Abstract
The swamp buffalo (Bubalus bubalis carabanensis) plays a crucial role in agriculture across Southeast Asia but is increasingly underutilized in regions like Sabah, Malaysia. This study presents the first comprehensive statewide genetic assessment of Sabahan swamp buffalo using mitochondrial cytochrome b (cytb) gene sequences to investigate maternal origins and assess genetic diversity. Blood samples were collected from 211 individuals across eight locations in Sabah. DNA extraction and PCR amplification successfully yielded 198 high-quality cytb sequences. Comparative analysis with 1745 publicly available sequences revealed that 197 Sabahan buffalo belong to the SA1 haplogroup, which is common across Southeast Asia. Only four haplotypes were identified, with one dominant haplotype found in 190 individuals, indicating remarkably low genetic diversity (haplotype diversity = 0.088; nucleotide diversity = 0.00031). An additional river buffalo haplotype detected in one phenotypically swamp buffalo suggests introgression from Murrah buffalo. Analysis of molecular variance (AMOVA) showed that 96.29% of genetic variation occurred within populations, with minimal differentiation among them. ΦST comparisons suggest Sabah’s buffalo are genetically closer to Chinese swamp buffalo populations than those from South Asia, supporting a maternal lineage via the China–Taiwan–Philippines dispersal route. Spatial autocorrelation analysis indicated localized gene flow, while demographic reconstruction via Bayesian Skyline Plot suggested long-term population stability with recent expansion. These findings highlight the limited maternal genetic variation within Sabahan swamp buffalo, likely stemming from a founder effect during introduction to Borneo and minimal subsequent genetic inflow. The study provides critical insight into the evolutionary history and population structure of Sabah’s swamp buffalo, with implications for conservation and livestock improvement strategies aimed at bolstering genetic diversity and productivity in the region.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-18694-4.
Subject terms: Evolution, Evolutionary genetics, Phylogenetics, Population genetics, Agricultural genetics, Evolutionary biology, Haplotypes, Population genetics, Sequencing
Introduction
The swamp buffalo (Bubalus bubalis carabanensis) is one of two domesticated water buffalo species, and is primarily found across Southeast Asia and China where it is famed for its draught capabilities1,2. Current evidence suggests that swamp buffaloes were domesticated from the Asian wild water (Bubalus arnee) somewhere around northern Thailand1–6. Archaeological finds of the earliest swamp buffalo skeletons in this region dates specimens to around 4000 years before present (YBP)1,7,8. Similarly, genetic studies estimate a domestication period between 900 and 7000 YBP5,9. Current assessment of genetic diversity across the swamp buffalo range consistently shows that genetic diversity is greatest around Thailand, a hallmark of livestock domestication centres2,5,10–12. These domestication dates and regions are further corroborated by the belief that swamp buffaloes were domesticated to aid the spread of rice (Oryza sativa) cultivation, around 4000–9000 YBP in the Yangzi river basin13–15. Post domestication, swamp buffaloes dispersed along two pathways. The first represents a northern route through China during the Shang dynasty, before appearing in Taiwan and the Philippines by 1500 YBP1,2,7,16. The second route was a migratory path from mainland Southeast Asia through Sumatra and onto southernly Asian islands1,2.
This historic range of Southeast Asia and China is where the majority of the global population of 37 million swamp buffaloes can be found1,17. However, despite historically being an incredibly useful livestock species, current trends show population declines1,17. Intensification and mechanisation of farming in the last century has led to the redundancy of swamp buffaloes’ draught capabilities, and therefore their replacement1. As a result, some areas (e.g., China, Philippines, Australia) are repurposing swamp buffalo for beef production18,19. Buffaloes exhibit remarkable adaptability to unforgiving environments and efficiently digest low-quality roughages20. Moreover, buffalo meat is notably rich in iron and conjugated linoleic acid (CLA), both of which are essential for maintaining good health20. However, raising swamp buffalo for meat production presents certain challenges, as prior selection of swamp buffaloes for short stature, easy handling, resilience, and endurance makes them an excellent draught species, but not highly fruitful for food production21,22. Farmers have overcome the low production rates recently by crossbreeding swamp buffalo with the second domestic water buffalo species, the river buffalo (Bubalus bubalis bubalis). Domesticated from B. arnee in the Indus Valley region around 6000 YBP for milk production, river buffaloes are far larger than swamp buffaloes1,23. As such, river buffaloes have been imported into Southeast Asia in attempts to improve traits such as increasing growth and reducing calving intervals in swamp buffalo22.
The Malaysian state of Sabah is one location attempting to generate a thriving buffalo industry through new breeding for beef and utilising crossbreeding with river buffalo. Buffaloes in Malaysia are often regarded as important assets but are generally undervalued by farmers as a livestock option, with goats and pigs often preferred24. In 2019, the buffalo population in Malaysia was estimated to be around 101,695, split by 46.9% across mainland Malaysia, 46.7% in Sabah, and 6.4% in Sarawak25. Again, swamp buffalo numbers are declining and Sabah alone has seen an 11.9% decline within five years prior25. The great share of Malaysia’s buffalo population in Sabah is reasoned to be due to the long-established tradition of breeding buffaloes as a supplementary source of income26. Buffaloes are often the livestock of choice for the integrated cattle and oil palm farming systems and can often be found throughout community lands across towns and villages26,27. The economic status of Malaysia is continuing to increase over time, and in continually developing, an increase in demand for livestock products is often seen in tandem with increased economic status17. In 2023, Malaysia imported approximately USD $41.3 million worth of bovine meat, with an annual average of 153 kilo tonnes28,29. In comparison, exports of bovine meat were worth USD $2.19 million in 2023, with just 1000 tonnes of beef exported28–30. The main sources of imported beef are Australia, India, Brazil and New Zealand, and the high cost of importing beef has contributed to reduced affordability to Malaysians26. An improved buffalo industry, with highly productive stock could provide greater benefits for domestic beef production.
This study presents the first statewide genetic analysis of domestic water buffalo in Sabah. Advances in livestock genetics have greatly enhanced productivity through genetic improvement of commercial stocks and identification of unique diversity for conservation31–34. As the first phase in the genetic characterisation of Sabahan buffalo, this research focuses on analysing mitochondrial diversity using the cytochrome b (cytb) gene. Cytb was selected over the D-loop/control region due to its moderate evolutionary rate and reduced susceptibility to homoplasy, which make it more suitable for robust phylogenetic inference and maternal lineage tracing35. Furthermore, cytb enables broad comparative analysis with existing datasets on swamp buffalo available in public genetic repositories. Mitochondrial DNA analysis was conducted to assess current genetic diversity, trace maternal origins, evaluate potential admixture between swamp and river buffalo, explore the relationship between gene flow and geographic distribution, and reconstruct the demographic history of the population.
