Abstract
Background
Myopia is a global public health concern affecting billions worldwide, with its prevalence rising at an alarming rate. High-quality RCTs for evaluating new interventions to slow myopia progression are needed. This systematic review aims to analyze the study designs and statistical practices of randomized controlled trials (RCTs) on myopia treatment published between 2019 and 2023.
Methods
A systematic search of PubMed and Embase was conducted in February 2024 and May 2025 using keyword-based and MeSH term-based queries, filtered for RCTs published between January 1, 2019, and December 31, 2023. All retrieved studies were manually screened to ensure they reported primary results from RCTs investigating myopia treatment or progression control, assessing at least one intervention for efficacy and/or safety.
Results
73 trials performed in more than 15 countries across 36 journals were identified. The most common interventions involved atropine (n = 19, 26.0%), lens (n = 22, 30.1%), surgery (n = 9, 12.3%), and light therapy (n = 8, 11.0%). These trials used one-eye design (n = 31, 42.5%), two-eye design (n = 27, 37.0%), paired-eye design (n = 9, 12.3%), mixed-eye design (n = 3, 4.1%). The most common primary outcomes were spherical equivalent refraction (n = 48, 65.8%), axial length (n = 45, 61.6%), and uncorrected distance visual acuity (n = 10, 13.7%). 66 trials (91.7%) utilized cycloplegia for measuring refractive error. Among 39 trials involving two eyes, inter-eye correlation was accounted for in the analysis of 23 trials (59.0%), with 8 (34.8%) using a paired t-test, 12 (52.2%) using a mixed effects model, and 3 (13.0%) using generalized estimating equations. Among 60 trials with missing data, 24 trials (40.0%) did not mention any statistical methods for missing data.
Conclusion
The methodological rigor of myopia RCTs can be improved by carefully considering eye study designs, employing robust statistical techniques, ensuring cycloplegia for refractive error measurements, and properly accounting for inter-eye correlation and missing data when applicable. Standardizing these practices will improve the quality of future myopia trials.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12886-025-04338-8.
Keywords: Myopia treatment, Randomized controlled trials, Statistical analysis, Trial design
Background
Myopia is a common eye condition affecting children and adults worldwide, especially in East Asia [1, 2]. A 2016 review predicted that by 2050, half of the world’s population would have myopia [3]. Myopia typically begins in childhood, and the earlier the onset, the more severe the condition. High myopia is associated with serious complications such as blindness, retinal detachment, and glaucoma [4]imposing significant financial burdens on both individuals and communities. As myopia remains a serious public health problem, finding effective interventions for myopia is increasingly important. The high prevalence of myopia has prompted researchers to find treatments to delay myopia progression. Many types of myopia treatments, such as atropine eye drops, orthokeratology lenses, surgery, and red-light therapy, have been investigated. As more treatments become available for evaluation, the number of randomized controlled trials (RCTs) on the efficacy and safety of myopia treatment has continued to increase (PubMed search, conducted Feb 2024).
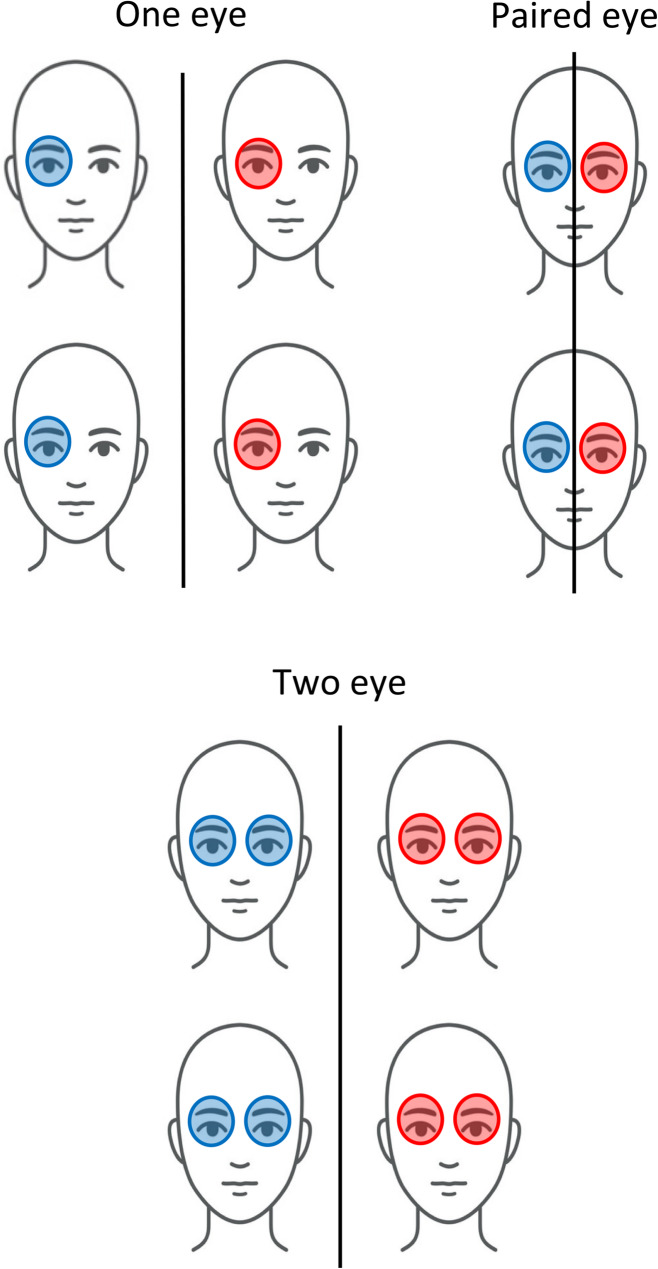
Unlike clinical trials of diseases in most organs, ophthalmic RCTs can enroll and analyze one or two eyes, which has implications for the trial design and statistical analysis. Ophthalmic RCTs can be categorized into four types: one-eye design, two-eye design, paired-eye design, and mixed-eye design (Fig. 1) [5]. In a one-eye design, only one eye per participant is included and analyzed. The one eye for study can be chosen in a variety of ways – it can be random, a certain eye (left or right), or the worse eye. In a two-eye design, both eyes of a participant are enrolled and analyzed. Two-eye designs can be further classified into the same group design, different group design, or mixed group design. In the same group design, both eyes of a participant receive the same treatment. In a different group, each eye receives different treatments. The paired-eye design is a special type of the different group design in which one eye of a participant receives a treatment and the other eye of the same participant receives a different treatment. In a mixed design, a mixture of one eye per participant and two eyes per participant is used.
Fig. 1.
Different study designs in ophthalmic trials. The blue and red tints represent different treatments or placebo. In one eye designs, only the results of one eye per participant are analyzed. In two eye designs, the results of both eyes per participant are analyzed. In paired eye designs, each person receives different treatments in each eye, and the results of both are collected for analysis
Based on the type of myopia RCT design chosen, it is necessary to apply the appropriate statistical analysis methods corresponding to the trial design. For example, in a two-eye design, inter-eye correlation should be adjusted in the statistical analysis due to high inter-eye correlation between the left and right eyes of a participant. There are a variety of statistical methods that can be used to adjust for inter-eye correlation, such as the generalized estimating equations or linear mixed effects model.
Review papers on myopia treatment RCTs have typically focused on the efficacy of a specific myopia treatment modality or across treatments [6–9] and not on the trial design and statistical methods. To the best of our knowledge, no review has systematically examined the study design characteristics and statistical analysis approaches for RCTs across various myopia treatments. To address this gap, we reviewed primary results from myopia treatment RCTs published over a 5-year period (between January 2019 and December 2023) across various journals. The objective of this review is to analyze the trial design characteristics and the statistical analysis approaches used in published myopia RCTs and identify areas for improvement in the design and analysis of future RCTs on myopia treatment.
Methods
A standardized protocol was developed by the two authors for the search criteria, screening, and data extraction of the study (Supplement 1). The review and screening processes were done twice to ensure accuracy.
Search criteria and screening
PubMed and Embase databases were used for a systematic retrospective review of RCTs related to myopia treatment.
Two types of PubMed searches were conducted to identify RCTs of myopia treatment. The first type involved a keyword search in which either “myopia,” “nearsightedness,” or “myopic astigmatism” is in the Title or Abstract. The second type involved a MeSH term search for myopia treatments. For both search types, filters were applied to limit the publication dates from January 1, 2019, to December 31, 2023 (e.g., 5-year period) and to include only randomized controlled trials provided by the PubMed filter. The PubMed search was last performed in March 2024 to confirm all trials were included. An Embase search was also conducted, using the MeSH term strategy and filters applied for the publication date and randomized controlled trials. The Embase search was last performed in May 2025.
