Abstract
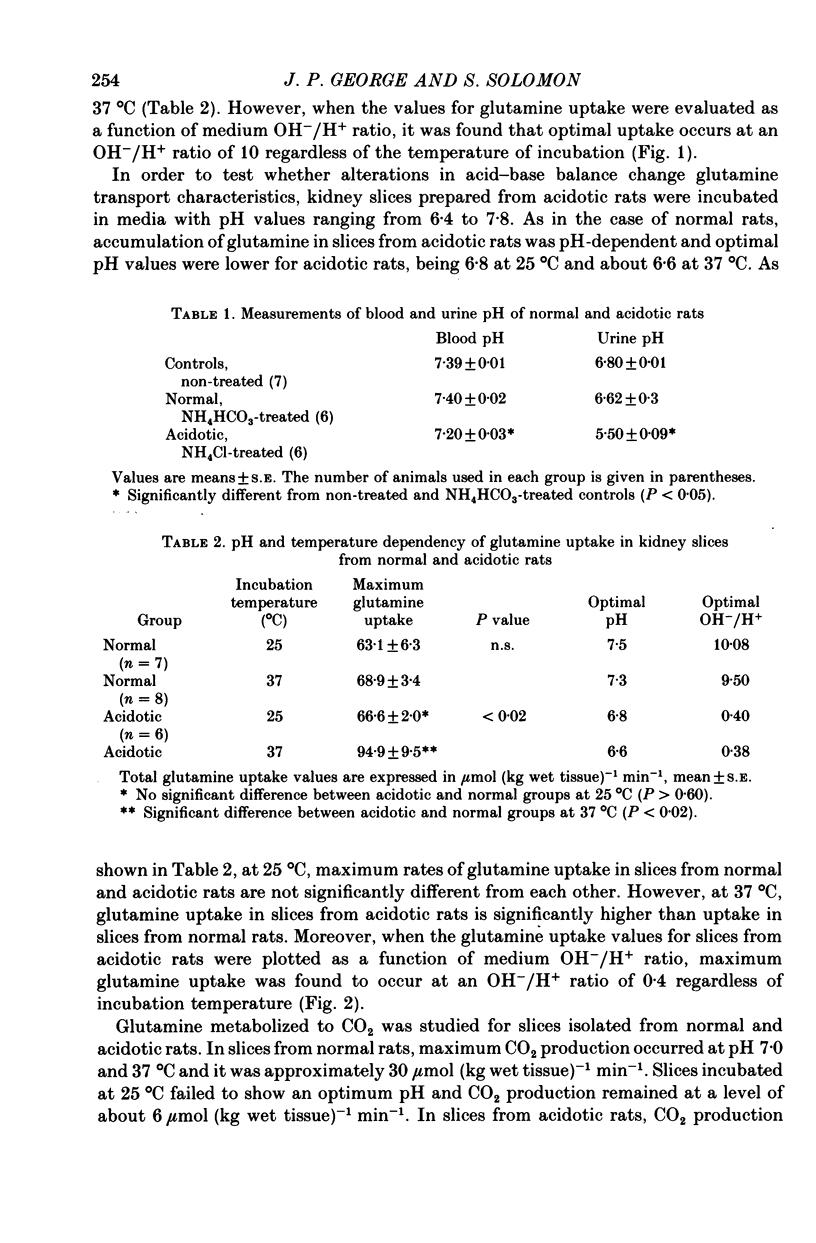
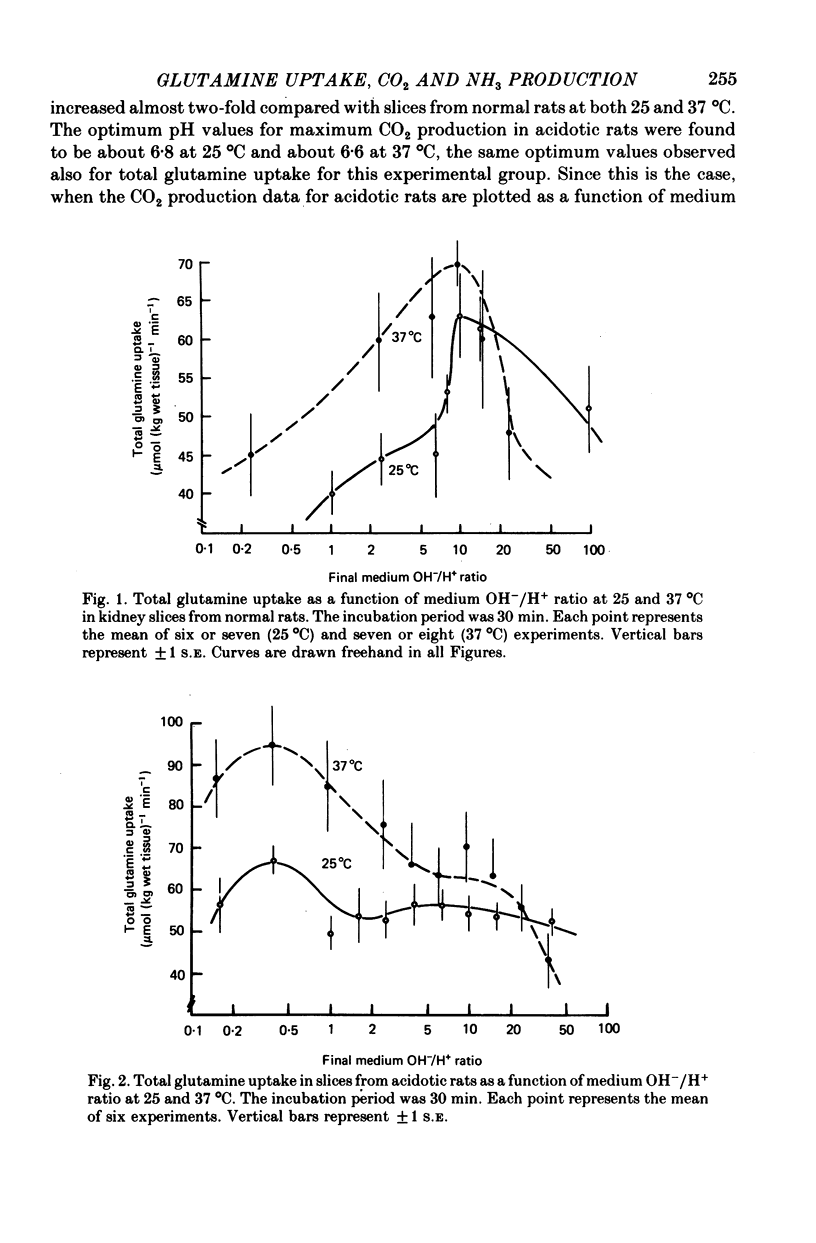
1. The effects of medium pH and temperature on glutamine uptake, NH3 production and CO2 production were examined using kidney cortex slices from normal and acidotic rats. 2. Uptake of glutamine by kidney slices from normal rats shows a pH optimum of 7.5 at 25 degrees C and 7.3 at 37 degrees C. Uptake is optimal, however, at a constant OH-/H+ ratio of 10. 3. In slices from acidotic rats greatest uptake was at pH 6.8 at 25 degrees C and 6.6 at 37 degrees C. Optimal OH-/H+ ratio was 0.4 and constant at both temperatures. 4. CO2 production from glutamine was greatest at pH 7.0 at 37 degrees C in slices from control rats. No pH optimum was detected at 25 degrees C. With slices from acidotic animals, optimal pH for CO2 production became identical with that for uptake. 5. Both basal and glutamine-stimulated NH3 production showed no optimal pH but were significantly higher in slices from acidotic rats compared with those from controls. 6. Dependence of glutamine penetration on optimal OH-/H+ ratio is considered to reflect a general membrane phenomenon which is produced by either an increase in carrier-substrate complexes or an increase in the number of carriers at this ratio. 7. Cellular penetration of glutamine does not appear to be a limiting factor in production of NH3 in vitro.