Materials and methods
Sample collection and DNA extraction
Blood samples were collected from 211 water buffaloes across eight locations of Sabah (Fig. 1). 7 of 8 populations sampled were indigenous swamp buffalo of Sabah. The other one population was a Department of Veterinary Services (DVS) Sabah buffalo research centre. The DVS population was comprised of a variety of swamp buffalo across Sabah, along with some buffaloes imported from Indonesia and Australia, and river-swamp hybrids from imported male Murrah buffalo. 5 ml of blood was extracted from each buffalo via the coccygeal vein and stored in EDTA, which was exclusively undertaken by qualified veterinarians from the Department of Veterinary Services Sabah, approved by Access License granted by the Sabah Biodiversity Council (license reference: JKM/MBS.1000-2/2 JLD.16 (5)). All methods are reported in accordance with ARRIVE guidelines. Of the 211 buffalo sampled, 210 were female and one was male. Among the females, 190 were swamp buffalo and 20 were crossbreeds resulting from swamp buffalo dams and a Murrah river buffalo sire. All crossbreed samples were taken from the DVS Sabah Buffalo Research Centre. The single male was a purebred Murrah. At the DVS Sabah Buffalo Research Centre, 106 buffalo were sampled, of which 24 had been imported from Australia. All buffaloes were adults, although their exact ages were not recorded. DNA was extracted using the Qiagen DNeasy Blood and Tissue Kit, following standard protocol (QIAGEN). DNA was quantified using the Qubit dsDNA Quantification Assay on a Qubit 3 Fluorometer (Invitrogen). A further 1745 publicly available swamp buffalo cytb sequences were retrieved from NCBI (FJ467648.1-FJ467917.1, KR009986.1-KR010168.1, MT182026.1-MT182644.1) from previous studies covering mainland Southeast Asia and China9,36,37. These were used to aid in determining the maternal origins of Sabahan swamp buffalo and as a comparison for the genetic diversity observed in Sabah.
Fig. 1.
Sabah sampling locations. The five divisions (bahagian) of Sabah coloured, key located at the bottom of the image. Sampling locations indicated by a location pin. Names of sampling locations stated, with their abbreviations in brackets. Italicised are the exact latitude and longitude GPS co-ordinates.
PCR and sequencing
Cytochrome b (cytb) sequences of 1403 bp were amplified using the primers BBub_CYTB_F (5’-TCAACCACGACCAATGATATGA-3’) and BBub_CYTB_R (5’-AGCTTTGGGTGTTGGTAGTG-3’). PCR reactions consisted of 25ul of 11 µl of 2 × Hot Start Taq 2X Master Mix (New England Biolabs), 0.75 µl of 0.25 uM of both BBub_CYTB_F and BBub_CYTB_R, 11 µl of RNAse free water and 1.5 µl of Buffalo DNA were added per PCR reaction. Sterile, RNAse free water was used in leu of buffalo DNA as the negative control. PCR conditions were adapted from Rosli et al.38. Initial denaturation took place at 95 °C for 15 min, followed by 35 cycles of: denaturation at 95 °C for 30 s, annealing at 65 °C for 30 s, and extension at 72 °C for 30 s. A final extension at 72 °C for 7 min at the end of the amplification cycles was included. PCR products were sequenced using Sanger sequencing (Eurofins PlateSeq Supreme service). DNA sequence chromatograms were trimmed, and base calls were confirmed by eye in Geneious Prime v2025.0.
Statistical analysis
Summary statistics of genetic variation (e.g. number of polymorphic sites) were calculated in DnaSP v6.12.0339. For the within Sabah analysis of population divergence, the samples were grouped by locality where they were collected (e.g. BE, KK), and the animals of the farm were kept as a separate group. For the between countries comparisons all the samples from Sabah were grouped together and compared to the buffaloes from other countries with their samples grouped by country of provenance. Arlequin v3.5.2.240 was used to carry out a molecular analysis of variance (AMOVA) within Sabah and between countries, as well as to estimate the population pairwise divergence ΦST . Populations for public data were defined as those used by Sun et al.37. Furthermore, groups for AMOVA were set as the geographic regions used by Lei et al.9, Zhang et al.37 and Sun et al.36. The relationship between haplotypes found in Sabah and their relationships to those previously published were assessed with a haplotype network built in PopART v1.7, using the TCS network model41. Subsequently, a map of haplotypes distributed across locations was built using the data from PopART.
The demographic history of Sabah’s buffalo population was studied using the Fu and Li’s Fs, and Tajima’s D summary statistics of genetic diversity that are sensitive to demographic changes42,43. A BSP analysis was used to model the effective female population size back through time, assuming a relaxed molecular clock to estimate the time of changes in effective population size over time accounting for the potential variation in substitution rate along the sequenced fragment using BEAST v2.7.7.044–46. The model of DNA sequence evolution used was Hasegawa, Kishino, and Yano (HKY) as identified as the best-fit substitution model in MEGA 12 (v.12.0.10)47,48. The clock model used was the optimised relaxed clock, with a mean clock rate set to 3.31 × 10−949. A total of 100,000,000 steps of the Markov Chain Monte Carlo algorithm were run discarding the first 10% of the steps as burn-in. Model outputs were checked visualised in Tracer (v.1.7.2) by ensuring the effective sample sizes (ESS) of parameters were > 200. BSP traces were plotted using ggplot250.
Spatial autocorrelation in GenAlEx v6.5 was used to determine the relationship between genetic similarity and geographic distance51. Pairwise ΦST calculated in Arlequin was used as the genetic distance, whilst geographic distance (km) was calculated within GenAlEx using latitudes and longitudes of each sampling location. Spatial autocorrelation was assessed for across (i) Sabah populations only, and (ii) across all swamp buffalo populations. Location coordinates for publicly obtained data were taken from their respective studies, and where missing, coordinates were approximated using the centre point of a sampling region9,36,37. For both analyses, a test of heterogeneity and correlograms plotting R-values against fixed-distance class intervals were carried out. A fixed-distance class of 150 km was used for the Sabah analysis, and 500 km for all swamp populations. To evaluate the significance of spatial patterns, 1000 bootstrap permutations were performed to generate 95% confidence intervals around r = 0 (depicting the null hypothesis) and to test whether observed R-values significantly differed from expected. Significance was declared when p < 0.0152.
Results
Dataset generation
DNA was successfully extracted for all 211 buffalo samples, with an average yield of 6.49 ng/µl (± 5.36). Furthermore, DNA amplification of cytb was successful for all samples. Sample sizes for each location can be found in Table 1. An example of a successful PCR is shown in Supplementary Fig. 1, where a clear band at 1403 bp can be seen following gel electrophoresis of the amplified DNA fragment. After sequencing, 198 of the 211 cytb sequences passed quality control. Following trimming of poor-quality sequence ends, a sequence length of 1099 bp was achieved across the Sabahan buffalo dataset.
Table 1.
