Abstract
We determined total and Cryptococcus neoformans glucuronoxylomannan (GXM)-reactive antibody repertoires of human immunodeficiency virus (HIV)-infected and HIV-uninfected Ugandans in a retrospective, case-control study of participants in a randomized controlled trial of pneumococcal vaccination. The study included 192 adults: 48 who subsequently developed cryptococcal meningitis (CM); (HIV+ CM+); 2 individuals who matched them in CD4+ T-cell level, stage of HIV disease, and age but did not develop CM (HIV+ CM−); and 48 HIV-uninfected individuals. Total serum immunoglobulin concentrations and titers of immunoglobulin M (IgM), IgG, and IgA to GXM, pneumococcal polysaccharides, and antibodies expressing certain VH3 idiotypes were determined with banked sera obtained before the development of cryptococcosis for HIV+ CM+ subjects. The results showed that HIV-infected subjects had significantly lower levels of IgM to GXM but higher levels of total immunoglobulin and IgG and IgA to GXM than those of HIV-uninfected subjects. HIV-infected subjects with a history of pneumonia had higher levels, and those with a history of herpes zoster had lower levels of GXM-binding antibodies than subjects with no history of either disease. Minimal to no cross-reactivity was demonstrated between antibodies to GXM and polysaccharides in a pneumococcal vaccine. No significant differences between the antibody repertoires of HIV+ CM+ and HIV+ CM− subjects were identified, but among subjects without a history of pneumonia, there was a trend towards lower VH3-positive antibody levels among HIV+ CM+ than among HIV+ CM− subjects. Our findings demonstrate an association between previous infectious diseases and differences in the total and GXM-reactive antibody repertoires of HIV-infected subjects and suggest the question of whether certain microbes modulate subsequent antibody responses to GXM deserves further study.
The central importance of intact CD4+ T-cell-mediated immunity in resistance to cryptococcosis is incontrovertible (48). However, the susceptibility to human immunodeficiency virus (HIV)-associated cryptococcosis is likely to depend on additional factors for the following reasons. First, the incidence of HIV-associated cryptococcosis was markedly less than that of profound CD4+ T-cell depletion, even at the height of the HIV epidemic (21). Second, since Cryptococcus neoformans is a ubiquitous, endemic fungus, and there is strong evidence that it is latent in humans (31, 59), exposure is unlikely to be an independent determinant of disease. Third, although C. neoformans can exploit host and/or environmental factors to enhance virulence, this does not explain why certain HIV-associated individuals develop cryptococcosis while others living in the same area with similar immunological profiles do not. Historically, serology has provided important insights into the epidemiology and pathogenesis of infectious diseases. Hence, our group has sought to identify the serological profiles of individuals who could be at risk for the development of cryptococcosis.
The importance of antibody-mediated immunity for natural resistance to cryptococcosis is uncertain. An increased risk for cryptococcosis has been noted in patients with hypogammaglobulinemia, and immunoglobulin defects have been noted in patients with X-linked immunodeficiency, hyperimmunoglobulin M (hyperIgM), and common variable immunodeficiency syndromes (33, 36, 37, 52, 61, 65). Although these syndromes are characterized by defects in acquired, T-cell-dependent antibody responses, they are complex disorders that also feature a central defect in the memory B-cell repertoire (3, 4). Similarly, the patients that appear to be at the highest risk for cryptococcosis often have B-cell defects in addition to impaired cell-mediated immunity, such as patients with HIV infection, and, in the pre-AIDS era, patients with B-cell or hematologic malignancies (39, 47). Mouse models of experimental cryptococcosis have also implicated B cells in resistance to C. neoformans (5, 56) while also demonstrating that antibody efficacy against lethal C. neoformans requires CD4+ T cells and/or mediators produced by T cells (7, 67).
Studies performed in the AIDS era have demonstrated the presence of glucuronoxylomannan (GXM)-reactive antibodies in sera from both HIV-infected and HIV-uninfected individuals, although qualitative and quantitative differences in the GXM-reactive antibody repertoires of HIV-infected and HIV-uninfected subjects have been identified (24, 25, 27, 35). The prevalence of cryptococcal meningitis (CM) in Africans with AIDS has been reported to be as high as 30% (30), which is three times higher than that reported for individuals with AIDS in the United States prior to the introduction of antiretroviral therapy (21). Since little is known about the response of Africans to C. neoformans, this study was undertaken to characterize GXM-reactive and total immunoglobulin profiles from HIV-infected Africans in an effort to identify serological parameters that could be associated with their high risk for cryptococcosis.
(Some of the data in this paper were presented at the 103rd meeting of the American Society for Microbiology, Washington, D.C., May 2003.)
MATERIALS AND METHODS
Subjects.
The sera used in this study were obtained from HIV-infected subjects who had been participants in a double-blind randomized placebo-controlled trial (RCT) of a 23-valent pneumococcal polysaccharide vaccine in Uganda, the results of which were reported previously (30). There were 61 subjects in the RCT who developed cryptococcosis during the course of that study; 48 of these subjects were identified retrospectively for inclusion in our study. The 13 subjects who were not included were excluded because there was insufficient serum available from them to study. A retrospective, case-control design was used in which each of the 48 subjects in the RCT who developed cryptococcosis (HIV+ CM+) was matched to 2 subjects in the RCT who did not develop cryptococcosis (HIV+ CM−) based on CD4 T-lymphocyte count, clinical stage of HIV disease, and age. In the RCT, cryptococcal disease was defined by the appropriate clinical findings and the recovery of C. neoformans from either cerebrospinal fluid or blood or a positive cryptococcal antigen test (Murex, United Kingdom) as described previously (29). The clinical and demographic characteristics of the subjects in the RCT who developed cryptococcosis were reported separately (29). At the time of recruitment into the RCT, a history of pneumonia, herpes zoster, or tuberculosis (TB) was recorded (64). A history of pneumonia required confirmatory evidence from a health clinic or hospital, a history of herpes zoster was identified by the presence of a typical scar, and a history of tuberculosis was confirmed by national TB program documentation. Of these clinical events, only a history of herpes zoster was associated with the development of cryptococcosis (29). Throughout the RCT, the diagnosis of pneumonia was made if there was an acute (<28 days) respiratory illness with new pulmonary parenchymal shadowing on a chest X ray; the diagnosis of herpes zoster was made with the appearance of the typical rash. Forty-eight HIV-uninfected control subjects of similar age to the HIV-infected subjects who were not enrolled in the vaccine RCT were recruited from attendees at the HIV testing clinics at which the RCT was conducted. The number of subjects studied was 192 (48 HIV+ CM+ plus 96 HIV+ CM− plus 48 HIV− subjects).
Sera.