All the papers identified were independently and manually screened based on the title and abstract by the two authors (Y.C. and G.S.Y.). Afterwards, papers were manually screened by the two authors based on the manuscript. The inclusion criteria were that papers had to be primary result trials involving study subjects with myopia and be RCTs specific to myopia treatment or delay of myopia progression, evaluate any one or more interventions that have outcomes measuring their efficacy and safety, and be published in the English language or have an English version of the publication. The papers had to compare differences in intervention(s) and/or include a lack of intervention. The exclusion criteria were papers that were not randomized, reported only secondary outcomes of myopia treatment, performed secondary analysis of data from previous trials, reported only the protocol or study design of the RCT (although referred only to collect trial information), reported methodological differences in interventions (i.e. different surgical methods for myopia treatment) or included additional follow-ups of trial participants beyond the primary endpoint. More details on the search criteria and eligibility process can be found in the supplemental (Supplement 1).
The systematic review was conducted in accordance with the PRISMA guidelines and was verified for adherence.
Data extraction
The two authors independently reviewed the eligible papers and collected information about the RCT design and statistical methods applied. All relevant data were recorded in a Google Form (Supplement 2). If the required information could not be found in the paper, other resources such as supplementary documents for the primary result paper, the ClinicalTrials.gov posting, or a published trial protocol or design paper were used. The following data were extracted:
Trial characteristics: type of intervention (drug, device/surgery, red light, etc.), journal published, country conducting the trial, nationality of the corresponding author, trial sponsor, trial registration number, number of clinical centers, number of treatment arms, length of enrollment period, inclusion criteria (including range of cycloplegic refractive error to be eligible and age range), cycloplegia usage and agent used for studies where spherical equivalent refraction was the primary outcome, secondary outcome, inclusion criteria, length of participant follow-up for primary outcome assessment.
Trial design: type of trial design (i.e. one-eye, two-eye, paired-eye, mixed design). For one-eye design, the selection process of the study eye. For trials involving both eyes, whether the two eyes of a participant were assigned to the same or different treatment groups (same-group, different group, mixed group design), blinding protocols for outcome measure assessment.
Sample size and statistical power: designed and actual sample size, planned statistical power, the statistical method used for sample size calculation.
Outcome Measures: number of primary outcomes (if not clearly defined, all outcomes were counted as primary outcomes), data type (continuous, binary, discrete, etc.) for outcomes, statistical tests used for comparing outcomes.
Inter-eye correlation for two-eye design: for trials involving two eyes (two-eye design, paired-eye design, and mixed-eye design), accountability of inter-eye correlation in statistical analysis, the statistical method used for adjusting for inter-eye correlation.
Missing data in primary outcome: for trials with missing data, the percentage with missing data in the primary outcome (calculated as the number of participants with missing data in the primary outcome divided by the total number of participants enrolled for RCT), handling of missing data in the statistical analysis for the primary outcome.
Multiplicity of comparisons: for applicable trials, correction for multiple comparisons from multiple arms or multiple primary outcomes, statistical methods used for correcting these multiple comparisons.
Statistical analyses
Descriptive analyses were performed to summarize the characteristics of myopia trial designs and statistical analyses used in the trials. Frequency and percentages were calculated for categorical data. Mean (with standard deviation (SD)) or median (with interquartile ranges (IQR)) were calculated for continuous data. Chi-square tests were used to assess factors potentially associated with adjustment for inter-eye correlation in statistical analysis (Supplement 3), sample size calculations (Supplement 4), and missing data methods (Supplement 5). P values less than 0.05 were considered statistically significant. All statistical analyses were performed using the software R.
Results
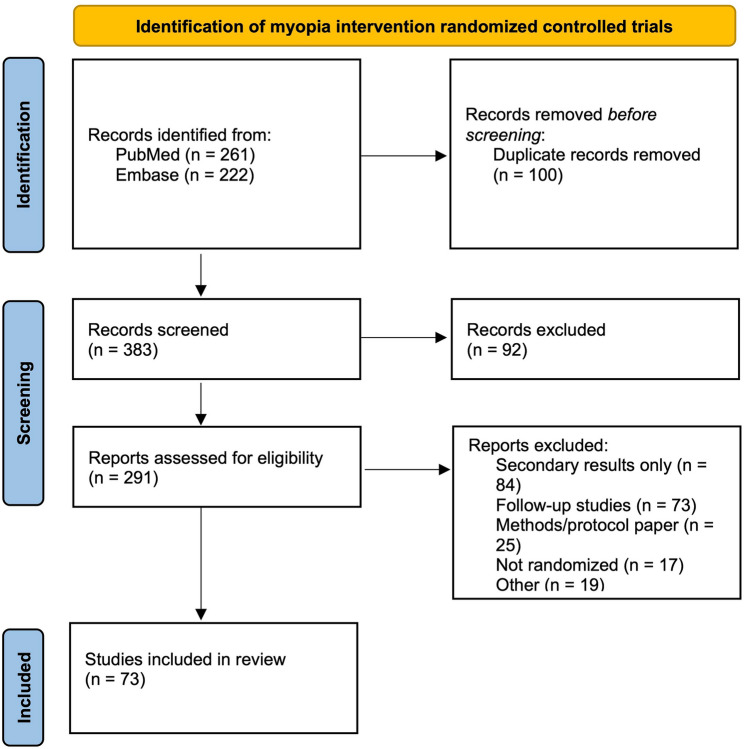
Through the search criteria and screening, 261 papers were identified in PubMed and 222 papers were identified through Embase (Fig. 2). 100 duplicate records were removed from 383 records. 92 of these records were excluded after initial screening. For eligibility screening, 84 papers were removed for reporting secondary results only, 73 were removed as a follow-up study, 25 were removed because they were methods/protocol papers, 17 were removed because they were not randomized, and 19 were removed for not meeting other eligibility criteria. After initial screening and eligibility screening, we identified 73 primary result papers reporting RCTs on myopia treatment published from January 1 st, 2019 to December 31 st, 2023 that meet the inclusion criteria [10–82] (Supplement 6).
Fig. 2.
PRISMA Flow Chart for Screening and Eligibility of Myopia RCTs
Trial characteristics
The characteristics of these 73 trials are summarized in Table 1.
Table 1.