Genetic diversity of cytb across Sabahan swamp buffalo populations, and with geographic regions outlined in Sun et al.3.
| n | No. haplotypes | No. polymorphic sites | Θ | π | |
|---|---|---|---|---|---|
| Population | |||||
| Beaufort | 16 | 1 | 0 | 0.000 ± 0.000 | 0.00000 |
| Beluran | 12 | 1 | 0 | 0.000 ± 0.000 | 0.00000 |
| Keningau | 16 | 2 | 1 | 0.125 ± 0.106 | 0.00011 |
| Kinabatangan | 8 | 1 | 0 | 0.000 ± 0.000 | 0.00000 |
| Kota Belud | 14 | 1 | 0 | 0.000 ± 0.000 | 0.00000 |
| Kota Kinabalu | 18 | 1 | 0 | 0.000 ± 0.000 | 0.00000 |
| Telupid | 74 | 2 | 22 | 0.027 ± 0.026 | 0.00058 |
| Australia* | 22 | 1 | 0 | 0.000 ± 0.000 | 0.00000 |
| Tawau | 18 | 2 | 1 | 0.471 ± 0.082 | 0.00043 |
| Geographic region | |||||
| Sabah | 198 | 4 | 23 | 0.088 ± 0.029 | 0.00031 |
| ML Yangtze | 492 | 30 | 37 | 0.529 ± 0.026 | 0.00331 |
| Upper Yangtze | 287 | 23 | 33 | 0.563 ± 0.032 | 0.00364 |
| SW China and NIC | 184 | 24 | 44 | 0.590 ± 0.042 | 0.00503 |
| SE China | 184 | 24 | 44 | 0.590 ± 0.042 | 0.00503 |
| SE Asia | 250 | 18 | 33 | 0.710 ± 0.023 | 0.00340 |
| South Asia | 105 | 16 | 47 | 0.744 ± 0.036 | 0.01012 |
Number of haplotypes, polymorphic sites, and diversities calculated in DnaSP (v6.12.03)9. Standard deviation shown after ±. * = Individuals originating from Australia but imported to Sabah, found at Telupid as part of government farm breeding programme, n = sample size, Θ = haplotype diversity, π = nucleotide diversity. Abbreviations as follows: ML Yangtze = Middle Lower Yangtze, SW China and NIC = Southwest China and North Indo China, SE China = Southeast China, SE Asia = Southeast Asia.
Genetic diversity of Sabah swamp buffalo
Four haplotypes were identified across Sabahan buffalo (Table 1). 23 polymorphic sites were found across these haplotypes. Across Sabah, haplotype diversity was 0.088 ± 0.029 (0.000–0.471) and nucleotide diversity was 0.00031 (0.00000–0.00058). The Australian imported buffalo possessed no variation as only one haplotype was found. Totals for each geographic region from the public data were also calculated (Table 1). A detailed breakdown of each of the 56 populations were also calculated (Supplementary Table 1). An AMOVA calculated using just Sabahan swamp buffalo found that 3.71% of genetic variation was accounted for among populations, whilst 96.29% of variation was accounted for within populations (Supplementary Table 2). An AMOVA was repeated on the full swamp buffalo dataset with regional mainland groups (Supplementary Table 3). Variation accounted for between groups was 24.52%. Among populations within each group accounted for 13.79% of variation, and again, as seen in the Sabahan populations alone, variation within populations accounted for 61.69% of all genetic variation.
The haplotype network shows that of the 198 Sabahan swamp buffalo tested, 197 were assigned to the SA1 group that was defined by Wang et al.5: I, II and III (Fig. 2). One swamp buffalo (SA_1683) had a river haplotype. This one individual solely accounted for 22 of the 23 polymorphic sites seen. Within the 197 assigned to the SA1, 190 swamp buffaloes were allocated to I, which is the main SA1 haplotype. Six individuals were appointed to II, and one individual was ascribed to III, both with one mutation difference each respectively (Fig. 2). The haplotype map shows low genetic diversity across Sabah as most populations exhibit a dominant haplotype (I) (Fig. 2). However, Tawau and Keningau show some variation, indicated by the presence of additional haplotypes. Telupid also shows an additional haplotype, but this was due to the one river haplotype found in one individual, the other 95 samples were all the I haplotype. All Australian imported samples were of the I haplotype. Counts of haplotypes per population can be found in Supplementary Table 4. The haplotype network of all 1943 sequences can be found in Supplementary Fig. 2.
Fig. 2.
Geographic distribution of haplotypes of Sabahan swamp buffalo. Pie charts calculated using PopART output. Abbreviations as follows; KU = Kota Belud, KK = Kota Kinabalu, BE = Beluran, Farm = Telupid government farm, KI = Kinabatangan, BF = Beaufort, KN = Keningau, TAW = Tawau. Key shown at the left-hand side. Top right panel depicts the cytb haplotype network of Sabahan swamp buffalo. I: n = 190, II: n = 6, III: n = 1, River: n = 1. Total number of sequences used = 198. River individual is present in the farm population. Where single mutations separate haplotypes, these have been noted on the thin black line. Thick black line denotes 23 mutations. Haplotype network calculated in PopART.
Genetic divergence
Between Sabah populations, average ΦST was 0.049 and ranged from − 0.034 (Telupid vs. Beluran) to 0.278 (Tawau vs. Beaufort) (Supplementary Table 5). Only Tawau consistently showed significant genetic differentiation to other Sabah populations (except Tawau vs. Kinabatangan). Pairwise ΦST values across all swamp buffalo population comparisons averaged 0.152 and ranged from 0.000 to 0.970 (Supplementary Table 5). Average ΦST was calculated between regional against Sabah. The results from most similar to most different were as follows: Sabah versus Sabah = 0.049, Sabah versus Upper Yangtze = 0.123, Sabah versus Middle Lower Yangtze = 0.125, Sabah vs Southeast China = 0.127, Sabah versus Southwest China and North Indochina = 0.193, Sabah versus South Asia = 0.700. Sabah populations were most similar to Chinese swamp buffalo regions than Southern Asian populations.
Isolation by distance
There was significant spatial autocorrelation across the dataset containing all swamp buffalo populations (Ω = 64.3, p < 0.001). This result suggests that there is relation between genetic similarity and geographic proximity. Correlation of haplotypes at short distances begins at r = 0.514 between 0 and 500 km, declining to − 0.021 by 1000–1500 km where r remains stable for the remaining distances (Fig. 3). Analysis of Sabah only populations found non-significant spatial autocorrelation (Ω = 12.3, p = 0.032) implying that dispersal within Sabah is not limited at the spatial scale. The Sabah result largely stems from the observation of most swamp buffaloes having the same mitochondrial haplotype (Fig. 2).
Fig. 3.
Spatial autocorrelation. Correlograms showing spatial autocorrelation (r) across geographic distance (km) between swamp populations. (A) Spatial autocorrelation on Sabahan populations only. Distance class size 150 km. (B) All swamp populations. Distance class size 500 km. Dashed lines show the 95% confidence interval surrounding r = 0 indicating no effect of geographic distance on r. 95% confidence error bar is shown for each value of r at each distance.