The sera used in this study were samples obtained from the HIV+ CM+ subjects described above before the development of cryptococcosis. All samples were tested for the presence of cryptococcal antigen to ensure that they represented precryptococcal disease samples in the case of the HIV+ CM+ groups and that occult disease was not present in the HIV+ CM− group. The serum samples from the HIV+ CM+ group had been collected from 1 to 6 months before the development of cryptococcosis. The sera were analyzed, and the HIV or cryptococcal disease status was not known to the technician. They were then separated from whole-blood samples by centrifugation, stored at −20°C, and heat treated for 30 min at 56°C prior to use.
Measurement of total immunoglobulin concentrations.
Total serum IgM, IgG, and IgA concentrations were determined using isotype-specific radial immunodiffusion kits (Kent Laboratories, Bellingham, WA) according to the manufacturer's instructions (27). Initially, all 192 samples were analyzed at a dilution of 1:2, and samples that were not within the range of the reference sera were reanalyzed at consecutive dilutions from 1:3 to 1:10. Total serum IgG2 concentrations were measured in every 10th sample, using anti-human IgG2 radial immunodiffusion kits (The Binding Site, Birmingham, England).
Measurement of antibodies to GXM.
Titers of IgM, IgG, and IgA to GXM were determined by enzyme-linked immunosorbent assay (ELISA) as described previously (35). Briefly, 96-well polystyrene plates (Costar; Corning Glass Works, Corning, NY) were coated for 3 h at room temperature with 1 μg/ml of a serotype A isolate of C. neoformans (SB4; provided by A. Casadevall, AECOM). The plates were then blocked overnight at 4°C with phosphate-buffered saline (PBS) containing 1% bovine serum albumin (1% BSA-PBS) (Fisher Biotech, Fairlawn, NJ) and washed with PBS containing 0.05% Tween 20 (Sigma, St. Louis, MO) prior to use. Serum samples were applied to the plates in duplicate at an initial dilution of 1:50 and then serially diluted 1:3 in 1% BSA-PBS. The plates were then incubated for 1 h at 37°C, washed, and incubated with 1 μg/ml alkaline phosphatase-labeled goat antihuman IgM, IgG, or IgA (Southern Biotech, Birmingham, AL) for 1 h at 37°C. After being washed, the plates were developed with p-nitrophenyl phosphate (Sigma) in bicarbonate buffer (pH 9.8). The absorbances were measured by an ELISA reader (MRX; Dynex Technologies, Chantilly, VA) at a wavelength of 405 nm. A mouse IgG1 monoclonal antibody (MAb) 18B7 (12), provided by A. Casadevall, AECOM, was used as a positive control, and wells with no sera were included as a negative control to determine the background absorbance of the reagents. The results were plotted on a semi-log scale after subtraction of 1.5 times the average background optical density (OD), and a titration curve was obtained. The titer was defined as the point at which the titration curve crossed an OD of 0.1.
Measurement of antibodies to pneumococcal capsular polysaccharides.
Levels of antibodies to pneumococcal capsular polysaccharides were determined in a subset of 17 HIV-infected subjects with a history of pneumonia but without a history of herpes zoster and 18 randomly selected HIV-infected subjects without a history of either pneumonia or herpes zoster. The antibody determinations were performed by ELISA using a pneumococcal capsular polysaccharide vaccine (Pneumovax; Merck, West Point, PA) with coated plates as described previously (1, 60). Briefly, plates were coated with 10 μg/ml of the vaccine diluted in PBS and incubated overnight at 4°C. After being blocked, the plates were (i) washed and incubated with serial dilutions of sera from the aforementioned subjects which had been absorbed with 1 μg/ml cell wall polysaccharide as described previously (1) beginning at a dilution of 1:50 for 1 h at 37°C, (ii) washed, and (iii) incubated with alkaline phosphatase-labeled goat antihuman IgM, IgG, and IgA (Southern Biotech), the mouse anti-human MAb D12 (see below), and then goat anti-mouse IgG. The positive control was a standard antipneumococcal serum (89SF; FDA). The negative control was wells without sera. After incubation with the secondary or tertiary antibodies, the plates were developed and titers were determined as described above. Inhibition studies with GXM were also performed. For these experiments, plates coated with 10 μg/ml of the pneumococcal vaccine were incubated with serum samples mixed with 0.5, 5, or 50 μg of SB4 GXM, and binding was detected with anti-human IgM or IgG as described above. The samples used produced a maximum OD of at least 0.6 (24 samples for IgM and 26 samples for IgG) and were used at a dilution that corresponded to the point on the binding curve representing 70% binding for the relevant isotype. After incubation, the plates were developed as described above with anti-human IgM and IgG. The percent inhibition was calculated as the OD with the inhibitor minus the OD without the inhibitor divided by the OD without the inhibitor times 100.
Idiotype expression.
The MAbs D12 and 16.84 are mouse anti-human anti-idiotypic reagents that bind to variable region determinants of human antibodies encoded by VH3 gene segments. These reagents have been used to analyze the VH3-positive antibody repertoires of HIV-infected and HIV-uninfected subjects in the United States (1, 27, 60). In this study, the levels of total and GXM-specific antibodies expressing the VH3 determinant recognized by D12 and 16.84 were determined by ELISA as described previously (27). For total idiotype determinations, ELISA plates coated with 5 μg/ml of the relevant MAb and blocked with 1% BSA-PBS were washed and incubated with the serum samples at an initial dilution of 1:100 and then serially diluted 1:3. For GXM-specific idiotype determinations, ELISA plates coated with GXM as described above were first incubated with serum samples used at a dilution of 1:50, washed, and then incubated with the MAbs as described above. After being washed, the plates were incubated with a combination of alkaline phosphatase-labeled goat anti-human IgM and IgG (Southern Biotech, Birmingham, AL) at a concentration of 1 μg/ml for 1 h at 37°C. After incubation, the plates were washed and developed, and the ODs were measured as described above. Negative controls were as described above, and titers were determined with a titration curve as detailed above.
Statistical analysis.
Anti-GXM antibody levels and idiotype antibody titers were log transformed to approximate normal distribution. The antibody levels in the HIV-infected subjects were compared to those of HIV-uninfected subjects and within the HIV-infected group, by cryptococcal disease status. Unadjusted analyses used Student's t test. Analyses adjusted for CD4+ T-cell count, clinical staging, and age in the HIV-infected group used conditional logistic regression analysis to estimate the association of antibody levels with cryptococcal status. Analysis of variance regression was used to compare antibody levels with multiple adjustments for possible confounding. Associations between continuous variables were investigated by linear regression analysis. All statistical analyses were performed using STATA version 7 (STATA Corp LP, College Station, Texas).
RESULTS
Subjects.
