Abstract
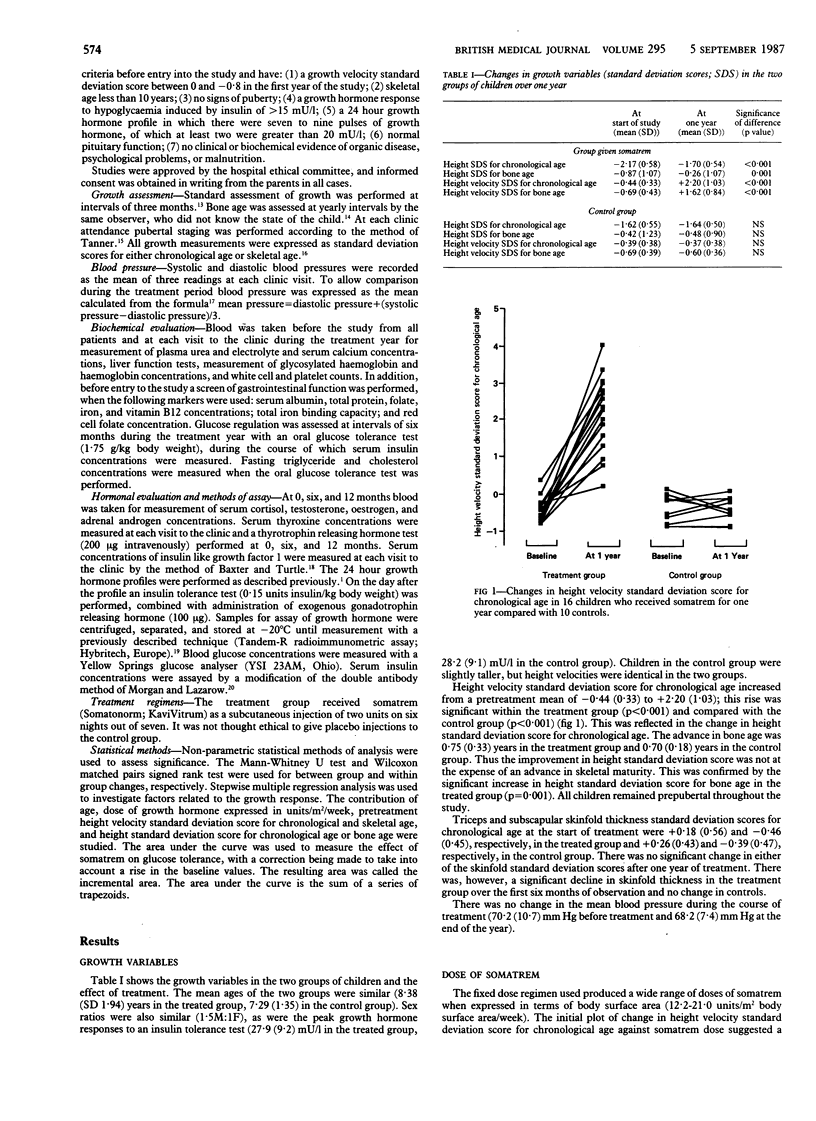
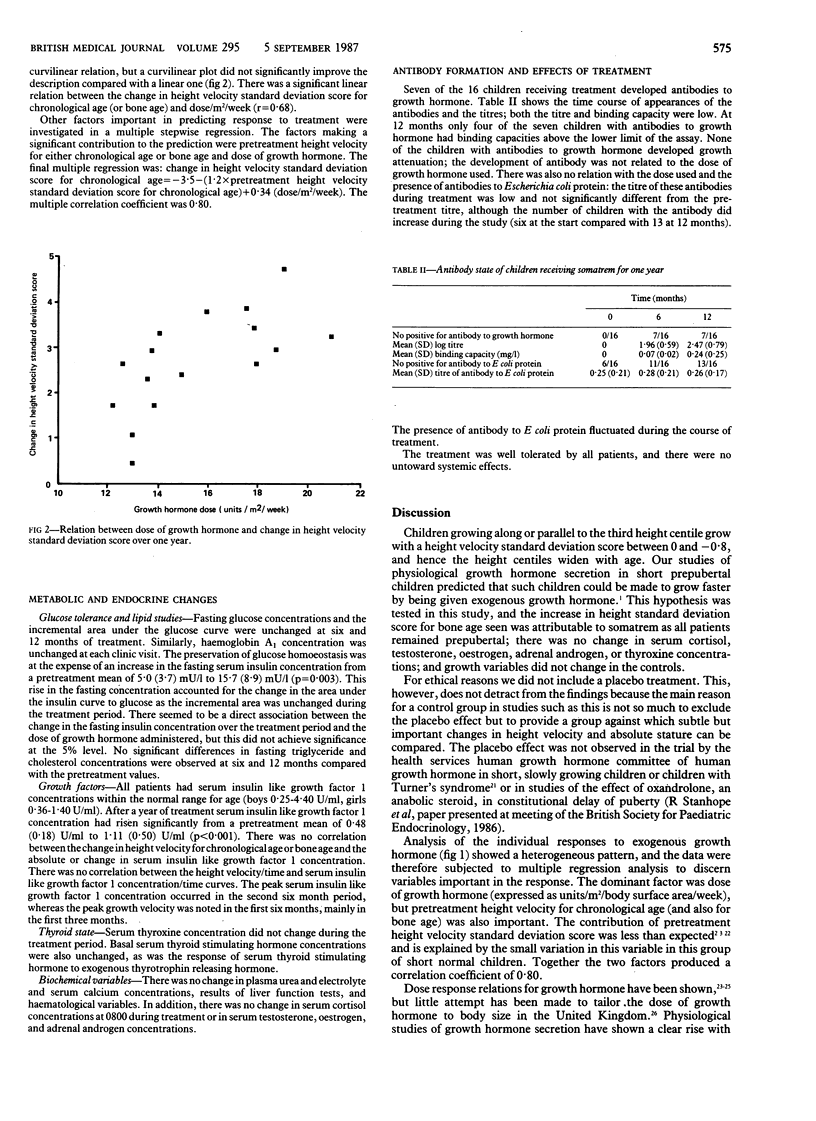
The growth of 26 short normal prepubertal children (mean age 8.4, height velocity standard deviation score for chronological age between +0.4 and -0.8) was studied for two years. Sixteen children were treated with somatrem (methionyl growth hormone) during the second year, and the remaining 10 children served as controls. During one year of treatment the height velocity standard deviation score for chronological age increased from the pretreatment mean of -0.44 (SD 0.33) to +2.20 (1.03). These values represented a change in height velocity from a pretreatment mean of 5.3 cm/year (range 4.6-6.9) to 7.4 cm/year (range 5.7-9.9). In the control group the height velocity standard deviation score was unchanged. Bone age advanced by 0.75 (0.33) years in the treated group compared with 0.70 (0.18) years in the control group. There was a significant increase in the height standard deviation score for bone age (0.63 (0.55] in the treated group. Multiple regression analysis of predictive factors contributing to the change in height velocity standard deviation score over the first year of treatment showed that the dose of growth hormone and pretreatment height velocity standard deviation score were important, together yielding a regression correlation coefficient of 0.80. The only metabolic side effect of treatment was an increase in fasting insulin concentration, which may be an important mediator of the anabolic effects of growth hormone. Treatment had no effect on thyroid function, blood pressure, or glucose tolerance. At the end of the treatment