Demographic history
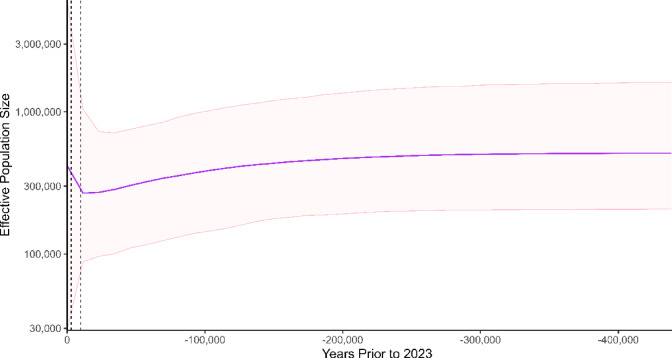
Significant changes in demographics were detected using a variety of statistics in the Sabahan buffalo population. Fu and Li’s Fs was − 1.404, which was not statistically significant to 95% CI. In contrast, Tajima’s D was − 2.53994, with a p-value of 0.001 to 95% CI (− 1.32, 2.06). This agrees with previous reports of Chinese and Indochinese buffalo populations9,53. Furthermore, BSP shows fluctuations in Sabahan swamp buffalo population size over the last 400,000 years. Between approximately 400,000–250,000 years ago, the population size was relatively stable (Fig. 4). An initial gradual decline in effective population size can be seen from approximately 250,000 years ago to 20,000 years ago, followed by a marked and sharp increase from approximately 20,000 years ago, continuing to 2023. However, wide 95% highest posterior density (HPD) intervals in the more recent past suggest greater uncertainty in recent demographic reconstructions.
Fig. 4.
Bayesian skyline plot of Sabahan swamp buffalo. Analysis of 198 Sabahan swamp buffalo cytb sequences of 1099 bp length, sampled in 2023 generated in BEAST (v.2.7.7.0) using the Hasegawa-Kishino-Yano (HKY) model with 100,000,000 replicates. X axis in units of years before 2023 CE, Y axis is equal to Neͳ. Clock model was the optimised relaxed clock, and the mean clock rate set to 3.31 × 10−9. Purple line is the median estimate, with the pink shaded area denoting the 95% confidence intervals. Dashed black vertical lines indicate the known domestication period of buffalo, 3000–10,000 years ago.
Discussion
This study presents the first statewide genetic analysis of Sabahan buffalo, revealing details surrounding their maternal origins and present genetic diversity. The average haplotype diversity across all Sabah populations was low at 0.088 ± 0.029 (0.000–0.471). This result is much lower than expected when compared to mainland populations, where the average haplotype diversity ranges between 0.267 and 1.009,36,37. Similarly, average nucleotide diversity of 0.00031 found across Sabah is also much lower than seen across mainland populations 0.00075–0.014699,36,37. Low nucleotide diversity in the cytochrome b gene was consistent with a previous study assessing d-loop nucleotide diversity (0.01906) at Sabah’s DVS farm across swamp and crossbred buffaloes54. The remarkably low diversity observed in Sabahan buffaloes is concerning for several reasons. First, despite a statewide sampling of buffalo populations, consistently low diversity was found across the dataset. Second, buffaloes from both beef and draught production systems were included, yet low diversity remained evident across these groups. Third, the reduced genetic diversity was not limited to buffaloes of Sabahan origin; it was also observed in animals at the DVS farm in Telupid, that has imported buffaloes from Australia and, previously, Indonesia.
The implications of low genetic diversity in Sabahan buffaloes remain uncertain based on this study. Mitochondrial data provides information from only maternal lineages, lacking insights into paternal lineages11. Whilst mitochondrial genomes are not neutral as once thought, the absence of nuclear DNA data limits understanding of genes associated with livestock function that has been integral in livestock genetic improvement33,55–58. Nevertheless, some inferences can be drawn. The abundance a single haplogroup, largely represented by a single, dominant haplotype suggests that the present-day mitochondrial diversity seen in the Sabahan buffalo, largely derive from few maternal lineages59. The low haplotype diversity likely reflects a historical bottleneck or founder event, wherein a small number of females contributed disproportionately to the gene pool, with this likely occurring through small numbers of buffalo being historically imported to the state26. This has been seen in Ovis canadensis mexicana in Tiburon Island, where the island population has significantly less genetic variation when compared to mainland populations60.
Consequently, these buffaloes may be closely related, and elevated levels of inbreeding could be widespread across the state. Whilst inbreeding is used in livestock to maintain lineages with desirable traits, Sabah lacks formal breeding programmes for swamp buffaloes. Therefore, Sabahan buffaloes may be at greater risk of inbreeding depression if breeding has not been linked to livestock performance and fitness61–64. Additionally, reduced genetic diversity restricts fluctuations in allele frequencies over time. As a result, Sabahan swamp buffaloes may face challenges adapting to climate change, such as increased susceptibility to heat stress and related health declines31. Meanwhile, between beef production and draught systems, low diversity may prevent Sabahan buffaloes becoming ‘genetically optimised’ for either system.
Sabah is actively seeking to develop its buffalo industry; however, the low genetic diversity observed in Sabahan buffaloes may hinder both production and economic goals. Swamp buffalo are yet to go through substantial selective breeding and genetic improvement for beef production. The limited genetic variation within the population may restrict the potential for selecting and developing improved lineages. Sabahan buffalo may provide the appearance of a ‘rare’ or local breed, whereby the lack of variation makes it difficult to genetically improve without population collapse32. A comparable case in Gloucester cattle—a native breed from the United Kingdom—demonstrated that while estimated breeding values could support development, the limited variation in genetic markers reduced the utility of incorporating genomic data65. In Sabahan buffalo, a previous study at the DVS farm assessed the heritability of birth weight66. The study found that heritability was low (0.29), indicating that other environmental factors affected birth weight, and therefore improvement may be difficult based on genetics alone66. Fortunately, Sabahan swamp buffalo are not a distinct breed and as greater diversity is present on mainland Asia, genetic diversity in Sabah could be restored through the importation of unrelated buffaloes from these genetically diverse populations2,5,9,37.
The low genetic diversity found across Sabah may have persisted since their arrival to Borneo. It appears that swamp buffaloes were domesticated to aid in rice cultivation as buffaloes excel over other draught animals in wet or waterlogged environments, such as muddy paddy fields24. Traditionally, swamp buffalo herds were driven across fields in order to slash weeds and stubble, thus preparing flooded land for rice cultivation67. The spread of rice cultivation across southeast Asia corresponds to around 4000 YBP, including in Borneo where evidence has been found in Sarawak68,69. However, Borneo does not possess optimal environments for rice production due to its mountainous rainforest environment70. It may not have been until rice cultivation methods were established, of which swamp buffalo may have been a part of, that rice and swamp buffaloes spread70. Demand for swamp buffalo before this may have been unlikely as, in Borneo, the hunting of bearded pigs (Sus barbatus) and banteng (Bos javanicus) is embedded in Bornean culture and may have fulfilled this demand for food71–73. It has been previously acknowledged that Sabah, and Malaysia as a whole, is lacking in imports of breeding stock to the ruminant industry26. Therefore, buffaloes may have only been imported to Borneo along with rice in small numbers, with few subsequent importations.