The clinical characteristics, WHO stage of disease, and baseline CD4 T-cell counts of the case and control subjects are shown in Table 1. As in the study of the clinical characteristics of the HIV-infected subjects who developed cryptococcosis during the RCT, we sought to identify relationships between aspects of the antibody repertoire and the illnesses used for staging purposes in the RCT. Among the 144 HIV-infected subjects, 26 had a history of pneumonia, and 41 had a history of herpes zoster prior to enrollment in the RCT. The median time from the diagnosis of pneumonia to the time serum samples were obtained from these subjects was 54 days (range, 9 to 731 days), and the median time from the diagnosis to the time serum samples were obtained from the subjects with herpes zoster was 114 days (range, 31 to 169 days). For the subjects studied herein, pneumonia occurred in 25% (12/48) of the subjects who developed cryptococcosis and in 15% (14/96) who did not. Herpes zoster occurred in the RCT in 35% (17/48) of subjects who developed cryptococcosis and in 25% (24/96) who did not. These differences were not statistically significant. No history of previous clinical illness was documented in the HIV-uninfected controls; hence, comparisons of HIV status and past clinical history were not possible.
TABLE 1.
Characteristics of the case (HIV+ CM+) and matched control (HIV+ CM−) subjects and unmatched HIV-uninfected controls
| Characteristic | Value for group
|
||
|---|---|---|---|
| HIV-infected subjects
|
HIV-uninfected subjects | ||
| HIV+ CM+ | HIV+ CM− | ||
| No. of subjects | 48 | 96 | 48 |
| No. of females (%) | 29 (60) | 70 (73) | 25 (52) |
| Median age in yr (range) | 26 (19-49) | 28 (16-48) | 25 (18-54) |
| Median CD4 count (range) | 37 (0-247) | 42 (0-242) | —a |
| No. with a history of herpes zoster (%) | 17 (35) | 24 (25) | — |
| No. with a history of pneumonia | 12 (25) | 14 (15) | — |
| No. with a history of tuberculosis | 4 (8) | 9 (9) | — |
| WHO stage | 1-3b | 1-4c | — |
—, not available and/or applicable.
Numbers of subjects among the HIV+ CM+ group were as follows: 1 (stage1), 12 (stage 2), and 35 (stage 3).
Numbers of subjects among the HIV+ CM− group were as follows: 2 (stage1), 29 (stage 2), 56 (stage 3), and 9 (stage 4).
Total immunoglobulin levels.
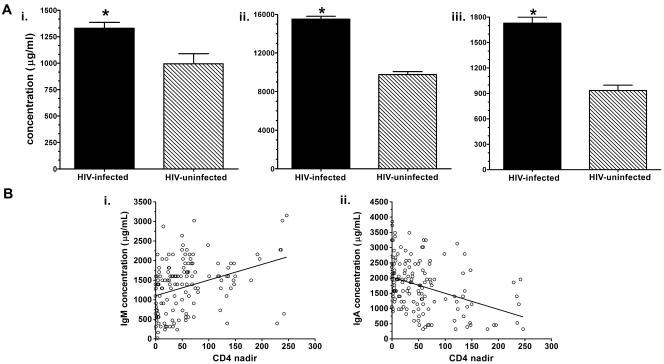
The total IgM, IgG, and IgA levels of the HIV-infected subjects were significantly higher than those of the HIV-uninfected controls (Fig. 1A). Among the HIV-infected subjects, there was a direct association between total IgM and CD4+ T-cell levels (Pearson's r = 0.03, P < 0.01) and an inverse correlation between total IgA and CD4+ T-cell levels (Pearson's r = −0.03, P < 0.01) (Fig. 1B). No significant differences in total immunoglobulin levels were observed between HIV+ CM+ and HIV+ CM− subjects. We sought associations between the clinical history of the HIV-infected subjects and total immunoglobulin levels and found that the level of total IgM was higher among subjects with a history of pneumonia (1,650 μg/ml) than those without a history of pneumonia (1,260 μg/ml) (P < 0.006, t test). There was no association between a history of herpes zoster and total immunoglobulin levels. There was no significant difference between the amount of IgG2 measured in the HIV-infected group and that in the HIV-uninfected group (data not shown).
FIG. 1.
(A) Total (i) IgM, (ii) IgG, and (iii) IgA concentrations of HIV-infected and HIV-uninfected Ugandans. The bar representing each group depicts the mean value of the antibody type designated on the x axis. *, P was <0.01, with a comparison of the groups by t test. (B) Scatter plots depicting the relationship between CD4+ T-cell levels (y axis) and (i) total IgG or (ii) IgA (x axis). Each circle represents one subject.
Antibodies to GXM.
Levels of GXM-reactive IgM, IgG, and IgA differed significantly by HIV status (Fig. 2). Compared to HIV-uninfected controls, HIV-infected subjects had significantly higher levels of GXM-specific IgG and IgA but a lower level of GXM-specific IgM. No significant differences in GXM-specific antibody levels were observed between HIV+ CM+ and HIV+ CM− subjects. There was no association between CD4+ T-cell level and GXM-specific IgM, IgG, or IgA levels. However, HIV-infected subjects with a history of pneumonia had significantly higher levels of GXM-specific IgM, IgG, and IgA than those with no history of pneumonia, whereas those with a history of herpes zoster had significantly lower levels of GXM-specific IgM and IgA than those with no history of herpes zoster (Fig. 3). These findings persisted in analyses adjusted for age, CD4+ T-cell level, gender, and clinical stage of HIV disease. However, reestimation of the relationship between GXM-specific antibody levels and cryptococcal disease using pneumonia and herpes zoster as confounders in the analysis of variance model did not alter the nonsignificant association between these parameters.
FIG. 2.
GXM-specific (A) IgM, (B) IgG, and (C) IgA levels of HIV-infected and HIV-uninfected Ugandans. The bar representing each group depicts the mean value for the antibody type designated on the x axis. *, P was <0.01, with a comparison of the groups by t test.
FIG. 3.
GXM-specific antibody levels of HIV-infected Ugandans with and without a past history of pneumonia (A) and herpes zoster (B). The bars represent the mean levels of GXM-specific antibody as designated on the x axis for the groups indicated in the legend. *, P was <0.04 (0.001 IgM, pneumonia groups; 0.007 IgM and 0.02 IgA, herpes zoster groups), with a comparison by t test of the groups with and without a history of the diseases noted below the graphs.
Antibodies to pneumococcal polysaccharides.
Levels of antibody to pneumococcal capsular polysaccharides in the vaccine used for the ELISA (PVX) were measured in 17 subjects with a history of pneumonia and 18 subjects without a history of pneumonia and compared to GXM-reactive antibodies to investigate for cross-reactivity. Levels of PVX-reactive IgM, IgG, IgA, and D12-specific MAb did not differ significantly between HIV-infected subjects that had a history of pneumonia and those that did not (Tables 2 and 3). Using Spearman's rank correlation coefficient, levels of GXM- and PVX-reactive antibodies were shown to be independent for IgM, IgG, IgA, and D12 isotype (P = 0.12, 0.31, 0.11, and 0.67, respectively) (Tables 2 and 3).
TABLE 2.