Full text
PDF


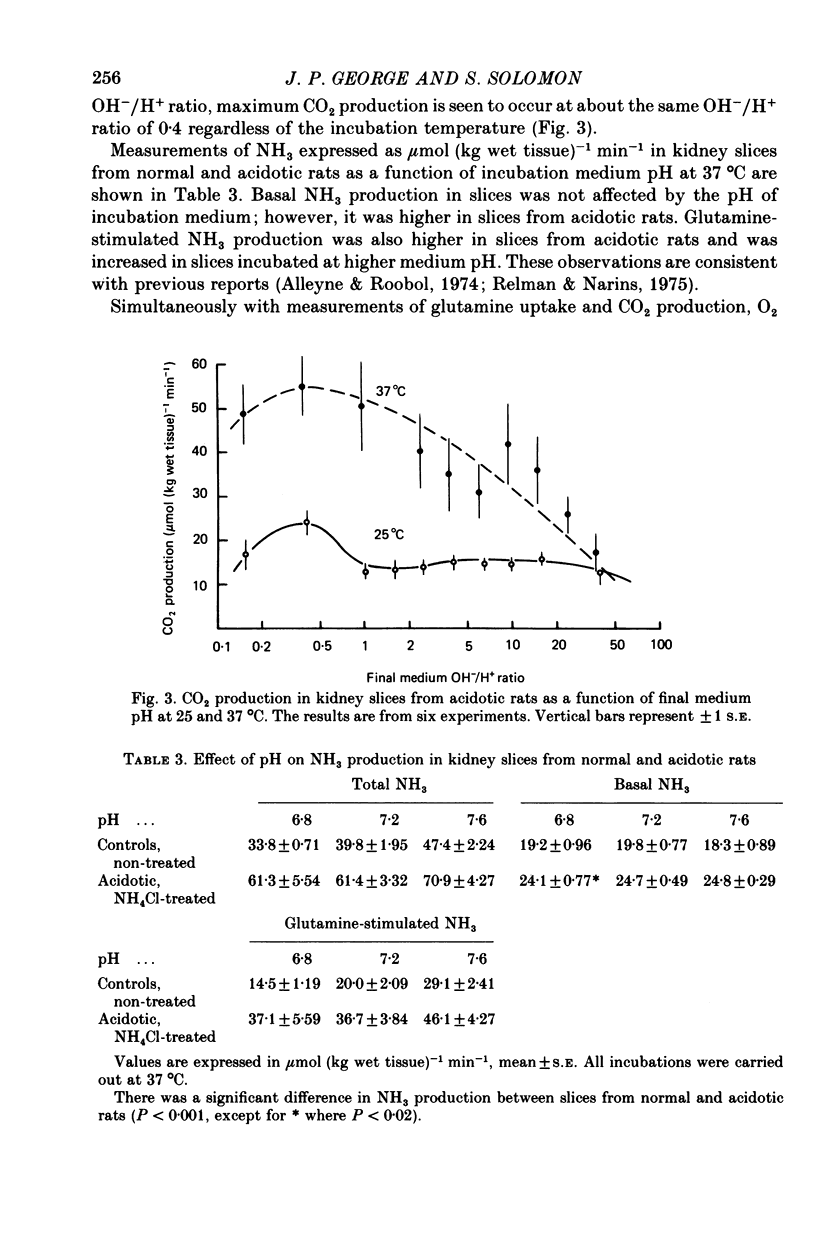





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alleyne G. A., Roobol A. Regulation of renal cortex ammoniagenesis. I. Stimulation of renal cortex ammoniagenesis in vitro by plasma isolated from acutely acidotic rats. J Clin Invest. 1974 Jan;53(1):117–121. doi: 10.1172/JCI107528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balagura-Baruch S., Shurland L. M., Welbourne T. C. Effects of alpha-ketoglutarate on renal ammonia release in the intact dog. Am J Physiol. 1970 Apr;218(4):1070–1075. doi: 10.1152/ajplegacy.1970.218.4.1070. [DOI] [PubMed] [Google Scholar]
- Curthoys N. P., Weiss R. F. Regulation of renal ammoniagenesis. Subcellular localization of rat kidney glutaminase isoenzymes. J Biol Chem. 1974 May 25;249(10):3261–3266. [PubMed] [Google Scholar]
- Damian A. C., Pitts R. F. Rates of glutaminase I and glutamine synthetase reactions in rat kidney in vivo. Am J Physiol. 1970 May;218(5):1249–1255. doi: 10.1152/ajplegacy.1970.218.5.1249. [DOI] [PubMed] [Google Scholar]
- George J. P., Solomon S. Dependence of glutamine metabolism and ammonia synthesis on sex in rat kidney slices. J Endocrinol. 1978 Dec;79(3):271–276. doi: 10.1677/joe.0.0790271. [DOI] [PubMed] [Google Scholar]
- Goldstein L. Glutamine transport by mitochondria isolated from normal and acidotic rats. Am J Physiol. 1975 Oct;229(4):1027–1033. doi: 10.1152/ajplegacy.1975.229.4.1027. [DOI] [PubMed] [Google Scholar]
- Goodman A. D., Fuisz R. E., Cahill G. F., Jr Renal gluconeogenesis in acidosis, alkalosis, and potassium deficiency: its possible role in regulation of renal ammonia production. J Clin Invest. 1966 Apr;45(4):612–619. doi: 10.1172/JCI105375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell B. J., Baumgardner F. W., Bondi K., Rahn H. Acid-base balance in cold-blooded vertebrates as a function of body temperature. Am J Physiol. 1970 Feb;218(2):600–606. doi: 10.1152/ajplegacy.1970.218.2.600. [DOI] [PubMed] [Google Scholar]
- Irias J. J., Greenberg R. E. Relationship of renal glucconeogenesis to control of ammonia formation. Am J Physiol. 1972 Oct;223(4):750–755. doi: 10.1152/ajplegacy.1972.223.4.750. [DOI] [PubMed] [Google Scholar]
- Kalra J., Brosnan J. T. The subcellular localization of glutaminase isoenzymes in rat kidney cortex. J Biol Chem. 1974 May 25;249(10):3255–3260. [PubMed] [Google Scholar]
- Kamm D. E., Asher R. R. Relation between glucose and ammonia production in renal cortical slices. Am J Physiol. 1970 Apr;218(4):1161–1165. doi: 10.1152/ajplegacy.1970.218.4.1161. [DOI] [PubMed] [Google Scholar]