Following low diversity estimates, four haplotypes were identified across the Sabahan samples. Three of these were swamp haplotypes, all of which are part of the SA1 haplogroup37. Unusually, one river haplotype was found within a phenotypically swamp buffalo. Typically, river buffaloes are imported through male lineages, whether importing live individuals or semen. Importation of river buffalo into swamp buffalo regions is common, particularly in the 1980s as the dairy cattle project was introduced30. Therefore, identification of a river haplotype indicates that female river buffaloes have been incorporated into crossbreeding practices. This haplotype is readily distinguishable due to the substantial genetic divergence between river and swamp buffaloes. Wang et al.5 estimated the divergence between river and swamp mitogenomes to be over 800,000 YBP, while more recent nuclear studies suggest a divergence time of approximately 240,000 YBP. Although both types were domesticated from the wild water buffalo (Bubalus arnee), their respective ancestral founders were already genetically distinct3,5,74. This prolonged period of separation, coupled with adaptation to different geographic regions (e.g., India versus Southeast Asia), and domestication for different products (milk production versus draught use), has led to considerably different genetic variation between the species3,74.
While crossbreeding river and swamp buffaloes may offer a short-term strategy to improve traits such as growth and body size in swamp buffaloes, the profound genetic differences may interfere with established selection patterns and phenotypic traits, potentially resulting in maladaptation to local environmental conditions31. If conducted without informed management, such crossbreeding could introduce long-term genetic complications. However, if carefully implemented, the introduction of novel genetic variation from river buffaloes may offer opportunities to develop new, beneficial traits in the Sabahan buffalo population75.
190 of samples here were of the primary SA1 haplotype. The other two swamp haplotypes were of a single mutation difference to this primary haplotype and found uniquely associated with single populations of Tawau and Keningau and are not found within the mainland populations. As such, ΦST results within Sabah populations showed there was low genetic differentiation between populations. Tawau was the only Sabahan population that differed significantly to the others. This is likely due to the additional II haplotype that is present only in this population, at a greater proportion than that of III in Keningau. Approximately half of the Sabahan buffalo sampled in this study derived from one population, being the DVS farm (Telupid). Local Sabah populations were assumed to comprise of buffalo local to Sabah, with no influence from imported genetics since buffaloes are bred locally. Meanwhile, the DVS farm contains a mixture of genetics from Sabah, Australia, and previous Indonesian imports. Therefore, it would be expected that this population does not reflect those of local Sabahan buffalo populations. If genetic improvement efforts at the DVS farm are successful, livestock at the DVS farm may become more desirable and valuable owing to greater productivity and economic output. This could promote the distribution of these improved animals across the state, potentially leading to the replacement of local genetic lineages by improved and imported genetics which could in turn have consequences of conservation of genetic resources in livestock. However, the impact of this replacement would be undetectable using mitochondrial data alone, as many of these animals share identical haplotypes. Therefore, further research using nuclear markers is essential to accurately assess the genetic diversity and structure of both local and farmed buffalo populations.
As the SA1 haplogroup was found in Sabah, it is difficult to confirm the origins of Sabahan buffalo because this haplogroup is prevalent across the swamp buffalo range. However, ΦST analysis here shows that it is more likely that Sabahan buffalo originated via the Chinese-Taiwan-Philippines pathway. ΦST scores were consistently lower verses Chinese populations than Southernly mainland Asia populations. This is due to the absence of rare haplogroups present in Chinese populations as only SA and SB haplogroups were captured in historical migrations37. Additional uptake of new haplotypes was not possible in China, as the only extant buffalo species at the time was Bubalus mephistopheles, which doesn’t appear to have been crossed with swamp buffalo76. Most notably, the population in Taiwan is almost completely comprised of SA1 haplogroup which may explain why cytb diversity is low in Sabah. Each migration away from a domestication centre captures a smaller proportion of genetic variation11,12. As such, rarer haplogroups were likely lost into China, and further diversity lost into Taiwan. This indicates that the Philippines and Sabah may only have a small pool of variation to capture. A previous study found that Malaysian buffalo tended to not cluster with Thai buffalo, further supporting a Chinese origin35. However, patterns of variation in Sabah may not be representative of wider Borneo, as Indonesian buffaloes possess different D-loop haplotypes in the north and south77.
The analysis of spatial autocorrelation across all swamp buffalo revealed that there was greater gene flow occurring at shorter distances (< 1000 km). Such relationship suggests that swamp buffalo are likely to breed locally or with nearby populations as, for example, local people exchange livestock in markets, and that animals tend to not be exchanged at greater distances53. Within the Sabah only analysis, potential high correlation is observed at the first distance (0–150 km), before becoming uncorrelated at 150–300 km. Mitochondrial diversity in Sabahan buffaloes is dominated by a single haplotype, therefore this result is unsurprising. The drop in correlation may be driven by Tawau, a population that is more distant compared to other sites and features another haplotype in abundance. AMOVA results may further support population structure results as most of the variation was assigned to within populations rather than among populations and groups. Whilst we may infer that Sabahan buffaloes derive from a Chinese migratory pathway, the generation of nuclear data would give greater resolution into understanding their relationship with mainland populations55.
Interestingly, within our samples collected were Australian imported swamp buffaloes. These are imported into Sabah to the DVS Buffalo Research Farm that is the Telupid population here. These individuals show improved beef production capabilities, thus aiding to improve native stock. All Australian individuals also showed an absence of diversity, as all were assigned into the SA1 haplogroup. Swamp buffalo were imported to Australia in the early nineteenth century from Timor and surrounding islands78,79. How these individuals effect Sabahan buffaloes genetic diversity is unclear from this study, however nuclear DNA from southern Indonesian islands would suggest Australian buffalo possess genetic diversity originating from the Thai-Sumatra migration pathway, therefore bringing a new influx of genetic diversity to Sabah2. These low haplotype and nucleotide diversities for each individual population, and the Australia subset of Telupid could be due to sampling bias, however, as there is little haplotype diversity across the whole state, this may indicate that it is not sampling bias80.
A BSP analysis was used to model the effective female population size back through time. Results largely reflected those of wider swamp buffalo studies, despite the smaller amount of data available here5,81. Swamp effective population size continually declined towards the domestication period (< 10,000 YBP). This may be due to overhunting of wild water buffalo prior to domestication, restricting animal resources for ancient people that triggered improved animal management leading to domestication82. Effective population size increases from that point, throughout the domestication period, and to the present day. In buffalo, this may be explained by accumulation of new haplotypes through uptake of new wild female buffaloes over time, or otherwise new mutations over time. This is well known in river buffalo where hybridisation with wild water buffalo is still occurring in the present day1,23,83. Results from statistics evaluating demographic changes (e.g., Tajima’s D) support a population expansion. Fu and Li’s D* and F* along with Tajima’s D all returned significant negative values indicating an excess of uncommon haplotypes9,84. Previous studies across swamp buffalo have showed that population expansion have occurred, such as in Chinese swamp buffalo9,53. However, Fu’s Fs is considered the most sensitive statistic and was the only non-significant result42. Therefore, it may be considered that Sabah’s buffalo population is relatively stable.