IgM, IgG, IgA, and VH3-positive antibody titers to 23-valent pneumococcal polysaccharide vaccine in HIV-infected subjects with and without a history of pneumonia
| Subjects (no.)b | Titera
|
|||
|---|---|---|---|---|
| IgM | IgG | IgA | D12 | |
| PN+ (17) | 3 (0.5) | 3.6 (0.4) | 2.7 (0.5) | 2.2 (0.4) |
| PN− (18) | 3 (0.4) | 3.4 (0.4) | 2.6 (0.6) | 2.5 (0.5) |
Displayed titers are log-transformed values with the standard deviation in parentheses.
No statistically significant differences were found between subjects with and without a history of pneumonia (PN+ and PN−, respectively) or between CM+ and CM− subjects (data not shown; t test).
TABLE 3.
Mean values of percent inhibition with soluble GXM for HIV-infected subjects with and without a history of pneumonia
| Concn of GXM-SB4 (μg/ml) | Mean % inhibition of indicated antibody (no. of subjects) in HIV-infected individuals who werea:
|
|||||
|---|---|---|---|---|---|---|
| PN+
|
PN−
|
|||||
| IgM (13) | IgG (12) | IgA (5) | IgM (13) | IgG (11) | IgA (2) | |
| 0.5 | 1.8 | 4.2 | 6.0 | 3.2 | 3.1 | 0 |
| 5 | 7.0 | 6.7 | 9.0 | 2.7 | 3.0 | 0 |
| 50 | 9.4 | 3.6 | 4.7 | 3.9 | 7.6 | 13.8 |
PN+, with a history of pneumonia; PN−, without a history of pneumonia.
Idiotype levels.
HIV-infected subjects had significantly higher levels of 16.84-positive antibodies than HIV-uninfected subjects (Fig. 4). There were no significant differences in 16.84- or D12-positive antibody levels between HIV+ CM+ and HIV+ CM− subjects. Among HIV-infected subjects, both 16.84-positive and D12-positive antibody levels correlated with total IgM levels (Pearson's r = 0.21, P < 0.01), and 16.84-positive antibody levels correlated with IgG (Pearson's r = 0.30, P < 0.01). Among HIV-infected subjects with a history of pneumonia, D12-positive antibody levels were significantly higher among those with a history of pneumonia than those without a history of pneumonia, with inverse titers of 680,000 and 390,000, respectively (P = 0.04, t test). There was also a trend towards higher levels of total 16.84-positive antibodies in the subjects with a history of pneumonia who developed cryptococcosis (HIV+ CM+) than in those who did not (P = 0.07, t test). This trend was reversed in the subjects without a history of pneumonia (P = 0.09), who had lower levels of 16.84-positive antibodies (Fig. 5).
FIG. 4.
VH3 (idiotype)-positive antibody levels of Ugandans. The bars represent the mean levels of 16.84- and D12-positive antibodies for the HIV-infected and HIV-uninfected groups. *, P was <0.01, with a comparison of the groups by t test.
FIG. 5.
16.84-(VH3 idiotype)-positive antibody levels of HIV+ CM+ and HIV+ CM− Ugandans based on a clinical history of pneumonia. The bars represent the mean levels of 16.84-positive antibodies in HIV+ CM+ and HIV+ CM− subjects with and without a history of pneumonia.
DISCUSSION
The GXM antibody responses of HIV-infected and HIV-uninfected individuals in the United States and France have been reported previously (2, 24, 25, 27, 35), but to our knowledge, this is the first study of the GXM antibody profiles of Africans. In accordance with studies of subjects in the United States (24, 27), we found significant differences between the GXM-reactive antibody levels of HIV-infected and HIV-uninfected Ugandans. Although we found no difference between the GXM-reactive or total immunoglobulin levels of HIV+ CM+ and HIV+ CM− subjects, there were unexpected differences between the antibody levels of HIV-infected subjects depending on whether or not there was a history of pneumonia or herpes zoster. Like groups in Europe and Africa (42, 51), we found a correlation between total IgM and CD4+ T-cell levels and an inverse relationship between total IgA and CD4+ T-cell levels among HIV-infected subjects. The finding in this and another study of subjects in the United States (27) that HIV-infected individuals had higher levels of total IgM, IgG, and IgA than HIV-uninfected individuals underscores the relationship between HIV-associated defects in cell-mediated immunity and hypergammaglobulinemia, which was first noted at the beginning of the HIV epidemic (41). HIV-associated hypergammaglobulinemia is characterized by an expanded population of naïve B cells (18, 23) and a reduced population of memory B cells (19, 20, 22). The HIV-infected subjects in this study had lower levels of GXM-reactive IgM than those of HIV-uninfected subjects, despite having higher levels of total IgM. This discordance could be consistent with a loss of GXM-specific memory IgM, since HIV infection is associated with a reduction in the CD27+ memory B-cell repertoire (19, 22). Deficiency of CD27+ IgM has been implicated in the susceptibility to Streptococcus pneumoniae (11, 40), an encapsulated pathogen that also causes an increased incidence of disease in HIV-infected individuals. Reduced numbers of CD27+ B cells have been reported to occur in patients with hyperIgM and common variable immunodeficiency (3, 4), conditions that have been associated with an increased incidence of cryptococcosis (36, 37, 65). GXM-specific human IgM MAbs prolonged survival in experimental cryptococcosis (28, 43, 44), but serological studies cannot identify memory IgM. Therefore, studies are planned to determine if GXM-reactive IgM is derived from memory B cells.
Our finding that HIV-infected subjects had higher levels of GXM-specific IgG than HIV-uninfected subjects is consistent with the results of two other studies from the United States (24, 27) but at variance with a study from France (25). Differences in study design and experimental methods preclude direct comparisons between these studies. One of the studies from the United States used a similar study design and experimental methods, but it did not include women or subjects with previous infectious diseases (27). Cryptococcosis was more common among men in the pre-AIDS era than among women (13) and in several cohorts of HIV-infected individuals in the United States and France than among Ugandans (26, 29, 34). Interestingly, higher levels of immunoglobulin have been reported among females than among males (38, 55). Women comprised 67% of the cohort in our study, but there were no differences in antibody levels based on gender in our adjusted analyses.
Our observation that levels of GXM-reactive IgG were higher among HIV-infected than HIV-uninfected subjects could reflect polyclonal B-cell expansion of preexisting B cells. However, GXM-reactive IgG and IgG to another antigen were not correlated in another study (24), and IgG reactivity with cryptococcal proteins was greater in HIV-infected than HIV-uninfected subjects (17). Although we cannot explain why HIV-infected individuals have higher levels of GXM-reactive IgG, the likelihood that HIV-associated cryptococcosis follows the reactivation of a latent state (31, 59) suggests the hypothesis that GXM-reactive IgG could reflect the status of the fungal load within a given individual and/or a response to exposure in the setting of cellular immunodeficiency. Longitudinal studies during which antibody levels are sampled in the same individuals over time could validate or refute this hypothesis. Although we found no difference in the GXM-reactive antibody levels of HIV+ CM+ and HIV+ CM− subjects in this study, our ability to detect differences could be hampered by the lack of availability of defined GXM antigens. Nonetheless, the development of cryptococcosis in individuals with high levels of GXM-reactive antibody could provide a clinical correlate for the observation that high antibody levels can abrogate GXM-specific antibody efficacy against C. neoformans in mice due to a prozone-like phenomenon (44, 62, 63).