- Law R. O. The inulin space, solute concentrations, and weight changes in rat renal medullary slices incubated in iso-osmolal media, and their modification during anoxia and hypothermia. J Physiol. 1975 May;247(1):37–54. doi: 10.1113/jphysiol.1975.sp010919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux G., Vinay P., Cartier P. Renal hemodynamics and ammoniagenesis. Characteristics of the antiluminal site for glutamine extraction. J Clin Invest. 1974 Mar;53(3):884–894. doi: 10.1172/JCI107629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OWEN E. E., ROBINSON R. R. Amino acid extraction and ammonia metabolism by the human kidney during the prolonged administration of ammonium chloride. J Clin Invest. 1963 Feb;42:263–276. doi: 10.1172/JCI104713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PITTS R. F. RENAL PRODUCTION AND EXCRETION OF AMMONIA. Am J Med. 1964 May;36:720–742. doi: 10.1016/0002-9343(64)90182-2. [DOI] [PubMed] [Google Scholar]
- Park Y. S., Hong S. K. Properties of toad skin Na-K-ATPase with special reference to effect of temperature. Am J Physiol. 1976 Nov;231(5 Pt 1):1356–1363. doi: 10.1152/ajplegacy.1976.231.5.1356. [DOI] [PubMed] [Google Scholar]
- Pilkington L. A., O'Donovan D. J. Metabolism of glutamine in cortex slices from dog kidney during acid-base alterations. Am J Physiol. 1971 Jun;220(6):1634–1639. doi: 10.1152/ajplegacy.1971.220.6.1634. [DOI] [PubMed] [Google Scholar]
- Pilkington L. A., Young T. K., Pitts R. F. Properties of renal luminal and antiluminal transport of plasma glutamine. Nephron. 1970;7(1):51–60. doi: 10.1159/000179807. [DOI] [PubMed] [Google Scholar]
- Preuss H. G., Baird K., Eastman S. T. The regulation of renal ammoniagenesis in the rat by extracellular factors. II. Ammoniagenesis by rat kidney slices incubating in normal acidotic sera. Metabolism. 1978 Nov;27(11):1639–1647. doi: 10.1016/0026-0495(78)90286-x. [DOI] [PubMed] [Google Scholar]
- Preuss H. G. Renal glutamate metabolism in acute metabolic acidosis. Nephron. 1969;6(3):235–246. doi: 10.1159/000179731. [DOI] [PubMed] [Google Scholar]
- Preuss H. G., Vivatsi-Manos O., Vertuno L. L. Effects of glutamine deamination on glutamine deamidation in rat kidney slices. J Clin Invest. 1973 Apr;52(4):755–764. doi: 10.1172/JCI107238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahn H. Body temperature and acid-base regulation. (Review article). Pneumonologie. 1974;151(2):87–94. doi: 10.1007/BF02097155. [DOI] [PubMed] [Google Scholar]
- Relman A. S., Narins R. G. The control of ammonia production in the rat. Med Clin North Am. 1975 May;59(3):583–593. doi: 10.1016/s0025-7125(16)32010-7. [DOI] [PubMed] [Google Scholar]
- Tannen R. L., Kunin A. S. Effect of potassium on ammoniagenesis by renal mitochondria. Am J Physiol. 1976 Jul;231(1):44–51. doi: 10.1152/ajplegacy.1976.231.1.44. [DOI] [PubMed] [Google Scholar]
- Vinay P., Lemieux G., Gougoux A. Characteristics of glutamine metabolism by rat kidney tubules: a carbon and nitrogen balance. Can J Biochem. 1979 Apr;57(4):346–356. doi: 10.1139/o79-044. [DOI] [PubMed] [Google Scholar]