Overall, this study found that swamp buffalo deriving from Sabah, Borneo possessed remarkably low diversity across the state, dominated by a single haplogroup. The identification of a river buffalo haplotype within a swamp buffalo indicates that there has been greater admixture besides importation of a few males or imported semen. The lack of other swamp haplogroups found suggests that Sabahan buffalo derive from the China-Taiwan-Philippines migration route as opposed to Thailand-Sumatra that possesses a greater variety of haplogroups. Our findings provide valuable insights into the evolutionary history and genetic structure of Sabahan swamp buffalo, contributing to conservation strategies and the sustainable management of this important livestock species.
Supplementary Information
Acknowledgements
This work was funded by the Natural Environment, Biotechnology and Biological Sciences and Medical Research Councils (NERC, BBSRC and MRC) [Grant No.: NE/X016714/1] as part of the One Health for One Environment: an A-Z Approach for Tackling Zoonoses (‘OneZoo’) Centre for Doctoral Training. It was also funded by the Southwest Biosciences Doctoral Training Partnership, Great Western Four (GW4) Doctoral Training Partnership, Department of Veterinary Services Sabah, Sabah Biodiversity Centre and Danau Girang Field Centre. This project was also supported using seed funding from Cardiff University’s GCRF QR Funding from the Higher Education Funding Council of Wales. The bioinformatic analyses for this paper were performed on the Cardiff University School of Biosciences Biocomputing Hub HPC/Cloud infrastructure. The authors would like to express the deepest of gratitude to the Department of Veterinary Services (DVS) Sabah, and SaBC for their instrumental help in facilitation of the project. Thanks also to the following people without whom the project would not have been achievable: Dr. Mischellena Samanthan Michael, Dr. Naomi Martin, Rosica Sipalan, Dr Noorsyakilah Binti Muhamud, Loinsing Kasang, Dr. Debra Epun, Raner Giswa, Romeo Cordova, John Thomas Mikat, Dr. Abel Jamaun, Dr. Cassandra Alexius, Daniel Juim, Dr. Dayang Ku Farah Ana Zaidun, Vinah Kuntiong, Henry Gunong. We would like to dedicate this paper to the memory of Mr. Roik Kuliban, who helped us with the fieldwork in the Kinabatangan region.
Author contributions
J.B. and L.D. wrote the main manuscript, prepared the figures, and analysed the data. L.D., K.J., and J.E. collected blood samples. J.B. and J.H. generated the Sabahan cytochrome b data. P.OtW., M.M., K.J., and B.G. were responsible for study design, securing of funding, and substantial revisions to the manuscript. All authors contributed vital edits and reviewed the manuscript.
Data availability
The datasets generated and/or analysed during the current study are not publicly available as the data forms part of an ongoing study and is currently being used for additional analyses. Data is available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All necessary permits and approvals were obtained for the collection, export, and import of biological samples involved in this research. Sampling was conducted under an Access License issued by the Sabah Biodiversity Council (license reference: JKM/MBS.1000-2/2 JLD.16 (5)), and export from Malaysia was carried out under an Export License from the same authority (license reference: JKM/MBS.1000-2/3 JLD.5 (52)). Import into the United Kingdom was authorized by the Animal and Plant Health Agency under an IV58 Import License (authorization number: ITIMP23.1193). All methods are reported in accordance with ARRIVE guidelines.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jessica Wyn Brocklebank and Luke Davies are joint first authors.
References
- 1.Zhang, Y., Colli, L. & Barker, J. S. F. Asian water buffalo: Domestication, history and genetics. Anim. Genet.51(2), 177–191 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Colli, L. et al. New insights on water buffalo genomic diversity and post-domestication migration routes from medium density SNP chip data. Front. Genet.9, 53 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun, T. et al. Genomic analyses reveal distinct genetic architectures and selective pressures in buffaloes. GigaScience.9(2), giz166 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maheswarappa, N. B., Muthupalani, M., Mohan, K., Banerjee, R., Sen, A. R., Barbuddhe, S. B. Water buffalo: Origin, emergence, and domestication. In Asiatic water buffalo 1–10 (Springer, Singapore, 2022).
- 5.Wang, S. et al. Whole mitogenomes reveal the history of Swamp buffalo: Initially shaped by glacial periods and eventually modelled by domestication. Sci. Rep.7(1), 4708 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cockrill, W. R. The water buffalo: A review. Br. Vet. J.137(1), 8–16 (1981). [DOI] [PubMed] [Google Scholar]
- 7.Epstein, H. Domestic animals of China (Africana Publishing Corporation, New York, 1971). [Google Scholar]
- 8.Higham, C. Early cultures of mainland Southeast Asia (Art Media Resources, Chicago, 2002). [Google Scholar]
- 9.Zhang, Y. et al. Strong and stable geographic differentiation of swamp buffalo maternal and paternal lineages indicates domestication in the China/Indochina border region. Mol. Ecol.25(7), 1530–1550 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Nagarajan, M., Nimisha, K. & Kumar, S. Mitochondrial DNA variability of domestic river buffalo (Bubalus bubalis) populations: Genetic evidence for domestication of river buffalo in Indian subcontinent. Genome Biol. Evol.7(5), 1252–1259 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruford, M. W., Bradley, D. G. & Luikart, G. DNA markers reveal the complexity of livestock domestication. Nat. Rev. Genet.4(11), 900–910 (2003). [DOI] [PubMed] [Google Scholar]
- 12.Groeneveld, L. F. et al. Genetic diversity in farm animals: A review. Anim. Genet.41(s1), 6–31 (2010). [DOI] [PubMed] [Google Scholar]
- 13.Kovach, M. J., Sweeney, M. T. & McCouch, S. R. New insights into the history of rice domestication. Trends Genet.23(11), 578–587 (2007). [DOI] [PubMed] [Google Scholar]
- 14.Sweeney, M. & McCouch, S. The complex history of the domestication of rice. Ann. Bot.100(5), 951–957 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fornasiero, A., Wing, R. A. & Ronald, P. Rice domestication. Curr. Biol.32(1), R20–R24 (2022). [DOI] [PubMed] [Google Scholar]
- 16.Amano, N., Piper, P. J., Hung, H.-C. & Bellwood, P. Introduced domestic animals in the Neolithic and metal age of the Philippines: Evidence from Nagsabaran Northern Luzon. J. Isl. Coast Archaeol.8(3), 317–335 (2013). [Google Scholar]
- 17.Food and Agriculture Organisation of the United Nations. Food and agriculture data. FAOSTAT. Available from: https://www.fao.org/faostat/en/#home. 2025.