Surprisingly, our data showed that GXM-reactive antibody levels of HIV-infected individuals differed depending on their history of previous clinical illness. Levels of GXM-reactive IgM and IgA were lower among subjects with a history of herpes zoster than among those without a history of herpes zoster but higher among subjects with a history of pneumonia than among those without a history of pneumonia. Although they emerged from a subgroup analysis and should be interpreted cautiously, these findings reveal associations between GXM-reactive antibody levels and clinical history. A history of herpes zoster, which was associated with the development of cryptococcal disease in the report by French et al. (30), could be a marker of the duration and/or stage of HIV infection (49), but differences in GXM-reactive antibody levels persisted in adjusted analyses. Although our study was not designed to address this question, our results suggest the hypothesis that low levels of GXM-reactive IgM and/or IgA could be associated with an increased risk for cryptococcosis in individuals with a history of herpes zoster. In support of this possibility, GXM-specific human IgM enhanced the survival of immunized and naïve mice after cryptococcal challenge (28, 43, 45).
The HIV-infected subjects in our study with a history of pneumonia had higher levels of total IgM, GXM-reactive, and VH3-positive antibodies than those without a history of pneumonia. However, those with a history of pneumonia did not have higher levels of antibodies to pneumococcal polysaccharides, and levels of antibodies to GXM and pneumococcal polysaccharides were not correlated. HIV-infected African children with acute pneumonia and adults with pneumococcal pneumonia had high levels of IgM and IgG (32, 46). Although pneumococcus was the causative agent in 46% of Kenyans with pneumonia with an identified etiology (58) and 30% of Ugandans (66), the etiology of most cases of pneumonia in Africans is unknown. Hence, in view of the incidence of cryptococcosis in the cohort that we studied, we cannot exclude the possibility that some of the patients with pneumonia in our cohort had cryptococcal pneumonia, a disease manifestation that may not be associated with positive fungal cultures or a detectable serum GXM level. We considered the possibility that cross-reactive antibodies were the cause of higher GXM-reactive antibody levels in subjects with a history of pneumonia. GXM-reactive and pneumococcal polysaccharide antibodies were found to manifest cross-reactivity in one, but no other, strain of mice (50). Our data showed that GXM-reactive and pneumococcal polysaccharide-reactive antibody levels were not correlated in subjects with or without a history of pneumonia and that there was little to no inhibition of the binding of antibodies to pneumococcal polysaccharides by GXM. Hence, our findings do not support the conclusion that higher levels of GXM-reactive antibody in subjects with a history of pneumonia reflected cross-reactivity with antibodies to pneumococcal polysaccharides. Further investigations of this question should use individual pneumococcal capsular polysaccharides, but such research was beyond the scope of this study.
VH3 is the predominant gene family used in antibodies to GXM and other capsular, including pneumococcal, polysaccharides (1, 2, 15, 27, 28, 44, 53). Studies of subjects in the United States have reported both increased and decreased VH3 expression, including D12- and 16.84-positive antibody levels, among HIV-infected individuals compared to HIV-uninfected individuals (1, 8-10, 15, 16, 27, 57, 60). In the study reported herein, we found that HIV-infected Africans had higher levels of 16.84-positive antibodies than HIV-uninfected Africans and that HIV-infected subjects with a history of pneumonia had higher levels than those without a history of pneumonia. Relevant to our study, HIV-infected children in the United States with a history of invasive pneumococcal disease had higher B-cell IgG VH3 expression than those without a history of invasive pneumococcal disease (15), underscoring the possible relationship between previous infectious diseases and VH3 repertoire expansion. Although, to our knowledge, VH3 expression has not been evaluated previously in Africans, our data suggest that it may differ from that of individuals from the United States. More studies are needed to answer this question, and, if differences are discovered, to determine whether genetic differences, previous infectious diseases, and/or other factors contribute to this phenomenon.
In subjects who developed cryptococcosis, our data also showed a trend towards an association between lower levels of 16.84-positive antibodies in subjects without a history of pneumonia than in those with a history of pneumonia. Lower levels of 16.84-positive antibodies among HIV-infected subjects that subsequently developed cryptococcosis were also found in a retrospective case-control study in the United States; however, subjects with a previous AIDS-defining illness were excluded, and a history of pneumonia was not recorded (27). GXM-specific 16.84-positive antibodies were not present in a high-enough titer for a comparison of the groups in the study reported herein. Nonetheless, the intriguing association between lower 16.84 antibody levels and cryptococcosis suggested by this and the aforementioned study calls for further analysis of the specificity and B-cell derivation of the 16.84-positive antibody repertoire.
Our observation that HIV-infected Africans had higher GXM-reactive antibody levels when they had a previous history of pneumonia but not herpes zoster or TB suggests that there could be disease-related influences on the antibody response to GXM. Hence, our data suggest that past infectious diseases may modulate subsequent immune responses, but more work is needed to validate this hypothesis and determine its biological significance for resistance and susceptibility to HIV-associated cryptococcosis. The most rigorous approach to address this question would be with a prospective, longitudinal study. Such a study would also provide valuable information about the influence of progressive cell-mediated immune defects on total and specific antibody responses. Since high and insufficient antibody levels have each been associated with deleterious effects and enhanced disease pathogenesis in experimental cryptococcosis (14), further examination of associations between GXM antibody levels and clinical history could provide insight into why the prevalence of cryptococcosis is higher in the developing world (6, 30, 54), where the burden of infectious diseases is substantially higher than in developed nations.
Acknowledgments
This research was supported by the National Institutes of Health grants RO1 AI 35370, 44374, and 45459 to L.-A.P., the United Kingdom Medical Research Council (grant G9323636), and TASO Uganda. N.F. is supported by a Career Development Fellowship from the Wellcome Trust (061230/Z/00/Z).
We thank Kausik Datta for his assistance in reviewing and preparing the manuscript.