Characteristics of myopia randomized controlled trials
| Trial Characteristics | N | Percentage |
|---|---|---|
| Publication Year | ||
| 2019 | 6 | 8.2% |
| 2020 | 10 | 13.7% |
| 2021 | 12 | 16.4% |
| 2022 | 23 | 31.5% |
| 2023 | 22 | 30.1% |
| Journal | ||
| Ophthalmology | 7 | 9.6% |
| JAMA Ophthalmology | 5 | 6.8% |
| American Journal of Ophthalmology | 4 | 5.5% |
| Scientific Reports | 4 | 5.5% |
| Acta Ophthalmology | 3 | 4.1% |
| BMC Ophthalmology | 3 | 4.1% |
| British Journal of Ophthalmology | 3 | 4.1% |
| Contact Lens Anterior Eye | 3 | 4.1% |
| Journal of Refractive Surgery | 3 | 4.1% |
| Ophthalmic and Physiological Optics | 3 | 4.1% |
| Translational Vision Science and Technology | 3 | 4.1% |
| Current Eye Research | 2 | 2.7% |
| Eye and Contact Lens | 2 | 2.7% |
| Indian Journal of Ophthalmology | 2 | 2.7% |
| International Ophthalmology | 2 | 2.7% |
| Journal of Clinical Medicine | 2 | 2.7% |
| Journal of Ophthalmology | 2 | 2.7% |
| JAMA | 2 | 2.7% |
| Asia-Pacific Journal of Ophthalmology | 1 | 1.4% |
| Clinical and Experimental Ophthalmology | 1 | 1.4% |
| Clinical and Experimental Optometry | 1 | 1.4% |
| Graefe’s Archive for Clinical and Experimental Ophthalmology | 1 | 1.4% |
| International Journal of Medical Sciences | 1 | 1.4% |
| International Journal of Ophthalmology | 1 | 1.4% |
| International Journal of Pharmaceutical and Clinical Research | 1 | 1.4% |
| International Journal of Research in Ayurveda and Pharmacy | 1 | 1.4% |
| Journal of Ocular Pharmacology and Therapeutics | 1 | 1.4% |
| Journal of the Pakistan Medical Association | 1 | 1.4% |
| Journal of Personalized Medicine | 1 | 1.4% |
| JAMA Open Network | 1 | 1.4% |
| JAMA Pediatrics | 1 | 1.4% |
| Japan Journal of Ophthalmology | 1 | 1.4% |
| Ophthalmic Epidemiology | 1 | 1.4% |
| Optometry and Vision Science | 1 | 1.4% |
| Saudi Journal of Ophthalmology | 1 | 1.4% |
| Technology and Health Care | 1 | 1.4% |
| Intervention | ||
| Atropine | 19 | 26.0% |
| 0.01% Atropine | 12 | |
| 0.05% Atropine | 1 | |
| Different Atropine Concentrations | 6 | |
| Combined Treatments | 10 | 13.7% |
| Lens | 22 | 30.1% |
| Orthokeratology | 7 | |
| Other lens | 15 | |
| Light Therapy | 8 | 11.0% |
| Social Intervention | 3 | 4.1% |
| Supplement | 2 | 2.7% |
| Surgery | 9 | 12.3% |
| Lasik vs. SMILE | 5 | |
| PRK vs. SMILE | 1 | |
| PRK vs. LASIK | 1 | |
| LASIK with accelerated cross-linking | 2 | |
| Country of the Trial | ||
| China | 37 | 50.7% |
| Hong Kong | 8 | 11.0% |
| India | 6 | 8.2% |
| Japan | 4 | 5.5% |
| United States | 4 | 5.5% |
| Denmark | 2 | 2.7% |
| Multiple countries | 2 | 2.7% |
| Singapore | 2 | 2.7% |
| Brazil | 1 | 1.4% |
| Egypt | 1 | 1.4% |
| Malaysia | 1 | 1.4% |
| Australia | 1 | 1.4% |
| Germany | 1 | 1.4% |
| Israel | 1 | 1.4% |
| Spain | 1 | 1.4% |
| Vietnam | 1 | 1.4% |
| Nationality of Corresponding Authors | ||
| China | 36 | 49.3% |
| Hong Kong | 8 | 11.0% |
| India | 6 | 8.2% |
| United States | 4 | 5.5% |
| Japan | 4 | 5.5% |
| Australia | 2 | 2.7% |
| Singapore | 2 | 2.7% |
| Multiple countries | 2 | 2.7% |
| Denmark | 2 | 2.7% |
| Brazil | 1 | 1.4% |
| Egypt | 1 | 1.4% |
| Germany | 1 | 1.4% |
| Malaysia | 1 | 1.4% |
| Israel | 1 | 1.4% |
| Spain | 1 | 1.4% |
| Vietnam | 1 | 1.4% |
| Funding source | ||
| Government only | 23 | 31.5% |
| Industry only | 14 | 19.2% |
| Government and University | 12 | 16.4% |
| Industry and University | 4 | 5.5% |
| University only | 4 | 5.5% |
| Government and Industry | 3 | 4.1% |
| Organization | 3 | 4.1% |
| N/A | 10 | 13.7% |
| Number of arms | ||
| 2 | 54 | 74.0% |
| 3 | 12 | 16.4% |
| 4 | 5 | 6.8% |
| 5 or greater | 2 | 2.7% |
| Number of clinical centers | ||
| 1 | 49 | 67.1% |
| 2 | 7 | 9.6% |
| 3 or greater | 13 | 17.8% |
| Not provided | 4 | 5.5% |
| Trial registration | ||
| Registered | 53 | 72.6% |
| Not registered | 20 | 27.4% |
| Enrollment period (months) | ||
| Less than 6 months | 11 | 15.1% |
| 6–12 months | 8 | 11.0% |
| 13–18 months | 10 | 13.7% |
| 19–24 months | 4 | 5.5% |
| Greater than 24 months | 14 | 19.2% |
| Not given | 26 | 35.6% |
6 trials were published in 2019 (8.2%), 10 trials in 2020 (13.7%), 12 trials in 2021 (16.4%), 23 trials in 2022 (31.5%), and 22 trials in 2023 (30.1%), noting an upward trend in myopia treatment trials over time. The primary result papers of these trials were published primarily in Ophthalmology (n = 7, 9.6%), JAMA Ophthalmology (n = 5, 6.8%), American Journal of Ophthalmology, Scientific Reports (n = 4 each, 5.5%), Acta Ophthalmology, BMC Ophthalmology, British Journal of Ophthalmology, Contact Lens Anterior Eye, Journal of Refractive Surgery, Ophthalmic and Physiological Optics, and Translational Vision Science and Technology (n = 3 each, 4.1%). The 73 papers encompassed various types of myopia treatment, such as SMILE vs. LASIK (n = 5, 6.8%), PRK vs. SMILE (n = 1, 1.4%), PRK vs. LASIK (n = 1, 1.4%), LASIK with accelerated cross-linking (n = 2, 2.7%), 0.01% atropine drops (n = 12, 16.4%), 0.05% atropine drops (n = 1, 1.4%), different atropine drop concentrations (n = 6, 8.2%), lens treatments (n = 22, 30.1%) such as orthokeratology, defocus incorporated multiple segments, implantable collamer lens, and dual focus contact lens, light therapy (n = 8, 11.0%), combined treatments (n = 10, 13.7%), social interventions like outdoor time and social media family education services (n = 3, 3.9%), and supplement use (n = 2, 2.7%). Mainland China had the most trials (n = 37, 50.7%) followed by Hong Kong (n = 8, 11.0%), India (n = 6, 8.2%), United States, Japan (n = 4 each, 5.5%), Denmark, Singapore, and multiple countries (n = 2 each, 2.7%), and various individual countries having one trial each (n = 8, 11.0%). Corresponding authors were from Mainland China (n = 36, 49.3%), Hong Kong (n = 8, 11.0%), India (n = 6, 8.2%), United States, Japan (n = 4 each, 5.5%), Australia, Singapore, multiple countries, Denmark, (n = 2 each, 2.7%), and various other individual countries (n = 7, 9.6%). The trials were funded by the government only (n = 23, 31.5%), industry only (n = 14, 19.2%), university only (n = 4, 5.5%), organization only (n = 3, 4.1%), government and university (n = 12, 16.4%), industry and university (n = 4, 5.5%), and government and industry (n = 3, 4.1%) – some trials did not list their funding sources (n = 10, 13.7%). The majority of trials had two arms (n = 54, 74.0%), some had three arms (n = 12, 16.4%), and a few had four or more arms (n = 7, 9.6%). Most trials were conducted at a single clinical center (n = 49, 67.1%). 53 trials (72.6%) were registered in a clinical trials database. 47 trials (64.4%) provided data on the number of months of the enrollment period (where participants were recruited and signed up to take part in the research), with a median (IQR) of 15.5 (7.5–22.5) months.
Design characteristics of trials
The design characteristics of the myopia RCTs are summarized in Table 2.
Table 2.
Design of myopia randomized controlled trials
| Design Characteristics | N | Percentage |
|---|---|---|
| Masking | ||
| No masking | 8 | 11.0% |
| Single masking | 19 | 26.0% |
| Double masking | 33 | 45.2% |
| Unknown | 13 | 17.8% |
| Eye Design | ||
| One-eye design | 31 | 42.5% |
| Two-eye design | 27 | 37.0% |
| Paired-eye design | 9 | 12.3% |
| Mixed-eye design | 3 | 4.1% |
| Unknown | 3 | 4.1% |
| Among one-eye design: how eye was chosen | 31 | |
| Certain eye (right/left) | 25 | 80.6% |
| Worse eye | 2 | 6.5% |
| Random eye | 2 | 6.5% |
| Other method | 2 | 6.5% |
| Follow-up period for primary outcome (months) | ||
| 1 month or less | 2 | 2.7% |
| 3 months | 5 | 6.8% |
| 6 months | 9 | 12.3% |
| 12 months | 30 | 41.1% |
| 18 months | 4 | 5.5% |
| 24 months | 18 | 24.7% |
| 36 months | 5 | 6.8% |
19 trials (26.0%) were single-masked, 33 trials (45.2%) were double-masked RCTs, and 21 trials (28.8%) had no masking or no masking information was provided. 31 trials (42.5%) used one-eye design, with 25 (80.6%) choosing a certain eye (left or right eye), 2 (6.5%) choosing the worse eye, 2 (6.5%) choosing an eye at random, and 2 (6.5%) using other methods (the eye that has myopia incidence, the eye that requires surgery, etc.). 27 trials (37.0%) were two-eye designs with two eyes in the same treatment group, and 9 trials (12.3%) were paired-eye designs. All but one paired-eye design trial had the two eyes assigned to two different groups. The paired-eye trial, where the two eyes were in the same group, involved one group receiving treatment A in one eye and treatment B in another eye, and another group receiving treatment A in one eye and treatment C in another eye [83]. 3 trials (4.1%) were mixed-eye designs, where either one or two eyes per participant were treated, and 3 trials (4.1%) did not include information about the eye design. The follow-up period for the primary outcome had a median of 12 (IQR: 6–24) months.
Inter-eye correlation
Inter-eye correlation used for myopia trials involving two eyes (two-eye, paired-eye, and mixed-eye trials) is summarized in Table 3.
