Miyake M., Hosoda Y., Nakano E., Mori Y., Takahashi A., Ooto S., Tamura H., Tabara Y., Yamashiro K., et al. Myopia prevalence and ocular biometry features in a general japanese population: the nagahama study. Ophthalmology. 2021;128:522–531. doi: 10.1016/j.ophtha.2020.08.023. [DOI] [PubMed] [Google Scholar]
- 7.Mccarty C.A., Livingston P.M., Taylor H.R. Prevalence of myopia in adults: implications for refractive surgeons. J. Refract. Surg. 1997;13:229–234. doi: 10.3928/1081-597X-19970501-08. [DOI] [PubMed] [Google Scholar]
- 8.Holden B.A., Fricke T.R., Wilson D.A., Jong M., Naidoo K.S., Sankaridurg P., Wong T.Y., Naduvilath T.J., Resnikoff S. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123:1036–1042. doi: 10.1016/j.ophtha.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Dolgin E. The myopia boom. Nature. 2015;519:276–278. doi: 10.1038/519276a. [DOI] [PubMed] [Google Scholar]
- 10.Populationstat World population. 2024. https://populationstat.com/
- 11.Ha A., Kim S.J., Shim S.R., Kim Y.K., Jung J.H. Efficacy and safety of 8 atropine concentrations for myopia control in children: a network meta-analysis. Ophthalmology. 2022;129:322–333. doi: 10.1016/j.ophtha.2021.10.016. [DOI] [PubMed] [Google Scholar]
- 12.Jonas J.B., Ang M., Cho P., Guggenheim J.A., He M.G., Jong M., Logan N.S., Liu M., Morgan I., Ohno-Matsui K., et al. Imi prevention of myopia and its progression. Investig. Ophthalmol. Vis. Sci. 2021;62:6. doi: 10.1167/iovs.62.5.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Logan N.S., Bullimore M.A. Optical interventions for myopia control. Eye. 2024;38:455–463. doi: 10.1038/s41433-023-02723-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tychsen L. Refractive surgery for children: excimer laser, phakic intraocular lens, and clear lens extraction. Curr. Opin. Ophthalmol. 2008;19:342–348. doi: 10.1097/ICU.0b013e328302cc89. [DOI] [PubMed] [Google Scholar]
- 15.Schmidinger G., Lackner B., Pieh S., Skorpik C. Long-term changes in posterior chamber phakic intraocular collamer lens vaulting in myopic patients. Ophthalmology. 2010;117:1506–1511. doi: 10.1016/j.ophtha.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 16.Shen Y., Chen X., Xian Y., Wang X., Wang X., Zhou X. Safety of intraocular pressure measurement using air-puff tonometer after implantable collamer lens implantation. J. Cataract Refract. Surg. 2022;48:900–905. doi: 10.1097/j.jcrs.0000000000000886. [DOI] [PubMed] [Google Scholar]
- 17.Agyekum S., Chan P.P., Adjei P.E., Zhang Y., Huo Z., Yip B.H.K., Ip P., Wong I.C.K., Zhang W., Tham C.C., et al. Cost-effectiveness analysis of myopia progression interventions in children. JAMA Netw. Open. 2023;6 doi: 10.1001/jamanetworkopen.2023.40986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raiskup F., Spoerl E. Corneal crosslinking with riboflavin and ultraviolet a. I. Principles. Ocul. Surf. 2013;11:65–74. doi: 10.1016/j.jtos.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Sun M., Zhang F., Li Y., Ouyang B., Wang M., Jiao X., Zhang L., Wang N. Evaluation of the safety and long-term scleral biomechanical stability of uva cross-linking on scleral collagen in rhesus monkeys. J. Refract. Surg. 2020;36:696–702. doi: 10.3928/1081597X-20200807-01. [DOI] [PubMed] [Google Scholar]
- 20.Chen X., Stojanovic A., Eidet J.R., Utheim T.P. Corneal collagen cross-linking (cxl) in thin corneas. Eye Vis. 2015;2:15. doi: 10.1186/s40662-015-0025-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang W., Duan A., Qi Y. Posterior scleral reinforcement to prevent progression of high myopia. Asia. Pac. J. Ophthalmol. 2019;8:366–370. doi: 10.1097/APO.0000000000000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qi Y., Duan A.L., You Q.S., Jonas J.B., Wang N. Posterior scleral reinforcement and vitrectomy for myopic foveoschisis in extreme myopia. Retina. 2015;35:351–357. doi: 10.1097/IAE.0000000000000313. [DOI] [PubMed] [Google Scholar]