REFERENCES
- 1.Abadi, J., J. Friedman, R. Jefferis, R. A. Mageed, and L. Pirofski. 1998. Human antibodies elicited by a pneumococcal vaccine express idiotypic determinants indicative of VH3 gene segment usage. J. Infect. Dis. 178: 707-716. [DOI] [PubMed] [Google Scholar]
- 2.Abadi, J., and L. Pirofski. 1999. Antibodies reactive with the cryptococcal capsular polysaccharide glucuronoxylomannan are present in sera from children with and without HIV infection. J. Infect. Dis. 180:915-919. [DOI] [PubMed] [Google Scholar]
- 3.Agematsu, K., T. Futatani, S. Hokibara, N. Kobayashi, M. Takamoto, S. Tsukada, H. Suzuki, S. Koyasu, T. Miyawaki, K. Sugane, A. Komiyama, and H. D. Ochs. 2002. Absence of memory B cells in patients with common variable immunodeficiency. Clin. Immunol. 103:34-42. [DOI] [PubMed] [Google Scholar]
- 4.Agematsu, K., H. Nagumo, K. Shinozaki, S. Hokibara, K. Yasui, K. Terada, N. Kawamura, T. Toba, S. Nonoyama, H. D. Ochs, and A. Komiyama. 1998. Absence of IgD-CD27(+) memory B cell population in X-linked hyper-IgM syndrome. J. Clin. Investig. 102:853-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aguirre, K. M., and L. L. Johnson. 1997. A role for B cells in resistance to Cryptococcus neoformans in mice. Infect. Immun. 65:525-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banerjee, U., K. Datta, T. Majumdar, and K. Gupta. 2001. Cryptococcosis in India: the awakening of a giant? Med. Mycol. 39:51-67. [DOI] [PubMed] [Google Scholar]
- 7.Beenhouwer, D. O., S. Shapiro, M. Feldmesser, A. Casadevall, and M. D. Scharff. 2001. Both Th1 and Th2 cytokines affect the ability of monoclonal antibodies to protect mice against Cryptococcus neoformans. Infect. Immun. 69:6445-6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berberian, L., L. Goodglick, T. J. Kipps, and J. Braun. 1993. Immunoglobulin VH3 gene products: natural ligands for HIV gp120. Science 261: 1588-1591. [DOI] [PubMed] [Google Scholar]
- 9.Berberian, L., J. Shukla, R. Jefferis, and J. Braun. 1994. Effects of HIV infection on VH3 (D12 idiotope) B cells in vivo. J. Acquir. Immune Defic. Syndr. 7:641-646. [PubMed] [Google Scholar]
- 10.Bessudo, A., L. Rassenti, D. Havlir, D. Richman, E. Feigal, and T. J. Kipps. 1998. Aberrant and unstable expression of immunoglobulin genes in persons infected with human immunodeficiency virus. Blood 92:1317-1323. [PubMed] [Google Scholar]
- 11.Carsetti, R., M. M. Rosado, S. Donnanno, V. Guazzi, A. Soresina, A. Meini, A. Plebani, F. Aiuti, and I. Quinti. 2005. The loss of IgM memory B cells correlates with clinical disease in common variable immunodeficiency. J. Allergy Clin. Immunol. 115:412-417. [DOI] [PubMed] [Google Scholar]
- 12.Casadevall, A., W. Cleare, M. Feldmesser, A. Glatman-Freedman, D. L. Goldman, T. R. Kozel, N. Lendvai, J. Mukherjee, L.-A. Pirofski, J. Rivera, A. L. Rosas, M. D. Scharff, P. Valadon, K. Westin, and Z. Zhong. 1998. Characterization of a murine monoclonal antibody to Cryptococcus neoformans polysaccharide that is a candidate for human therapeutic studies. Antimicrob. Agents Chemother. 42:1437-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casadevall, A., and J. R. Perfect (ed.). 1998. Human cryptococcosis, p. 407-457. In Cryptococcus neoformans, 1st ed. American Society of Microbiology, Washington, D.C.
- 14.Casadevall, A., and L. Pirofski. 2005. Insights into mechanisms of antibody-mediated immunity from studies with Cryptococcus neoformans. Curr. Mol. Med. 5:421-423. [DOI] [PubMed] [Google Scholar]
- 15.Chang, Q., J. Abadi, M. Rosenberg, and L. A. Pirofski. 2004. VH3 gene expression in children with HIV infection. J. Infect. 49:274-282. [DOI] [PubMed] [Google Scholar]
- 16.Chang, Q., P. Alpert, J. Abadi, and L. Pirofski. 2000. A pneumococcal capsular polysaccharide vaccine induces a repertoire shift with increased VH3 expression in peripheral B cells from HIV-uninfected, but not HIV-infected individuals. J. Infect. Dis. 181:1313-1321. [DOI] [PubMed] [Google Scholar]
- 17.Chen, L.-C., D. L. Goldman, T. L. Doering, L.-A. Pirofski, and A. Casadevall. 1999. Antibody response to Cryptococcus neoformans proteins in rodents and humans. Infect. Immun. 67:2218-2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chong, Y., H. Ikematsu, I. Ariyama, K. Chijiwa, W. Li, K. Yamaji, S. Kashiwagi, and J. Hayashi. 2001. Evidence of B cell clonal expansion in HIV type 1-infected patients. AIDS Res. Hum. Retrovir. 17:1507-1515. [DOI] [PubMed] [Google Scholar]
- 19.Chong, Y., H. Ikematsu, K. Kikuchi, M. Yamamoto, M. Murata, M. Nishimura, S. Nabeshima, S. Kashiwagi, and J. Hayashi. 2004. Selective CD27+ (memory) B cell reduction and characteristic B cell alteration in drug-naive and HAART-treated HIV type 1-infected patients. AIDS Res. Hum. Retrovir. 20:219-226. [DOI] [PubMed] [Google Scholar]
- 20.Chong, Y., H. Ikematsu, M. Yamamoto, M. Murata, K. Yamaji, M. Nishimura, S. Nabeshima, S. Kashiwagi, and J. Hayashi. 2004. Increased frequency of CD27− (naive) B cells and their phenotypic alteration in HIV type 1-infected patients. AIDS Res. Hum. Retrovir. 20:621-629. [DOI] [PubMed] [Google Scholar]
- 21.Currie, B. P., and A. Casadevall. 1994. Estimation of the prevalence of cryptococcal infection among patients infected with the human immunodeficiency virus in New York City. Clin. Infect. Dis. 19:1029-1033. [DOI] [PubMed] [Google Scholar]
- 22.De Milito, A., C. Morch, A. Sonnerborg, and F. Chiodi. 2001. Loss of memory (CD27) B lymphocytes in HIV-1 infection. AIDS 15:957-964. [DOI] [PubMed] [Google Scholar]
- 23.De Milito, A., A. Nilsson, K. Titanji, R. Thorstensson, E. Reizenstein, M. Narita, S. Grutzmeier, A. Sonnerborg, and F. Chiodi. 2004. Mechanisms of hypergammaglobulinemia and impaired antigen-specific humoral immunity in HIV-1 infection. Blood 103:2180-2186. [DOI] [PubMed] [Google Scholar]