Table 3.
Inter-eye correlation accounted for in myopia randomized controlled trials involving two eyes
| Inter-eye correlation | N | Percentage |
|---|---|---|
| Trials involving two eyes | 39 | |
| Adjusted | 23 | 59.0% |
| Not adjusted | 16 | 41.0% |
| Among trials adjusting inter-eye correlation, what method | 23 | |
| Paired t-test | 8 | 34.8% |
| Mixed effects model | 12 | 52.2% |
| Generalized estimating equations | 3 | 13.0% |
| Among trials not adjusting inter-eye correlation, what method | 16 | |
| Analyzing two eyes separately | 11 | 68.8% |
| Average of two eyes | 5 | 31.3% |
Of the two-eye, paired-eye, and mixed-eye trials that involved two eyes (n = 39, 53.4%), 23 trials (59.0%) adjusted for inter-eye correlation in statistical analysis, with 8 trials (34.8%) using a paired-t-test, 12 (52.2%) using a linear mixed effects model, and 3 (13.0%) using generalized estimating Eq. 16 trials (41.0%) that involved two eyes did not account for inter-eye correlation, with 11 trials (68.8%) analyzing the two eyes into one analysis, and 5 trials (31.3%) taking the average of two eyes.
Primary and secondary outcomes
The primary outcomes for myopia trials are summarized in Table 4.
Table 4.
Primary outcomes measured in myopia randomized controlled trials
| Primary Outcomes | N | Percentage |
|---|---|---|
| One primary outcome | 27 | 37.0% |
| Two primary outcomes | 24 | 32.9% |
| More than two primary outcomes | 22 | 30.1% |
| Spherical equivalent refraction | 48 | 65.8% |
| Axial length | 45 | 61.6% |
| Uncorrected distance visual acuity | 10 | 13.7% |
| Best corrected visual acuity | 10 | 13.7% |
| Corneal thickness/Anterior chamber depth | 6 | 8.2% |
| Endothelial cell count/density | 3 | 4.1% |
| Corneal curvature | 2 | 2.7% |
| Efficacy index | 2 | 2.7% |
| Spherical/Higher order aberrations | 2 | 2.7% |
A wide variety of primary outcomes were reported. 27 (37.0%) trials had only one primary outcome, 24 trials (32.9%) Had two primary outcomes, and 22 trials (30.1%) had more than two primary outcomes. The primary outcomes where they were reported in at least two trials were spherical equivalent (n = 48, 65.8%), axial length (n = 45, 61.6%), uncorrected distance visual acuity (n = 10, 13.7%), best corrected visual acuity (n = 10, 13.7%), corneal thickness or anterior chamber depth (n = 6, 8.2%), endothelial cell count or density (n = 3, 4.1%), corneal curvature, efficacy index, and spherical/higher order abberations (n = 2 each, 2.7%).
A total of 99 different secondary outcomes were used in these myopia RCTs, in which the most common secondary outcomes were best corrected visual acuity (n = 13, 16.9%), axial length (n = 11, 14.3%), and spherical equivalent (n = 8, 10.4%).
Use of cycloplegia in myopia trials
The use of cycloplegic agents for measurement of refractive errors is summarized in Table 5.
Table 5.
Cycloplegia used in myopia RCTs that measured SER in inclusion criteria, primary, or secondary outcome (n = 72)
| Cycloplegia | N | Percentage |
|---|---|---|
| Did not mention cycloplegic | 6 | 8.3% |
| Cycloplegic | 66 | 91.7% |
| Cyclopentolate | 25 | 37.9% |
| Tropicamide | 7 | 10.6% |
| Homatropine | 2 | 3.0% |
| Topiramate | 1 | 1.5% |
| Combined agent or based on age | 17 | 25.8% |
| Not given | 14 | 21.2% |
Among 72 trials that had spherical refractive measurements in their primary outcome, secondary outcome, or inclusion criteria, 66 trials (91.7%) stated that refractive error measurements were taken under cycloplegia, while 6 trials (8.3%) provided no information or did not use cycloplegia. Of the trials using cycloplegia, 25 trials used cyclopentolate (37.9%), 7 used tropicamide (10.6%), 2 used homatropine (3.0%), 1 used topiramate (1.5%), and 14 trials (21.2%) did not indicate what cycloplegic agent was used. 17 trials (25.8%) used combined cycloplegic agents or used different cycloplegic agents based on the age of the participant.
Sample size calculations in trials
The characteristics of sample size calculations used in trials are summarized in Table 6.
Table 6.
Sample size and statistical power calculations used in myopia randomized controlled trials
| Sample Size Calculations | N | Percentage |
|---|---|---|
| Provided full sample size calculation information | 50 | 68.5% |
| Did not provide sample size calculation | 21 | 28.8% |
| Did not provide statistical power | 23 | 31.5% |
| Did not provide sample size calculation methods | 20 | 27.4% |
| Did not provide sample size, power, and methods | 18 | 24.7% |
| Among those providing sample size calculation methods, what method used | 53 | |
| Two sample t-test | 46 | 86.8% |
| Paired t-test | 7 | 13.2% |
| Among those providing statistical power | 50 | |
| 80% power | 21 | 42.0% |
| > 80% and < 90% power | 2 | 4.0% |
| >90% power | 27 | 54.0% |
50 trials (68.5%) provided complete information on the sample size, the statistical power, and the statistical method used for sample size calculations. 21 trials (28.8%) did not provide information about the sample size calculation, 23 trials (31.5%) did not provide statistical power, and 20 trials (27.4%) did not provide information on the statistical method used for sample size calculations. 18 trials (24.7%) did not provide any information about sample size, power, or statistical methods used. Among the trials that provided sample size calculations, the median (IQR) calculated sample size was 37 (24–61) participants per treatment group. Among the 53 trials (72.6%) that provided the statistical method used for sample size calculations, 46 trials (86.8%) used the two-sample t-test and 7 trials (13.2%) used the paired t-test. Among the 50 trials (68.5%) that provided information about statistical power, 21 trials (42.0%) were designed with 80% power, 2 trials (4.0%) were designed with > 80% and < 90% power, and 27 trials (54.0%) were designed with > 90% power.
Trials with one-eye and two-eye designs (p = 0.001), or trials that are registered (p = 0.0005) had a greater proportion of trials providing both sample size power and the statistical test used than other trials. (Supplement 4).
Missing data in primary outcomes
The handling of missing data in primary outcomes in myopia trials is summarized in Table 7.
Table 7.
Missing data in primary outcome(s) and methods used in myopia randomized controlled trials
| Missing Data | N | Percentage |
|---|---|---|
| Missing data in primary outcome absent | 13 | 17.8% |
| Missing data present | 60 | 82.2% |
| Statistical method provided for handling missing data | 36 | 60.0% |
| Per protocol | 16 | 44.4% |
| Intention to treat | 10 | 27.8% |
| Multiple imputation | 2 | 5.6% |
| Linear mixed effects model | 2 | 5.6% |
| Intention to treat and per protocol | 2 | 5.6% |
| Generalized estimating equations | 2 | 5.6% |
| Markov chain Monte Carlo, intention to treat | 1 | 2.8% |
| Last value carried forward | 1 | 2.8% |
| No statistical method given | 24 | 40.0% |
60 trials (82.2%) had missing data in their primary outcome, of which the median (IQR) percentage of missing data per participant is 13.8% (7.6% − 23.6%). Among those with missing data, 36 trials (60.0%) provided the method used to handle missing data, with 16 trials (44.4%) applying per protocol, 10 (27.8%) applying intention-to-treat, 2 (5.6%) using multiple imputations, 2 (3.6%) using Linear mixed effects model, 2 (3.6%) using generalized estimating equations, 1 (2.8%) using last value carried forward, and 3 (8.3%) using combined methods. 24 trials (40.0%) did not provide a statistical method for handling missing data.
One-eye and two-eye designed trials were more likely to provide statistical methods used for missing data than other designs (p = 0.0451).
Correction of multiple comparisons
Multiple comparisons and correction methods in myopia trials are summarized in Table 8.
Table 8.
Correction for multiple comparisons used in myopia randomized controlled trials
| Correction for Multiple Comparisons | N | Percentage |
|---|---|---|
| Trials with more than one primary outcome | 46 | 63.0% |
| Bonferroni procedure correction | 8 | 17.4% |
| No correction | 38 | 82.6% |
| Trials with more than two arms | 19 | 26.0% |
| Bonferroni procedure correction | 8 | 42.1% |
| No correction | 11 | 57.9% |
| Trials with primary and secondary that used multiple comparisons | 4 | 5.5% |
| Bonferroni procedure correction | 3 | 75.0% |
| Holm-Sidak correction | 1 | 25.0% |
46 trials (63.0%) had more than one primary outcome. Of those, 8 trials (17.4%) corrected for multiple comparisons from more than one primary outcome, all using the Bonferroni procedure. 19 trials (26.0%) had more than two arms. Of those, 8 trials (42.1%) corrected for multiple comparisons, all using the Bonferroni procedure. There were 4 trials (5.5%) with one primary outcome and two arms that used multiple comparison methods because of multiple secondary outcomes. Of those 4 trials, 3 (75.0%) used Bonferroni and 1 (25.0%) used Holm-Sidak correction.