- 18.Naveena, B. M. & Kiran, M. Buffalo meat quality, composition, and processing characteristics: Contribution to the global economy and nutritional security. Anim. Front.4(4), 18–24 (2014). [Google Scholar]
- 19.(PDF) Trends in buffalo production in Asia. ResearchGate. Available from: https://www.researchgate.net/publication/41394240_Trends_in_buffalo_production_in_Asia. (2024)
- 20.Wanapat, M. & Chanthakhoun, V. Buffalo production for emerging market as a potential animal protein source for global population. Buffalo Bull.34(2), 169–180 (2015). [Google Scholar]
- 21.Budiarto A, Ciptadi G, Hakim L, Karima HN, Hisam MH. The productivity of female swamp buffaloes (Bubalus bubalis) in East Java, Indonesia. In IOP Conference Series: Earth and Environmental Science, vol. 247 012029 (2019).
- 22.Pineda, P. S., Flores, E. B., Herrera, J. R. V. & Low, W. Y. Opportunities and challenges for improving the productivity of swamp buffaloes in Southeastern Asia. Front. Genet.12, 629861 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar, S., Nagarajan, M., Sandhu, J. S., Kumar, N. & Behl, V. Phylogeography and domestication of Indian river buffalo. BMC Evol Biol.7(1), 1–8 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.FAO Regional Office for Asia and the Pacific. Water buffalo: An asset undervalued. In Food and Agriculture Organization Report No.: 1 (2000).
- 25.Khalex, A. H. et al. Buffalo in Borneo, Sarawak: A review of the current status of the indigenous buffalo industry. J. Buffalo Sci.10, 32–40 (2021). [Google Scholar]
- 26.Ariff, O., Sharifah, N. & Hafidz, A. Status of beef industry of Malaysia. Malays. Soc. Anim. Prod.18(2), 1–21 (2015). [Google Scholar]
- 27.Ahmad, A. R. & Mohd Nasir, A. S. The practices and factors affecting the implementation of integrated cattle and oil palm farming system in Malaysia. Humanit. Soc. Sci. Rev.8(4), 693–700 (2020). [Google Scholar]
- 28.Agriculture and Horticulture Development Board. Trade and production: Malaysia. Trade and Production: Malaysia. Available from: https://ahdb.org.uk/trade-and-policy/cptpp/malaysia. (2025)
- 29.Simoes, A. & Hidalgo, C. The economic complexity observatory: An analytical tool for understanding the dynamics of economic development. | Request PDF. In Scalable Integration of Analytics and Visualization (San Francisco, California 2011). Available from: https://www.researchgate.net/publication/221605462_The_Economic_Complexity_Observatory_An_Analytical_Tool_for_Understanding_the_Dynamics_of_Economic_Development.
- 30.Eli, N., Weng, K. K. & Oming, M. Regulation of livestock farming in Sabah: Issues and challenges. Malay Civiliz (2000). Available from: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.486.3545&rep=rep1&type=pdf.
- 31.Kristensen, T. N., Hoffmann, A. A., Pertoldi, C. & Stronen, A. V. What can livestock breeders learn from conservation genetics and vice versa?. Front. Genet.6, 38 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Biscarini, F., Nicolazzi, E. L., Stella, A., Boettcher, P. J. & Gandini, G. Challenges and opportunities in genetic improvement of local livestock breeds. Front. Genet.6, 33 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meuwissen, T., Hayes, B. & Goddard, M. Accelerating improvement of livestock with genomic selection. Annu. Rev. Anim. Biosci.1(1), 221–237 (2013). [DOI] [PubMed] [Google Scholar]
- 34.Georges, M., Charlier, C. & Hayes, B. Harnessing genomic information for livestock improvement. Nat. Rev. Genet.20(3), 135–156 (2019). [DOI] [PubMed] [Google Scholar]
- 35.Lau, C. H. et al. Genetic diversity of Asian water buffalo (Bubalus bubalis): Mitochondrial DNA D-loop and cytochrome b sequence variation. Anim. Genet.29(4), 253–264 (1998). [DOI] [PubMed] [Google Scholar]
- 36.Lei, C. Z. et al. Genetic diversity of mitochondrial cytochrome b gene in Chinese native buffalo. Anim. Genet.42(4), 432–436 (2011). [DOI] [PubMed] [Google Scholar]
- 37.Sun, T. et al. Genetic diversity of mitochondrial cytochrome b gene in swamp buffalo. Anim. Genet.51(6), 977–981 (2020). [DOI] [PubMed] [Google Scholar]
- 38.Rosli, M. K. A. et al. Optimization of PCR conditions to amplify Cyt b, COI and 12S rRNA gene fragments of Malayan gaur (Bos gaurus hubbacki) mtDNA. Genet. Mol. Res. GMR10(4), 2554–2568 (2011). [DOI] [PubMed] [Google Scholar]
- 39.Rozas, J. et al. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol.34(12), 3299–3302 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Excoffier, L. & Lischer, H. E. L. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour.10(3), 564–567 (2010). [DOI] [PubMed] [Google Scholar]
- 41.Clement, M., Snell, Q., Walke, P., Posada, D. & Crandall, K. TCS: Estimating gene genealogies. In Proceedings 16th International Parallel and Distributed Processing Symposium 7 (2002). Available from: https://ieeexplore.ieee.org/document/1016585.