- 23.Dong J., Zhu Z., Xu H., He M. Myopia control effect of repeated low-level red-light therapy in chinese children: a randomized, double-blind, controlled clinical trial. Ophthalmology. 2023;130:198–204. doi: 10.1016/j.ophtha.2022.08.024. [DOI] [PubMed] [Google Scholar]
- 24.Jiang Y., Zhu Z., Tan X., Kong X., Zhong H., Zhang J., Xiong R., Yuan Y., Zeng J., Morgan I.G., He M. Effect of repeated low-level red-light therapy for myopia control in children: a multicenter randomized controlled trial. Ophthalmology. 2022;129:509–519. doi: 10.1016/j.ophtha.2021.11.023. [DOI] [PubMed] [Google Scholar]
- 25.Xiong R., Zhu Z., Jiang Y., Kong X., Zhang J., Wang W., Kiburg K., Yuan Y., Chen Y., Zhang S., et al. Sustained and rebound effect of repeated low-level red-light therapy on myopia control: a 2-year post-trial follow-up study. Clin. Exp. Ophthalmol. 2022;50:1013–1024. doi: 10.1111/ceo.14149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu M., Liu Y., Fang D., Li M., Fu T., Yao K., Wang P., Sun X., Xiang Y. Safety of repeated low-level red-light therapy for children with myopia. Photodiagnosis Photodyn. Ther. 2024;47 doi: 10.1016/j.pdpdt.2024.104198. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y., Xiong R., Yang S., Zhu Z., Li H., Xiang K., Congdon N., Wang W., He M. Safety of repeated low-level red-light therapy for myopia: a systematic review. Asia. Pac. J. Ophthalmol. 2024;13 doi: 10.1016/j.apjo.2024.100124. [DOI] [PubMed] [Google Scholar]
- 28.Liu G., Rong H., Liu Y., Wang B., Du B., Song D., Wei R. Effectiveness of repeated low-level red light in myopia prevention and myopia control. Br. J. Ophthalmol. 2024;108:1299–1305. doi: 10.1136/bjo-2023-324260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao K., Tian L., Ma D.L., Zhao S.Q., Li A., Jin Z.B., Jie Y. Daily low-level red light for spherical equivalent error and axial length in children with myopia: a randomized clinical trial. JAMA Ophthalmol. 2024;142:560–567. doi: 10.1001/jamaophthalmol.2024.0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He X., Wang J., Zhu Z., Xiang K., Zhang X., Zhang B., Chen J., Yang J., Du L., Niu C., et al. Effect of repeated low-level red light on myopia prevention among children in china with premyopia: a randomized clinical trial. JAMA Netw. Open. 2023;6 doi: 10.1001/jamanetworkopen.2023.9612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang H., Cui M., Jie Y., Chen T., Kang M., Bai W., Wang B., Wang Y. Efficacy of repeated low-level red-light therapy in the prevention and control of myopia in children. Photodiagnosis Photodyn. Ther. 2024;47 doi: 10.1016/j.pdpdt.2024.104216. [DOI] [PubMed] [Google Scholar]
- 32.Xu Y., Cui L., Kong M., Li Q., Feng X., Feng K., Zhu H., Cui H., Shi C., Zhang J., Zou H. Repeated low-level red light therapy for myopia control in high myopia children and adolescents: a randomized clinical trial. Ophthalmology. 2024;131:1314–1323. doi: 10.1016/j.ophtha.2024.05.023. [DOI] [PubMed] [Google Scholar]
- 33.Zhou X., Zhang S., Yang F., Yang Y., Huang Q., Huang C., Qu J., Zhou X. Decreased choroidal blood perfusion induces myopia in guinea pigs. Investig. Ophthalmol. Vis. Sci. 2021;62:30. doi: 10.1167/iovs.62.15.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou X., Zhang S., Zhang G., Chen Y., Lei Y., Xiang J., Xu R., Qu J., Zhou X. Increased choroidal blood perfusion can inhibit form deprivation myopia in guinea pigs. Investig. Ophthalmol. Vis. Sci. 2020;61:25. doi: 10.1167/iovs.61.13.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou X., Pardue M.T., Iuvone P.M., Qu J. Dopamine signaling and myopia development: what are the key challenges. Prog. Retin. Eye Res. 2017;61:60–71. doi: 10.1016/j.preteyeres.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ostrin L.A., Schill A.W. Red light instruments for myopia exceed safety limits. Ophthalmic Physiol. Opt. 2024;44:241–248. doi: 10.1111/opo.13272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu H., Yang Y., Guo J., Peng J., Zhao P. Retinal damage after repeated low-level red-light laser exposure. JAMA Ophthalmol. 2023;141:693–695. doi: 10.1001/jamaophthalmol.2023.1548. [DOI] [PubMed] [Google Scholar]