Discussion
We reviewed 73 primary result papers from RCTs on myopia treatment for trial design and statistical analysis methods. We found that some trials used substandard trial designs and suboptimal statistical analyses. Key areas for improvement included carefully explaining the rationale behind study eye designs, appropriately handling inter-eye correlations, explicitly detailing sample size and power calculations, properly addressing missing data and methods used for primary outcomes, and correcting for multiple comparisons, when necessary.
One-eye and two-eye designs in ophthalmic RCTs each have distinct advantages and disadvantages. A one-eye design is simpler to analyze statistically, as it involves only one eye per participant. When interventions have potential risks or side effects, a one-eye design may be considered more ethical, as it minimizes harm. However, this design limits the amount of information that can be gathered and does not mimic the future application of treatment, which likely applies to both eyes. The choice of which eye to treat - the right eye, left eye, or the worse eye - may affect outcomes and introduce bias. Variability between the eyes, such as differences in intraocular pressure or ocular asymmetry, may impact the results depending on which eye is selected.
In contrast, two-eye designs allow for more data collection, which increases the statistical power and precision of the study’s results. However, the data from both eyes of the same participant are likely to be similar to each other than data from two separate participants, potentially reducing the generalizability of the findings. Furthermore, there may be situations where both eyes of a participant do not meet the eligibility criteria for the myopia treatment RCT, limiting the applicability of a two-eye design.
Deciding between one-eye or two-eye designs in ophthalmic trials depends on many factors, including the type of treatment (systemic or local), the safety profile of the treatment (high-risk treatment may start with one eye treatment), symmetry of the ocular condition being studied, and costs. The decision impacts the statistical method used. Previous studies have shown that approximately half of ophthalmic RCTs tend to use a one-eye design, and approximately a quarter use two-eye or paired-eye designs [5, 84, 85]. In our review of myopia RCTs, we found a slightly greater number of trials using a two-eye or paired-eye design (n = 36, 49.3%) than a one-eye design (n = 31, 42.5%), likely due to the high symmetry of myopia in two eyes and the non-invasive nature of most myopia treatments. Myopia researchers must carefully justify what type of eye design to utilize and appropriately analyze the data based on the design chosen.
In our review, among 39 trials that used two eyes from some or all participants (a two-eye design, paired-eye design, or mixed-eye design), 16 trials (41.0%) did not account for inter-eye correlation, either by ignoring it and analyzing the two eyes separately or taking the average of the two eyes. While taking the average of the two eyes can eliminate the need to adjust for the inter-eye correlation, it may lead to loss of statistical information. It is prudent to analyze data from both eyes by accounting for inter-eye correlation. Many trials involving surgical treatments of myopia were adjusted for inter-eye correlation. Many surgical trials were paired-eye designs, which naturally accounted for inter-eye correlation through the commonly used paired t-test. When using two-eye designs with two eyes receiving the same treatment, the adjustment for inter-eye correlation is less straightforward, but it is still equally important to account for it. In such cases, we recommend methods such as generalized estimating equations or mixed effects models, which appropriately model within-subject correlation.
When a patient’s myopic progression is measured, it is considered the gold standard to measure refractive error under cycloplegic conditions [86, 87]. Cyclopentolate and tropicamide are commonly used in children to induce cycloplegia [88]. In our review, some trials (n = 6, 8.3%) did not explicitly mention whether cycloplegia was used, and among the 66 trials that reported using cycloplegia, 14 trials (21.2%) did not specify what type of cycloplegic agent was used. Trials used a variety of cycloplegic agents – some varied agents based on age, while others used combination regimens [20, 64, 71]. Some trials had a protocol to wait a certain amount of time after cycloplegic agents were added. Regardless of the cycloplegic agent or protocol, the primary concern is ensuring that adequate cycloplegia is induced. This can be assessed by evaluating the pupils’ reactions to light or measuring the amplitude of accommodation. In myopia RCTs, it is especially prudent to use cycloplegia and assess it when the primary outcome, secondary outcome, or inclusion criteria include refractive error. Establishing a standard cycloplegic treatment regimen for the measurement of refraction across myopia RCTs may improve comparability of findings.
Power and sample size estimations are important for establishing statistical validity and minimizing Type I and Type II errors. In our review, 31.5% of trials (n = 23) did not report the statistical power, 28.8% (n = 21) of trials did not report the sample size estimation, and 24.7% of trials (n = 18) did not provide information on the statistical method used for both sample size and power calculations. While some studies accounted for dropout rates - a best practice to ensure adequate statistical power - this was not consistently reported across studies [12, 35]. Interestingly, surgical trials were less likely to disclose details on sample size calculations, dropout rates, statistical power, and methods used, highlighting a potential gap and room for improvement in future trials. In contrast, registered trials were more likely to provide sample size information, possibly because of trial registration requirements. One-eye and two-eye designs were also more likely to provide sample size and statistical power. It is encouraged that myopia RCTs report full information regarding the power, sample size estimations, and statistical methods used.
In our review, over 80% of trials had missing data for primary outcomes, and of those with missing data, various methods were employed to address it. The majority of these trials utilized per-protocol analysis, which excluded subjects with incomplete data (n = 16, 44.4%). Other approaches included intention-to-treat analysis (n = 10, 27.8%), which incorporates data from all participants regardless of protocol adherence, multiple imputation (n = 2, 5.6%), and last value carried forward methods (n = 1, 2.8%). While per-protocol analysis is a common and simple approach for handling missing data, it may introduce bias with the exclusion of randomized participants. Employing a statistical method such as multiple imputation can offer a robust solution to mitigate bias from removing missing data, especially if the proportion of missing data is substantial. However, if the proportion of missing data is negligible, a per-protocol approach is adequate. Furthermore, 24 of the 60 trials with missing data (40.0%) did not provide a statistical method for handling missing data. This demonstrates a lack of transparency, and we recommend that future myopia trials mention the statistical methods used for treatment of missing data.
When multiple statistical comparisons are performed, it is important to control the Type I error rate at 5%. Multiple comparisons can arise from having more than two treatment arms or having multiple outcomes. Our review found that 56 trials (76.6%) of trials had multiple arms or multiple primary outcomes, of which only 16 trials (28.6%) were corrected for multiple comparisons. This demonstrates the need to incorporate appropriate multiple comparison correction methods to avoid overstating the statistical significance.
Many different primary outcomes have been used in RCTs. However, many trials did not explicitly state what the primary outcome or secondary outcomes were used in the trial, while many outcome measures were reported in the primary results paper. A well-designed trial should pre-specify the primary outcome(s) and secondary outcomes, and the primary result paper should report them accordingly.
This study has several limitations. First, not all eligible RCTs on myopia treatments may have been retrieved through our search strategy. While PubMed and Embase are widely used databases, not all global RCTs may be indexed within them. However, both of these databases provided robust search functionalities, including MeSH terms, specific keyword searches, and date filters, which improve retrieval accuracy. To maximize the inclusion of relevant studies, we tested multiple keyword combinations and MeSH terms before finalizing the strategy (Supplement 1). The final search approaches were agreed upon by both authors to ensure that the most comprehensive dataset was collected, and the searches were done independently. Second, the study was not prospectively registered. However, rigorous measures were taken to improve methodological reliability. All retrieved papers were independently searched and screened twice by two authors to ensure eligibility and reproducibility, and any discrepancies were discussed in a systematic manner. Data collection was performed independently, with verification at each stage to minimize bias. Details regarding the screening and eligibility processes are discussed in Supplement 1.
Conclusions
Overall, this review aimed to explore the current state of the methods of RCTs for myopia treatment and identify areas for improvement in the design and statistical analysis of future myopia trials. As myopia is a disease involving two eyes, it is important to consider how many eyes are included in the trial, and when two eyes are enrolled in a trial, to adjust for inter-eye correlation. Furthermore, sample size, statistical power, and statistical methods should be mentioned in the trial design to ensure that the trial has good power to detect meaningful treatment effects. In the analysis of data, correction for multiple comparisons from multiple arms or multiple outcomes should be considered. Missing data in outcome measures should be minimized in the conduct of the trial, and a statistical approach for dealing with missing data should be specified. We hope that this review can raise awareness in improving the design and statistical analysis of RCTs for myopia treatment in the ophthalmic community.