- 24.DeShaw, M., and L. Pirofski. 1995. Antibodies to Cryptococcus neoformans capsular polysaccharide glucuronoxylomannan are ubiquitous in the serum of HIV+ and HIV− individuals. Clin. Exp. Immunol. 99:425-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dromer, F., P. Aucouturier, J.-P. Clauvel, G. Saimot, and P. Yeni. 1988. Cryptococcus neoformans antibody levels in patients with AIDS. Scan. J. Infect. Dis. 20:283-285. [DOI] [PubMed] [Google Scholar]
- 26.Dromer, F., S. Mathoulin, B. Dupont, A. Laporte, et al. 1996. Epidemiology of cryptococcosis in France: a 9-year survey (1985-1993). Clin. Infect. Dis. 23:82-90. [DOI] [PubMed] [Google Scholar]
- 27.Fleuridor, R., R. H. Lyles, and L. Pirofski. 1999. Quantitative and qualitative differences in the serum antibody profiles of HIV-infected persons with and without Cryptococcus neoformans meningitis. J. Infect. Dis. 180:1526-1536. [DOI] [PubMed] [Google Scholar]
- 28.Fleuridor, R., Z. Zhong, and L. Pirofski. 1998. A human IgM monoclonal antibody prolongs survival of mice with lethal cryptococcosis. J. Infect. Dis. 178:1213-1216. [DOI] [PubMed] [Google Scholar]
- 29.French, N., K. Gray, C. Watera, J. Nakiyingi, E. Lugada, M. Moore, D. Lalloo, J. A. Whitworth, and C. F. Gilks. 2002. Cryptococcal infection in a cohort of HIV-1-infected Ugandan adults. AIDS 16:1031-1038. [DOI] [PubMed] [Google Scholar]
- 30.French, N., J. Nakinyingi, L. M. Carpenter, E. Lugada, C. Watera, K. Moi, M. Moore, D. Antvelink, D. Mulder, E. N. Janoff, J. Whiworth, and C. F. Gilks. 2000. 23-Valent pneumococcal polysaccharide vaccine in HIV-1-infected Ugandan adults: double-blind, randomised and placebo controlled trial. Lancet 355:2106-2111. [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Hermoso, D., G. Janbon, and F. Dromer. 1999. Epidemiologic evidence for dormant Cryptococcus neoformans infection. J. Clin. Microbiol. 37:3204-3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gordon, S. B., D. E. Miller, R. B. Day, T. Ferry, D. S. Wilkes, C. T. Schnizlein-Bick, E. E. Zijlstra, R. C. Read, M. E. Molyneux, and H. L. Twigg III. 2003. Pulmonary immunoglobulin responses to Streptococcus pneumoniae are altered but not reduced in human immunodeficiency virus-infected Malawian adults. J. Infect. Dis. 188:666-670. [DOI] [PubMed] [Google Scholar]
- 33.Gupta, S., M. Ellis, T. Cesario, M. Ruhling, and B. Vayuvegula. 1987. Disseminated cryptococcal infection in a patient with hypogammaglobulinemia and normal T cell functions. Am. J. Med. 82:129-131. [DOI] [PubMed] [Google Scholar]
- 34.Hajjeh, R. A., L. A. Conn, D. S. Stephens, W. Baughman, R. Hamill, E. Graviss, P. G. Pappas, C. Thomas, A. Reingold, G. Rothrock, L. C. Hutwager, A. Schuchat, M. E. Brandt, R. W. Pinner, and the Cryptococcal Active Surveillance Group. 1999. Cryptococcosis: population-based multivariate active surveillance and risk factors in human immunodeficiency virus-infected persons. J. Infect. Dis. 179:449-454. [DOI] [PubMed] [Google Scholar]
- 35.Houpt, D. C., G. S. Pfrommer, B. J. Young, T. A. Larson, and T. R. Kozel. 1994. Occurrences, immunoglobulin classes, and biological activities of antibodies in normal human serum that are reactive with Cryptococcus neoformans glucuronoxylomannan. Infect. Immun. 62:2857-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iseki, M., M. Anzo, N. Yamashita, and N. Matsuo. 1994. Hyper-IgM immunodeficiency with disseminated cryptococcosis. Acta Paediatr. 83:780-782. [DOI] [PubMed] [Google Scholar]
- 37.Jo, E. K., H. S. Kim, M. Y. Lee, M. Iseki, J. H. Lee, C. H. Song, J. K. Park, T. J. Hwang, and H. Kook. 2002. X-linked hyper-IgM syndrome associated with Cryptosporidium parvum and Cryptococcus neoformans infections: the first case with molecular diagnosis in Korea. J. Korean Med. Sci. 17:116-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kacprazak-Bergman, I. 1994. Sexual dimorphism of heritability of immunoglobulin levels. Ann. Hum. Biol. 21:563-569. [DOI] [PubMed] [Google Scholar]
- 39.Kaplan, M. H., P. P. Rosen, and D. Armstrong. 1977. Cryptococcosis in a cancer hospital. Cancer 39:2265-2274. [DOI] [PubMed] [Google Scholar]
- 40.Kruetzmann, S., M. M. Rosado, H. Weber, U. Germing, O. Tournilhac, H. H. Peter, R. Berner, A. Peters, T. Boehm, A. Plebani, I. Quinti, and R. Carsetti. 2003. Human immunoglobulin M memory B cells controlling Streptococcus pneumoniae infections are generated in the spleen. J. Exp. Med. 197: 939-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lane, C. H., H. Masur, L. C. Edgar, G. Whalen, A. H. Rook, and A. S. Fauci. 1983. Abnormalities of B cell activation and immunoregulation in patients with AIDS. N. Engl. J. Med. 309:453-458. [DOI] [PubMed] [Google Scholar]
- 42.Lugada, E. S., J. Mermin, B. Asjo, F. Kaharuza, R. Downing, N. Langeland, V. Ormaasen, J. Bruun, A. C. Awor, and E. Ulvestad. 2004. Immunoglobulin levels amongst persons with and without human immunodeficiency virus type 1 infection in Uganda and Norway. Scand. J. Immunol. 59:203-208. [DOI] [PubMed] [Google Scholar]
- 43.Maitta, R., K. Datta, and L. Pirofski. 2004. Efficacy of immune sera from human immunoglobulin transgenic mice immunized with a peptide mimotope of Cryptococcus neoformans glucuronoxylomannan. Vaccine 22:4062-4068. [DOI] [PubMed] [Google Scholar]
- 44.Maitta, R. W., K. Datta, Q. Chang, R. X. Luo, B. Witover, K. Subramaniam, and L.-A. Pirofski. 2004. Protective and nonprotective human immunoglobulin M monoclonal antibodies to Cryptococcus neoformans glucuronoxylomannan manifest different specificities and gene use profiles. Infect. Immun. 72:4810-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maitta, R. W., K. Datta, A. Lees, S. S. Belouski, and L.-A. Pirofski. 2004. Immunogenicity and efficacy of Cryptococcus neoformans capsular polysaccharide glucuronoxylomannan peptide mimotope-protein conjugates in human immunoglobulin transgenic mice. Infect. Immun. 72:196-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mazengera, L. R., K. J. Nathoo, and B. J. Zegers. 1999. Serum immunoglobulin levels in paediatric patients with pneumonia. Cent. Afr. J. Med. 45:300-302. [DOI] [PubMed] [Google Scholar]