- 38.Pineles S.L., Kraker R.T., Vanderveen D.K., Hutchinson A.K., Galvin J.A., Wilson L.B., Lambert S.R. Atropine for the prevention of myopia progression in children: a report by the american academy of ophthalmology. Ophthalmology. 2017;124:1857–1866. doi: 10.1016/j.ophtha.2017.05.032. [DOI] [PubMed] [Google Scholar]
- 39.van der Sande E., Polling J.R., Tideman J.W.L., Meester-Smoor M.A., Thiadens A.A.H.J., Tan E., De Zeeuw C.I., Hamelink R., Willuhn I., Verhoeven V.J.M., et al. Myopia control in mendelian forms of myopia. Ophthalmic Physiol. Opt. 2023;43:494–504. doi: 10.1111/opo.13115. [DOI] [PubMed] [Google Scholar]
- 40.Lawrenson J.G., Shah R., Huntjens B., Downie L.E., Virgili G., Dhakal R., Verkicharla P.K., Li D., Mavi S., Kernohan A., et al. Interventions for myopia control in children: a living systematic review and network meta-analysis. Cochrane Database Syst. Rev. 2023;2 doi: 10.1002/14651858.CD014758.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wen D., Mcalinden C., Flitcroft I., Tu R., Wang Q., Alió J., Marshall J., Huang Y., Song B., Hu L., et al. Postoperative efficacy, predictability, safety, and visual quality of laser corneal refractive surgery: a network meta-analysis. Am. J. Ophthalmol. 2017;178:65–78. doi: 10.1016/j.ajo.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 42.Guber I., Mouvet V., Bergin C., Perritaz S., Othenin-Girard P., Majo F. Clinical outcomes and cataract formation rates in eyes 10 years after posterior phakic lens implantation for myopia. JAMA Ophthalmol. 2016;134:487–494. doi: 10.1001/jamaophthalmol.2016.0078. [DOI] [PubMed] [Google Scholar]
- 43.Dong X., Liu J., Bu J. The efficacy of modified posterior scleral reinforcement with round scleral patches in chinese children with high myopia. Graefes Arch. Clin. Exp. Ophthalmol. 2020;258:1543–1547. doi: 10.1007/s00417-020-04646-3. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y.H., Xin C., Li X.X., Yang K., Liu S.M., Qiao L.Y. Posterior scleral reinforcement surgery effectively slows the rate of high myopic progression in children. J. Fr. Ophtalmol. 2024;47 doi: 10.1016/j.jfo.2024.104213. [DOI] [PubMed] [Google Scholar]
- 45.Cao K., Wang J., Zhang J., Yusufu M., Jin S., Zhu G., He H., Qi Y., Wan X.H. The effectiveness and safety of posterior scleral reinforcement with vitrectomy for myopic foveoschisis treatment: a systematic review and meta-analysis. Graefes Arch. Clin. Exp. Ophthalmol. 2020;258:257–271. doi: 10.1007/s00417-019-04550-5. [DOI] [PubMed] [Google Scholar]
- 46.Wollensak G., Spoerl E., Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am. J. Ophthalmol. 2003;135:620–627. doi: 10.1016/s0002-9394(02)02220-1. [DOI] [PubMed] [Google Scholar]
- 47.Wollensak G. Crosslinking treatment of progressive keratoconus: new hope. Curr. Opin. Ophthalmol. 2006;17:356–360. doi: 10.1097/01.icu.0000233954.86723.25. [DOI] [PubMed] [Google Scholar]
- 48.Zhang F., Lai L. Advanced research in scleral cross-linking to prevent from progressive myopia. Asia. Pac. J. Ophthalmol. 2021;10:161–166. doi: 10.1097/APO.0000000000000340. [DOI] [PubMed] [Google Scholar]