Supplementary Information
Acknowledgements
Not applicable.
Abbreviations
- IQR
Interquartile range
- RCT
Randomized controlled trials
- SD
Standard deviation
- SER
Spherical equivalent refraction
Authors’ contributions
YC acquired, analyzed, and interpreted the data and drafted the manuscript. GSY conceived the project, acquired and analyzed the data, and made substantial revisions to the manuscript. All authors read and approved the final manuscript.
Funding
Youvin Chung did not receive any funding.
Dr. Gui Shuang received funding from the NIH Vision Core grant P30-EY01583–26 and Research to Prevent Blindness (New York, New York).
Data availability
The dataset supporting the conclusions of this article is included within the article in Supplement 3.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pan CW, Ramamurthy D, Saw SM. Worldwide prevalence and risk factors for myopia. Ophthalmic Physiol Opt Jan. 2012;32(1):3–16. 10.1111/j.1475-1313.2011.00884.x. [DOI] [PubMed] [Google Scholar]
- 2.Morgan IG, Ohno-Matsui K, Saw SM, Myopia. Lancet. May 5 2012;379(9827):1739-48. 10.1016/s0140-6736(12)60272-4 [DOI] [PubMed]
- 3.Holden BA, Fricke TR, Wilson DA, et al. Global prevalence of myopia and high myopia and Temporal trends from 2000 through 2050. Ophthalmology May. 2016;123(5):1036–42. 10.1016/j.ophtha.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Haarman AEG, Enthoven CA, Tideman JWL, Tedja MS, Verhoeven VJM, Klaver CCW. The complications of myopia: A review and Meta-Analysis. Invest Ophthalmol Vis Sci Apr. 2020;9(4):49. 10.1167/iovs.61.4.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee CF, Cheng AC, Fong DY. Eyes or subjects: are ophthalmic randomized controlled trials properly designed and analyzed? Ophthalmology Apr. 2012;119(4):869–72. 10.1016/j.ophtha.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 6.Ha A, Kim SJ, Shim SR, Kim YK, Jung JH. Efficacy and safety of 8 Atropine concentrations for myopia control in children: A network Meta-Analysis. Ophthalmology Mar. 2022;129(3):322–33. 10.1016/j.ophtha.2021.10.016. [DOI] [PubMed] [Google Scholar]
- 7.Lanca C, Pang CP, Grzybowski A. Effectiveness of myopia control interventions: A systematic review of 12 randomized control trials published between 2019 and 2021. Front Public Health. 2023;11:1125000. 10.3389/fpubh.2023.1125000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang J, Liao Y, Yan N, et al. Efficacy of repeated Low-Level Red-Light therapy for slowing the progression of childhood myopia: A systematic review and Meta-analysis. Am J Ophthalmol Aug. 2023;252:153–63. 10.1016/j.ajo.2023.03.036. [DOI] [PubMed] [Google Scholar]
- 9.Zhu Z, Chen Y, Tan Z, Xiong R, McGuinness MB, Müller A. Interventions recommended for myopia prevention and control among children and adolescents in china: a systematic review. Br J Ophthalmol Feb. 2023;107(2):160–6. 10.1136/bjophthalmol-2021-319306. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal S, Saxena A, Saxena S. Mridula. Evaluation of the safety and effectiveness of Low-Dose Atropine eye drops in managing myopia progression among. Indian Child 01/01. 2023;15:254–63. [Google Scholar]
- 11.Ang M, Farook M, Htoon HM, Mehta JS. Randomized clinical trial comparing femtosecond LASIK and Small-Incision lenticule extraction. Ophthalmology Jun. 2020;127(6):724–30. 10.1016/j.ophtha.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Bao J, Huang Y, Li X, et al. Spectacle lenses with aspherical lenslets for myopia control vs Single-Vision spectacle lenses: A randomized clinical trial. JAMA Ophthalmol May. 2022;1(5):472–8. 10.1001/jamaophthalmol.2022.0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chamberlain P, Peixoto-de-Matos SC, Logan NS, Ngo C, Jones D, Young G. A 3-year randomized clinical trial of misight lenses for myopia control. Optom Vis Sci Aug. 2019;96(8):556–67. 10.1097/opx.0000000000001410. [DOI] [PubMed] [Google Scholar]
- 14.Chan HHL, Choi KY, Ng ALK, et al. Efficacy of 0.01% Atropine for myopia control in a randomized, placebo-controlled trial depends on baseline electroretinal response. Sci Rep Jul. 2022;8(1):11588. 10.1038/s41598-022-15686-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen H, Wang W, Liao Y, et al. Low-intensity red-light therapy in slowing myopic progression and the rebound effect after its cessation in Chinese children: a randomized controlled trial. Graefes Arch Clin Exp Ophthalmol Feb. 2023;261(2):575–84. 10.1007/s00417-022-05794-4. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Xiong R, Chen X, et al. Efficacy comparison of repeated Low-Level red light and Low-Dose Atropine for myopia control: A randomized controlled trial. Transl Vis Sci Technol Oct. 2022;3(10):33. 10.1167/tvst.11.10.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chia A, Ngo C, Choudry N, Yamakawa Y, Tan D. Atropine ophthalmic solution to reduce myopia progression in pediatric subjects: the randomized, Double-Blind multicenter phase II APPLE study. Asia Pac J ophthalmol (Phila). Jul-Aug. 2023;01(4):370–6. 10.1097/apo.0000000000000609. [DOI] [PubMed] [Google Scholar]
- 18.Chiang B, Valerio GS, Manche EE, Prospective. Randomized contralateral eye comparison of Wavefront-Guided laser in situ keratomileusis and small incision lenticule extraction refractive surgeries. Am J Ophthalmol May. 2022;237:211–20. 10.1016/j.ajo.2021.11.013. [DOI] [PubMed] [Google Scholar]
- 19.Cui C, Li X, Lyu Y, et al. Safety and efficacy of 0.02% and 0.01% Atropine on controlling myopia progression: a 2-year clinical trial. Sci Rep Nov. 2021;15(1):22267. 10.1038/s41598-021-01708-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dong J, Zhu Z, Xu H, He M. Myopia control effect of repeated Low-Level Red-Light therapy in Chinese children: A randomized, Double-Blind, controlled clinical trial. Ophthalmology Feb. 2023;130(2):198–204. 10.1016/j.ophtha.2022.08.024. [DOI] [PubMed] [Google Scholar]
- 21.Dong R, Zhang Y, Yuan Y, Liu Y, Wang Y, Chen Y. A prospective randomized self-controlled study of LASIK combined with accelerated cross-linking for high myopia in chinese: 24-month follow-up. BMC Ophthalmol Jun. 2022;24(1):280. 10.1186/s12886-022-02491-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fang J, Huang Z, Long Y, et al. Retardation of myopia by multifocal soft contact lens and orthokeratology: A 1-Year randomized clinical trial. Eye Contact Lens Aug. 2022;1(8):328–34. 10.1097/icl.0000000000000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia-Del Valle AM, Blázquez V, Gros-Otero J, et al. Efficacy and safety of a soft contact lens to control myopia progression. Clin Exp Optom Jan. 2021;104(1):14–21. 10.1111/cxo.13077. [DOI] [PubMed] [Google Scholar]
- 24.Guo B, Cheung SW, Kojima R, Cho P. One-year results of the variation of orthokeratology lens treatment zone (VOLTZ) study: a prospective randomised clinical trial. Ophthalmic Physiol Opt Jul. 2021;41(4):702–14. 10.1111/opo.12834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo L, Fan L, Tao J, et al. Use of topical 0.01% Atropine for controlling near Work-Induced transient myopia: A randomized, Double-Masked, Placebo-Controlled study. J Ocul Pharmacol Ther Mar. 2020;36(2):97–101. 10.1089/jop.2019.0062. [DOI] [PubMed] [Google Scholar]
- 26.He S, Luo Y, Chen P, et al. Prospective, randomized, contralateral eye comparison of functional optical zone, and visual quality after SMILE and FS-LASIK for high myopia. Transl Vis Sci Technol Feb. 2022;1(2):13. 10.1167/tvst.11.2.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He X, Sankaridurg P, Wang J, et al. Time outdoors in reducing myopia: A School-Based cluster randomized trial with objective monitoring of outdoor time and light intensity. Ophthalmology Nov. 2022;129(11):1245–54. 10.1016/j.ophtha.2022.06.024. [DOI] [PubMed] [Google Scholar]