- 42.Fu, Y. X. Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics147(2), 915–925 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tajima, F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics123(3), 585–595 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suchard, M. A. et al. Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evol.4(1), vey016 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barido-Sottani, J. et al. Taming the BEAST—A community teaching material resource for BEAST 2. Syst. Biol.67(1), 170–174 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bouckaert, R. et al. BEAST 2.5: An advanced software platform for Bayesian evolutionary analysis. PLOS Comput Biol15(4), e1006650 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hasegawa, M., Kishino, H. & Yano, T. Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. J Mol Evol.22(2), 160–174 (1985). [DOI] [PubMed] [Google Scholar]
- 48.Kumar, S. et al. MEGA12: Molecular Evolutionary Genetic Analysis Version 12 for Adaptive and Green Computing. Mol Biol Evol.41(12), msae263 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Atkulwar, A., Farah, S., Gadhikar, Y. & Baig, M. Mitochondrial DNA diversity in wild gaur (Bos gaurus gaurus): Evidence from extant and historical samples. Mitochondrial DNA Part B5(2), 1556–1560 (2020). [Google Scholar]
- 50.Rambaut, A., Drummond, A. J., Xie, D., Baele, G. & Suchard, M. A. Posterior summarization in Bayesian phylogenetics using tracer 1.7. Syst Biol.67(5), 901–904 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peakall, R. & Smouse, P. E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research—An update. Bioinformatics28(19), 2537–2539 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Banks, S. C. & Peakall, R. Genetic spatial autocorrelation can readily detect sex-biased dispersal. Mol Ecol.21(9), 2092–2105 (2012). [DOI] [PubMed] [Google Scholar]
- 53.Yue, X. P. et al. Phylogeography and domestication of Chinese swamp buffalo. PLoS ONE8(2), e56552 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shaari, N. A. L. et al. Karyotypic and mtDNA based characterization of Malaysian water buffalo. BMC Genet.20(1), 1–6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hurst, G. D. D. & Jiggins, F. M. Problems with mitochondrial DNA as a marker in population, phylogeographic and phylogenetic studies: the effects of inherited symbionts. Proc. R. Soc. B Biol. Sci.272(1572), 1525–1534 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Camus, M. F. & Dhawanjewar, A. S. Multilevel selection on mitochondrial genomes. Curr. Opin. Genet. Dev.80, 102050 (2023). [DOI] [PubMed] [Google Scholar]
- 57.Koch, R. E. et al. Integrating mitochondrial aerobic metabolism into ecology and evolution. Trends Ecol. Evol.36(4), 321–332 (2021). [DOI] [PubMed] [Google Scholar]
- 58.Goddard, M. E. & Hayes, B. J. Genomic selection. J. Anim. Breed. Genet.124(6), 323–330 (2007). [DOI] [PubMed] [Google Scholar]
- 59.Carroll, S. P. & Fox, C. W. (eds) Conservation biology: Evolution in action (Oxford University Press, Oxford, 2008). [Google Scholar]
- 60.Hedrick, P. W., Gutierrez-Espeleta, G. A. & Lee, R. N. Founder effect in an island population of bighorn sheep. Mol. Ecol.10(4), 851–857 (2001). [DOI] [PubMed] [Google Scholar]
- 61.Howard, J. T., Pryce, J. E., Baes, C. & Maltecca, C. Invited review: Inbreeding in the genomics era: Inbreeding, inbreeding depression, and management of genomic variability. J. Dairy Sci.100(8), 6009–6024 (2017). [DOI] [PubMed] [Google Scholar]
- 62.Leroy, G. Inbreeding depression in livestock species: review and meta-analysis. Anim. Genet.45(5), 618–628 (2014). [DOI] [PubMed] [Google Scholar]
- 63.Gutiérrez-Reinoso, M. A., Aponte, P. M. & García-Herreros, M. A review of inbreeding depression in dairy cattle: Current status, emerging control strategies, and future prospects. J. Dairy Res.89(1), 3–12 (2022). [DOI] [PubMed] [Google Scholar]
- 64.Doekes, H. P., Bijma, P. & Windig, J. J. How depressing is inbreeding? A meta-analysis of 30 years of research on the effects of inbreeding in livestock. Genes12(6), 926 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pollott, G. E., Charlesworth, A. & Wathes, D. C. Possibilities to improve the genetic evaluation of a rare breed using limited genomic information and multivariate BLUP. Animal8(5), 685–694 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Soh, S. S. et al. Short communication: Heritability estimation of birth weight of swamp buffalo in Sabah, Malaysia. J. Buffalo Sci.9, 24–28 (2020). [Google Scholar]
- 67.Jackson, J. C. Rice cultivation in west Malaysia: Relationships between culture history, customary practices and recent developments. J. Malays. Branch R. Asiat. Soc.45(2), 76–96 (1972). [Google Scholar]
- 68.Deng, Z. et al. Validating earliest rice farming in the Indonesian Archipelago. Sci. Rep.10(1), 10984 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jones, S. E., Hunt, C. O., Barton, H., Lentfer, C. J. & Reimer, P. J. Forest disturbance, arboriculture and the adoption of rice in the Kelabit Highlands of Sarawak, Malaysian Borneo. Holocene23(11), 1528–1546 (2013). [Google Scholar]
- 70.Barton, H. The reversed fortunes of sago and rice, Oryza sativa, in the rainforests of Sarawak, Borneo. Quat Int.249, 96–104 (2012). [Google Scholar]
- 71.Kurz, D. J. et al. Transformation and endurance of Indigenous hunting: Kadazandusun–Murut bearded pig hunting practices amidst oil palm expansion and urbanization in Sabah, Malaysia. People Nat.3(5), 1078–1092 (2021). [Google Scholar]
- 72.Kurz, D. J. et al. Socio-ecological factors shape the distribution of a cultural keystone species in Malaysian Borneo. Npj Biodivers.2(1), 1–9 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gardner, P. C., Goossens, B., Bakar, S. B. A. & Bruford, M. W. Hunting pressure is a key contributor to the impending extinction of Bornean wild cattle. Endanger. Species Res.45, 225–235 (2021). [Google Scholar]
- 74.Luo, X. et al. Understanding divergent domestication traits from the whole-genome sequencing of swamp- and river-buffalo populations. Natl. Sci. Rev.7(3), 686–701 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cruz, L. C. Transforming swamp buffaloes to producers of milk and meat through crossbreeding and backcrossing. Wartazoa19, 103–116 (2018). [Google Scholar]
- 76.Yang, D. Y., Liu, L., Chen, X. & Speller, C. F. Wild or domesticated: DNA analysis of ancient water buffalo remains from north China. J. Archaeol. Sci.35(10), 2778–2785 (2008). [Google Scholar]
- 77.Wibowo, A., Putra, W. P. B. & Summpunn, P. The phylogeny of Bornean swamp buffalo (Bubalus bubalis) analysis based on D-loop mitochondrial DNA sequence variation. Trop. Anim. Sci. J.46(2), 139–145 (2023). [Google Scholar]
- 78.Mcmahon, C. R. et al. Genetic structure of introduced swamp buffalo subpopulations in tropical Australia. Austral. Ecol.38(1), 46–56 (2013). [Google Scholar]
- 79.Albrecht, G., McMahon, C. R., Bowman, D. M. J. S. & Bradshaw, C. J. A. Convergence of culture, ecology, and ethics: Management of feral swamp buffalo in Northern Australia. J. Agric. Environ. Ethics22(4), 361–378 (2009). [Google Scholar]
- 80.Prihandini, P. W., Primasari, A., Luthfi, M., Efendy, J. & Pamungkas, D. Genetic diversity of mitochondrial DNA cytochrome b in Indonesian native and local cattle populations. J. Ilmu. Ternak Dan Vet.25(2), 39 (2020). [Google Scholar]
- 81.Finlay, E. K. et al. Bayesian inference of population expansions in domestic bovines. Biol. Lett.3(4), 449–452 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Diamond, J. Evolution, consequences and future of plant and animal domestication. Nature418(6898), 700–707 (2002). [DOI] [PubMed] [Google Scholar]
- 83.Kandel, R. C. et al. Population and demography of Asian wild buffalo (Bubalus arnee Kerr, 1792) at Koshi Tappu wildlife reserve Nepal. J. Emerg. Trends Econ. Manag. Sci.9, 212–218. 10.10520/EJC-11e3d785da (2018). [Google Scholar]
- 84.Schmidt D, Pool J. The effect of population history on the distribution of the Tajima’s D statistic. Popul. Engl. Ed. 1–8 (2002).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available as the data forms part of an ongoing study and is currently being used for additional analyses. Data is available from the corresponding author on reasonable request.