- 47.Melzer, M., M. Colbridge, F. Keenan, D. Stainsby, and E. L. Ong. 1998. Cryptococcosis: an unusual opportunistic infection complicating B cell lymphoproliferative disorders. J. Infect. 36:220-222. [DOI] [PubMed] [Google Scholar]
- 48.Mitchell, T. G., and J. R. Perfect. 1995. Cryptococcosis in the era of AIDS—100 years after the discovery of Cryptococcus neoformans. Clin. Microbiol. Rev. 8:515-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morgan, D., C. Mahe, S. Malamba, M. Okongo, B. Mayanja, and J. Whitworth. 2001. Herpes zoster and HIV-1 infection in a rural Ugandan cohort. AIDS 15:223-229. [DOI] [PubMed] [Google Scholar]
- 50.Parra, C., J. M. Gonzalez, E. Castaneda, and S. Fiorentino. 2005. Anti-glucuronoxylomannan IgG1 specific antibodies production in Cryptococcus neoformans resistant mice. Biomedica 25:110-119. [PubMed] [Google Scholar]
- 51.Phillips, A. N., C. A. Sabin, J. Elford, M. Bofill, C. A. Lee, and G. Janossy. 1993. CD8 lymphocyte counts and serum immunoglobulin A levels early in HIV infection as predictors of CD4 lymphocyte depletion during 8 years of follow-up. AIDS 7:975-980. [DOI] [PubMed] [Google Scholar]
- 52.Pires, R. D. J., III, M. C. Guimaraes, M. J. Moya, F. R. Oliveira, P. Louzada, and R. Martinez. 2000. Hypogammaglobulinemia as predisposing factor for Cryptococcus neoformans infection: regarding two cases. Rev. Soc. Bras. Med. Trop. 33:603-608. [PubMed] [Google Scholar]
- 53.Pirofski, L. 2001. Polysaccharides, mimotopes and vaccines for encapsulated pathogens. Trends Microbiol. 9:445-452. [DOI] [PubMed] [Google Scholar]
- 54.Pitisuttithum, P., S. Tansuphasawadikul, A. J. Simpson, P. A. Howe, and N. J. White. 2001. A prospective study of AIDS-associated cryptococcal meningitis in Thailand treated with high-dose amphotericin B. J. Infect. 43:226-233. [DOI] [PubMed] [Google Scholar]
- 55.Ritchie, R. F., G. E. Palomaki, L. M. Neveux, O. Navolotskaia, T. B. Ledue, and W. Y. Craig. 1998. Reference distributions for immunoglobulins A, G, and M: a practical, simple, and clinically relevant approach in a large cohort. J. Clin. Lab. Anal. 12:363-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rivera, J., O. Zaragoza, and A. Casadevall. 2005. Antibody-mediated protection against Cryptococcus neoformans pulmonary infection is dependent on B cells. Infect. Immun. 73:1141-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scamurra, R. W., D. J. Miller, M. Abrahamsen, V. Kapur, S. M. Wahl, E. C. Milner, and E. N. Janoff. 2000. Impact of HIV-1 infection on VH3 gene repertoire of naive human B cells. J. Immunol. 164:5482-5491. [DOI] [PubMed] [Google Scholar]
- 58.Scott, J. A., A. J. Hall, C. Muyodi, B. Lowe, M. Ross, B. Chohan, K. Mandaliya, E. Getambu, F. Gleeson, F. Drobniewski, and K. Marsh. 2000. Aetiology, outcome, and risk factors for mortality among adults with acute pneumonia in Kenya. Lancet 355:1225-1230. [DOI] [PubMed] [Google Scholar]
- 59.Spitzer, E. D., S. G. Spitzer, L. F. Freundlich, and A. Casadevall. 1993. Persistence of initial infection in recurrent Cryptococcus neoformans meningitis. Lancet 341:595-596. [DOI] [PubMed] [Google Scholar]
- 60.Subramaniam, K. S., R. Segal, R. H. Lyles, M. C. Rodriguez-Barradas, and L. A. Pirofski. 2003. Qualitative change in antibody responses of human immunodeficiency virus-infected individuals to pneumococcal capsular polysaccharide vaccination associated with highly active antiretroviral therapy. J. Infect. Dis. 187:758-768. [DOI] [PubMed] [Google Scholar]
- 61.Tabone, M. D., G. Leverger, J. Landman, C. Aznar, L. Boccon-Gibon, and G. Lasfargues. 1994. Disseminated lymphonodular cryptococcosis in a child with X-linked hyper-IgM immunodeficiency. Pediatr. Infect. Dis. J. 13:77-79. [DOI] [PubMed] [Google Scholar]
- 62.Taborda, C., and A. Casadevall. 2001. Immunoglobulin M efficacy against Cryptococcus neoformans: mechanism, dose dependence, and prozone-like effects in passive protection experiments. J. Immunol. 166:2100-2107. [DOI] [PubMed] [Google Scholar]
- 63.Taborda, C., J. Rivera, O. Zaragoza, and A. Casadevall. 2003. More is not necessarily better: prozone-like effects in passive immunization with IgG. J. Immunol. 170:3621-3630. [DOI] [PubMed] [Google Scholar]
- 64.The WHO International Collaborating Group for the Study of the WHO Staging System. 1993. Proposed World Health Organization staging system for HIV infection and disease: preliminary testing by an international collaborative cross-sectional study. AIDS 7:711-718. [PubMed] [Google Scholar]
- 65.Winkelstein, J. A., M. C. Marino, H. Ochs, R. Fuleihan, P. R. Scholl, R. Geha, E. R. Stiehm, and M. E. Conley. 2003. The X-linked hyper-IgM syndrome: clinical and immunologic features of 79 patients. Medicine (Baltimore) 82:373-384. [DOI] [PubMed] [Google Scholar]
- 66.Yoshimine, H., K. Oishi, F. Mubiru, H. Nalwoga, H. Takahashi, H. Amano, P. Ombasi, K. Watanabe, M. Joloba, T. Aisu, K. Ahmed, M. Shimada, R. Mugerwa, and T. Nagatake. 2001. Community-acquired pneumonia in Ugandan adults: short-term parenteral ampicillin therapy for bacterial pneumonia. Am. J. Trop. Med. Hyg. 64:172-177. [DOI] [PubMed] [Google Scholar]
- 67.Yuan, R., A. Casadevall, and M. D. Scharff. 1997. T cells cooperate with passive antibody to modify the course of Cryptococcus neoformans in mice. Proc. Natl. Acad. Sci. USA 94:2483-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]