- 28.He X, Wang J, Zhu Z, et al. Effect of repeated Low-level red light on myopia prevention among children in China with premyopia: A randomized clinical trial. JAMA Netw Open Apr. 2023;3(4):e239612. 10.1001/jamanetworkopen.2023.9612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hieda O, Hiraoka T, Fujikado T, et al. Efficacy and safety of 0.01% Atropine for prevention of childhood myopia in a 2-year randomized placebo-controlled study. Jpn J Ophthalmol May. 2021;65(3):315–25. 10.1007/s10384-021-00822-y. [DOI] [PubMed] [Google Scholar]
- 30.Hu P, Tao L. Comparison of the clinical effects between digital keratoplasty and traditional orthokeratology lenses for correcting juvenile myopia. Technol Health Care. 2023;31(6):2021–9. 10.3233/thc-220893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang W, Ji Y, Zheng S, et al. The effectiveness and rotational stability of vertical implantation of the implantable collamer lens for the treatment of myopia. J Refract Surg Oct. 2022;38(10):641–7. 10.3928/1081597x-20220831-01. [DOI] [PubMed] [Google Scholar]
- 32.Hvid-Hansen A, Jacobsen N, Møller F, Bek T, Ozenne B, Kessel L. Myopia control with Low-Dose Atropine in European children: Six-Month results from a randomized, Double-Masked, Placebo-Controlled, multicenter study. J Pers Med Feb. 2023;14(2). 10.3390/jpm13020325. [DOI] [PMC free article] [PubMed]
- 33.Jakobsen TM, Møller F. Control of myopia using orthokeratology lenses in Scandinavian children aged 6 to 12 years. Eighteen-month data from the Danish randomized study: clinical study of Near-sightedness; treatment with orthokeratology lenses (CONTROL study). Acta Ophthalmol Mar. 2022;100(2):175–82. 10.1111/aos.14911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jethani J. Efficacy of low-concentration Atropine (0.01%) eye drops for prevention of axial myopic progression in premyopes. Indian J Ophthalmol Jan. 2022;70(1):238–40. 10.4103/ijo.IJO_1462_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang Y, Zhu Z, Tan X, et al. Effect of repeated Low-Level Red-Light therapy for myopia control in children: A multicenter randomized controlled trial. Ophthalmology May. 2022;129(5):509–19. 10.1016/j.ophtha.2021.11.023. [DOI] [PubMed] [Google Scholar]
- 36.Jorge FA, Taguchi F, Campos M. The 18-Month outcomes of a contralateral, randomized, prospective clinical trial comparing photorefractive keratectomy and SMILE for myopia. J Refract Surg Mar. 2023;39(3):180–6. 10.3928/1081597x-20230113-01. [DOI] [PubMed] [Google Scholar]
- 37.Kinoshita N, Konno Y, Hamada N, et al. Efficacy of combined orthokeratology and 0.01% Atropine solution for slowing axial elongation in children with myopia: a 2-year randomised trial. Sci Rep Jul. 2020;29(1):12750. 10.1038/s41598-020-69710-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kohnen T, Lwowski C, Hemkeppler E, et al. Comparison of Femto-LASIK with combined accelerated Cross-linking to Femto-LASIK in high myopic eyes: A prospective randomized trial. Am J Ophthalmol Mar. 2020;211:42–55. 10.1016/j.ajo.2019.10.024. [DOI] [PubMed] [Google Scholar]
- 39.Kong XH, Zhao Y, Chen Z, et al. A randomized controlled trial of the effect of 0.01% Atropine eye drops combined with auricular acupoint stimulation on myopia progression. J Ophthalmol. 2021;2021:5585441. 10.1155/2021/5585441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lam CSY, Tang WC, Tse DY, et al. Defocus incorporated multiple segments (DIMS) spectacle lenses slow myopia progression: a 2-year randomised clinical trial. Br J Ophthalmol Mar. 2020;104(3):363–8. 10.1136/bjophthalmol-2018-313739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lau JK, Wan K, Cho P. Orthokeratology lenses with increased compression factor (OKIC): A 2-year longitudinal clinical trial for myopia control. Cont Lens Anterior Eye Feb. 2023;46(1):101745. 10.1016/j.clae.2022.101745. [DOI] [PubMed] [Google Scholar]
- 42.Lee SS, Lingham G, Blaszkowska M, et al. Low-concentration Atropine Eyedrops for myopia control in a multi-racial cohort of Australian children: A randomised clinical trial. Clin Exp Ophthalmol Dec. 2022;50(9):1001–12. 10.1111/ceo.14148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Q, Guo L, Zhang J, et al. Effect of School-Based family health education via social media on children’s myopia and parents’ awareness: A randomized clinical trial. JAMA Ophthalmol Nov. 2021;1(11):1165–72. 10.1001/jamaophthalmol.2021.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li SM, Ran AR, Kang MT, et al. Effect of text messaging parents of School-Aged children on outdoor time to control myopia: A randomized clinical trial. JAMA Pediatr Nov. 2022;1(11):1077–83. 10.1001/jamapediatrics.2022.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu X, Wang P, Xie Z, et al. One-year myopia control efficacy of cylindrical annular refractive element spectacle lenses. Acta Ophthalmol Sep. 2023;101(6):651–7. 10.1111/aos.15649. [DOI] [PubMed] [Google Scholar]
- 46.Mori K, Torii H, Fujimoto S, et al. The effect of dietary supplementation of Crocetin for myopia control in children: A randomized clinical trial. J Clin Med Aug. 2019;7(8). 10.3390/jcm8081179. [DOI] [PMC free article] [PubMed]
- 47.Mori K, Torii H, Hara Y, et al. Effect of Violet Light-Transmitting eyeglasses on axial elongation in myopic children: A randomized controlled trial. J Clin Med Nov. 2021;22(22). 10.3390/jcm10225462. [DOI] [PMC free article] [PubMed]
- 48.Peng T, Jiang J. Efficiency and related factors of multifocal soft contact lenses in controlling myopia. Eye Contact Lens Dec. 2023;1(12):535–41. 10.1097/icl.0000000000001043. [DOI] [PubMed] [Google Scholar]
- 49.Qian Y, Chen X, Naidu RK, Zhou X. Comparison of efficacy and visual outcomes after SMILE and FS-LASIK for the correction of high myopia with the sum of myopia and astigmatism from – 10.00 to -14.00 dioptres. Acta Ophthalmol Mar. 2020;98(2):e161–72. 10.1111/aos.14078. [DOI] [PubMed] [Google Scholar]
- 50.Raffa LH, Allinjawi K, Sharanjeet K, Akhir SM, Mutalib HA. Myopia control with soft multifocal contact lenses: 18-month follow-up. Saudi J Ophthalmol Oct-Dec. 2021;35(4):325–31. 10.4103/1319-4534.347305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rappon J, Chung C, Young G, et al. Control of myopia using diffusion optics spectacle lenses: 12-month results of a randomised controlled, efficacy and safety study (CYPRESS). Br J Ophthalmol Nov. 2023;107(11):1709–15. 10.1136/bjo-2021-321005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rateb M, Gad AAM, Tohamy D, Elmohamady MN. A prospective comparative study between implantable Phakic intraocular contact lens and implantable collamer lens in treatment of myopia in adults. J Ophthalmol. 2022;2022:9212253. 10.1155/2022/9212253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ren Q, Yang B, Liu L, Cho P. Orthokeratology in adults and factors affecting success: study design and preliminary results. Cont Lens Anterior Eye Dec. 2020;43(6):595–601. 10.1016/j.clae.2020.03.016. [DOI] [PubMed] [Google Scholar]
- 54.Repka MX, Weise KK, Chandler DL, et al. Low-Dose 0.01% Atropine eye drops vs placebo for myopia control: A randomized clinical trial. JAMA Ophthalmol Aug. 2023;1(8):756–65. 10.1001/jamaophthalmol.2023.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sankaridurg P, Bakaraju RC, Naduvilath T, et al. Myopia control with novel central and peripheral plus contact lenses and extended depth of focus contact lenses: 2 year results from a randomised clinical trial. Ophthalmic Physiol Opt Jul. 2019;39(4):294–307. 10.1111/opo.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sankaridurg P, Weng R, Tran H, et al. Spectacle lenses with highly aspherical lenslets for slowing myopia: A randomized, Double-Blind, Cross-Over clinical trial: parts of these data were presented as a poster at the annual research in vision and ophthalmology meeting, 2022. Am J Ophthalmol Mar. 2023;247:18–24. 10.1016/j.ajo.2022.10.021. [DOI] [PubMed] [Google Scholar]
- 57.Saxena R, Dhiman R, Gupta V, et al. Atropine for the treatment of childhood myopia in india: multicentric randomized trial. Ophthalmology Sep. 2021;128(9):1367–9. 10.1016/j.ophtha.2021.01.026. [DOI] [PubMed] [Google Scholar]