- 49.Hoang Q.V., Wen Q., Paik D.C., Chun Y.Y., Silverman R., Nagasaki T., Trokel S.L., Zyablitskaya M. Scleral growth stunting via sub-tenon injection of cross-linking solutions in live rabbits. Br. J. Ophthalmol. 2023;107:889–894. doi: 10.1136/bjophthalmol-2021-319427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hu Y., Ding X., Jiang J., Yu M., Chen L., Zhai Z., Zhang H., Fang B., Wang H., Yu S., et al. Long-term axial length shortening in myopic orthokeratology: incident probability, time course, and influencing factors. Investig. Ophthalmol. Vis. Sci. 2023;64:37. doi: 10.1167/iovs.64.15.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berntsen D.A., Ticak A., Sinnott L.T., Chandler M.A., Jones J.H., Morrison A., Jones-Jordan L.A., Walline J.J., Mutti D.O., BLINK Study Group Peripheral defocus, pupil size, and axial eye growth in children wearing soft multifocal contact lenses in the blink study. Investig. Ophthalmol. Vis. Sci. 2023;64:3. doi: 10.1167/iovs.64.14.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohno-Matsui K. Proposed classification of posterior staphylomas based on analyses of eye shape by three-dimensional magnetic resonance imaging and wide-field fundus imaging. Ophthalmology. 2014;121:1798–1809. doi: 10.1016/j.ophtha.2014.03.035. [DOI] [PubMed] [Google Scholar]
- 53.Neelam K., Cheung C.M.G., Ohno-Matsui K., Lai T.Y.Y., Wong T.Y. Choroidal neovascularization in pathological myopia. Prog. Retin. Eye Res. 2012;31:495–525. doi: 10.1016/j.preteyeres.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 54.Wu H., Chen W., Zhao F., Zhou Q., Reinach P.S., Deng L., Ma L., Luo S., Srinivasalu N., Pan M., et al. Scleral hypoxia is a target for myopia control. Proc. Natl. Acad. Sci. USA. 2018;115:E7091–E7100. doi: 10.1073/pnas.1721443115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang L., Yi K., Sun Q., Chen Z., Xiang Y., Ren W., Wu P., He S., Yang Y., Feng L., et al. Palladium nanocrystals regulates scleral extracellular matrix remodeling in myopic progression by modulating the hypoxia signaling pathway nrf-2/ho-1. J. Control. Release. 2024;373:293–305. doi: 10.1016/j.jconrel.2024.07.031. [DOI] [PubMed] [Google Scholar]
- 56.Huang Y.Y., Nagata K., Tedford C.E., Mccarthy T., Hamblin M.R. Low-level laser therapy (lllt) reduces oxidative stress in primary cortical neurons in vitro. J. Biophotonics. 2013;6:829–838. doi: 10.1002/jbio.201200157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Salehpour F., Mahmoudi J., Kamari F., Sadigh-Eteghad S., Rasta S.H., Hamblin M.R. Brain photobiomodulation therapy: a narrative review. Mol. Neurobiol. 2018;55:6601–6636. doi: 10.1007/s12035-017-0852-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhu Q., Cao X., Zhang Y., Zhou Y., Zhang J., Zhang X., Zhu Y., Xue L. Repeated low-level red-light therapy for controlling onset and progression of myopia-a review. Int. J. Med. Sci. 2023;20:1363–1376. doi: 10.7150/ijms.85746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cohen Y., Peleg E., Belkin M., Polat U., Solomon A.S. Ambient illuminance, retinal dopamine release and refractive development in chicks. Exp. Eye Res. 2012;103:33–40. doi: 10.1016/j.exer.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 60.Wang M., Schaeffel F., Jiang B., Feldkaemper M. Effects of light of different spectral composition on refractive development and retinal dopamine in chicks. Investig. Ophthalmol. Vis. Sci. 2018;59:4413–4424. doi: 10.1167/iovs.18-23880. [DOI] [PubMed] [Google Scholar]
- 61.Watts N.S., Taylor C., Rucker F.J. Temporal color contrast guides emmetropization in chick. Exp. Eye Res. 2021;202 doi: 10.1016/j.exer.2020.108331. [DOI] [PubMed] [Google Scholar]
- 62.Zhou W., Liao Y., Wang W., Sun Y., Li Q., Liu S., Tang J., Li L., Wang X. Efficacy of different powers of low-level red light in children for myopia control. Ophthalmology. 2024;131:48–57. doi: 10.1016/j.ophtha.2023.08.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
All original data will be made available from the lead contact upon request.
-
•
All original code will be made available from the lead contact upon request.
-
•
Any additional information required to analyze the data reported in this article is available from the lead contact upon request.