- 58.Sen S, Yadav H, Jain A, Verma S, Gupta P. Effect of Atropine 0.01% on progression of myopia. Indian J Ophthalmol Sep. 2022;70(9):3373–6. 10.4103/ijo.IJO_256_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sharma I, Das GK, Rohatgi J, Sahu PK, Chhabra P, Bhatia R. Low dose Atropine in preventing the progression of childhood myopia: A randomised controlled trial. Curr Eye Res Apr. 2023;48(4):402–7. 10.1080/02713683.2022.2162925. [DOI] [PubMed] [Google Scholar]
- 60.Shekar V, K S. MYOPIA MANAGEMENT WITH AYURVEDIC OCULAR VH. THERAPEUTICS: A CLINICAL STUDY. Int J Res Ayurveda Pharm. 2022;12/05:13:23–7. 10.7897/2277-4343.1306151. [Google Scholar]
- 61.Tan Q, Ng AL, Cheng GP, Woo VC, Cho P. Combined 0.01% Atropine with orthokeratology in childhood myopia control (AOK) study: A 2-year randomized clinical trial. Cont Lens Anterior Eye Feb. 2023;46(1):101723. 10.1016/j.clae.2022.101723. [DOI] [PubMed] [Google Scholar]
- 62.Tian L, Cao K, Ma DL, et al. Six-month repeated irradiation of 650 Nm low-level red light reduces the risk of myopia in children: a randomized controlled trial. Int Ophthalmol Oct. 2023;43(10):3549–58. 10.1007/s10792-023-02762-7. [DOI] [PubMed] [Google Scholar]
- 63.Tse JSH, Cheung JKW, Wong GTK, et al. Integrating clinical data and tear proteomics to assess efficacy, ocular surface status, and biomarker response after orthokeratology lens wear. Transl Vis Sci Technol Sep. 2021;1(11):18. 10.1167/tvst.10.11.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Walline JJ, Walker MK, Mutti DO, et al. Effect of high add power, medium add power, or Single-Vision contact lenses on myopia progression in children: the BLINK randomized clinical trial. Jama Aug. 2020;11(6):571–80. 10.1001/jama.2020.10834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wei S, Li SM, An W, et al. Safety and efficacy of Low-Dose Atropine Eyedrops for the treatment of myopia progression in Chinese children: A randomized clinical trial. JAMA Ophthalmol Nov. 2020;1(11):1178–84. 10.1001/jamaophthalmol.2020.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weng R, Lan W, Bakaraju R, et al. Efficacy of contact lenses for myopia control: insights from a randomised, contralateral study design. Ophthalmic Physiol Opt Nov. 2022;42(6):1253–63. 10.1111/opo.13042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xia L, Zhao H, Wang Y. Effect of 0.01% Atropine on diopter and optic axis in adolescents and children with myopia. J Pak Med Assoc. Mar 2023;73(3):656–8. 10.47391/jpma.6241. [DOI] [PubMed]
- 68.Xu S, Li Z, Zhao W, et al. Effect of atropine, orthokeratology and combined treatments for myopia control: a 2-year stratified randomised clinical trial. Br J Ophthalmol Nov. 2023;22(12):1812–7. 10.1136/bjo-2022-321272. [DOI] [PubMed] [Google Scholar]
- 69.Yam JC, Li FF, Zhang X, et al. Two-Year clinical trial of the Low-Concentration Atropine for myopia progression (LAMP) study: phase 2 report. Ophthalmology Jul. 2020;127(7):910–9. 10.1016/j.ophtha.2019.12.011. [DOI] [PubMed] [Google Scholar]
- 70.Yam JC, Zhang XJ, Zhang Y, et al. Effect of Low-Concentration Atropine Eyedrops vs placebo on myopia incidence in children: the LAMP2 randomized clinical trial. Jama Feb. 2023;14(6):472–81. 10.1001/jama.2022.24162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang X, Bi H, Li L, et al. The effect of relative corneal refractive power shift distribution on axial length growth in myopic children undergoing orthokeratology treatment. Curr Eye Res May. 2021;46(5):657–65. 10.1080/02713683.2020.1820528. [DOI] [PubMed] [Google Scholar]
- 72.Yu S, Du L, Ji N, et al. Combination of orthokeratology lens with 0.01% Atropine in slowing axial elongation in children with myopia: a randomized double-blinded clinical trial. BMC Ophthalmol Nov. 2022;15(1):438. 10.1186/s12886-022-02635-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yuval C, Otzem C, Laura BS, et al. Evaluating the effect of a myopia control spectacle lens among children in israel: 12-Month results. Am J Ophthalmol Jan. 2024;257:103–12. 10.1016/j.ajo.2023.08.019. [DOI] [PubMed] [Google Scholar]
- 74.Zadnik K, Schulman E, Flitcroft I, et al. Efficacy and safety of 0.01% and 0.02% Atropine for the treatment of pediatric myopia progression over 3 years: A randomized clinical trial. JAMA Ophthalmol Oct. 2023;1(10):990–9. 10.1001/jamaophthalmol.2023.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang Y, Li T, Li Z, Dai M, Wang Q, Xu C. Clinical outcomes of single-step transepithelial photorefractive keratectomy and off-flap epipolis-laser in situ keratomileusis in moderate to high myopia: 12-month follow-up. BMC Ophthalmol May. 2022;23(1):234. 10.1186/s12886-022-02443-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao Q, Hao Q. Clinical efficacy of 0.01% Atropine in retarding the progression of myopia in children. Int Ophthalmol Mar. 2021;41(3):1011–7. 10.1007/s10792-020-01658-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao Q, Hao Q. Comparison of the clinical efficacies of 0.01% Atropine and orthokeratology in controlling the progression of myopia in children. Ophthalmic Epidemiol Oct. 2021;28(5):376–82. 10.1080/09286586.2021.1875010. [DOI] [PubMed] [Google Scholar]
- 78.Zhao X, Zhang L, Ma J, et al. Comparison of Wavefront-Guided femtosecond LASIK and optimized SMILE for correction of Moderate-to-High astigmatism. J Refract Surg Mar. 2021;37(3):166–73. 10.3928/1081597x-20201230-01. [DOI] [PubMed] [Google Scholar]
- 79.Zhou L, Tong L, Li Y, Williams BT, Qiu K. Photobiomodulation therapy retarded axial length growth in children with myopia: evidence from a 12-month randomized controlled trial evidence. Sci Rep Feb. 2023;27(1):3321. 10.1038/s41598-023-30500-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou W, Liao Y, Wang W, et al. Efficacy of different powers of Low-Level red light in children for myopia control. Ophthalmology Jan. 2024;131(1):48–57. 10.1016/j.ophtha.2023.08.020. [DOI] [PubMed] [Google Scholar]
- 81.Zhu Q, Tang GY, Hua ZJ, et al. 0.05% Atropine on control of myopia progression in Chinese school children: a randomized 3-year clinical trial. Int J Ophthalmol. 2023;16(6):939–46. 10.18240/ijo.2023.06.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhu Q, Yin J, Li X, et al. Effects of Long-Term wear and discontinuation of orthokeratology lenses on the eyeball parameters in children with myopia. Int J Med Sci. 2023;20(1):50–6. 10.7150/ijms.79496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hashemi H, Alvani A, Aghamirsalim M, Miraftab M, Asgari S. Comparison of transepithelial and conventional photorefractive keratectomy in myopic and myopic astigmatism patients: a randomized contralateral trial. BMC Ophthalmol Feb. 2022;11(1):68. 10.1186/s12886-022-02293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dong R, Ying GS. Characteristics of design and analysis of ophthalmic randomized controlled trials: A review of ophthalmic papers 2020–2021. Ophthalmol Sci Jun. 2023;3(2):100266. 10.1016/j.xops.2022.100266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Glassman AR, Melia M. Randomizing 1 eye or 2 eyes: a missed opportunity. JAMA Ophthalmol Jan. 2015;133(1):9–10. 10.1001/jamaophthalmol.2014.3600. [DOI] [PubMed] [Google Scholar]
- 86.Bagheri A, Feizi M, Shafii A, Faramarzi A, Tavakoli M, Yazdani S. Effect of cycloplegia on corneal biometrics and refractive state. J Ophthalmic Vis Res Apr-Jun. 2018;13(2):101–9. 10.4103/jovr.jovr_196_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cheung SW, Chan R, Cheng RC, Cho P. Effect of cycloplegia on axial length and anterior chamber depth measurements in children. Clin Exp Optom Nov. 2009;92(6):476–81. 10.1111/j.1444-0938.2009.00419.x. [DOI] [PubMed] [Google Scholar]
- 88.Ho M, Morjaria P. Cycloplegic refraction in children. Community Eye Health. 2024;37(122):14–5. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset supporting the conclusions of this article is included within the article in Supplement 3.