year seven of the 16 treated children had developed antibodies to growth hormone, but they were present in low titre with low binding capacity and in no child was growth attenuated. Biosynthetic growth hormone improved the height velocity of children growing along or parallel to the third height centile, but the effects on height prognosis need to be assessed over a longer period.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baxter R. C., Brown A. S., Turtle J. R. Radioimmunoassay for somatomedin C: comparison with radioreceptor assay in patients with growth-hormone disorders, hypothyroidism, and renal failure. Clin Chem. 1982 Mar;28(3):488–495. [PubMed] [Google Scholar]
- Bercu B. B., Shulman D., Root A. W., Spiliotis B. E. Growth hormone (GH) provocative testing frequently does not reflect endogenous GH secretion. J Clin Endocrinol Metab. 1986 Sep;63(3):709–716. doi: 10.1210/jcem-63-3-709. [DOI] [PubMed] [Google Scholar]
- Binoux M., Lassarre C., Hardouin N. Somatomedin production by rat liver in organ culture. III. Studies on the release of insulin-like growth factor and its carrier protein measured by radioligand assays. Effects of growth hormone, insulin and cortisol. Acta Endocrinol (Copenh) 1982 Mar;99(3):422–430. [PubMed] [Google Scholar]
- Blethen S. L., Chasalow F. I. Use of a two-site immunoradiometric assay for growth hormone (GH) in identifying children with GH-dependent growth failure. J Clin Endocrinol Metab. 1983 Nov;57(5):1031–1035. doi: 10.1210/jcem-57-5-1031. [DOI] [PubMed] [Google Scholar]
- Brook C. G., Hindmarsh P. C., Healy M. J. A better way to detect growth failure. Br Med J (Clin Res Ed) 1986 Nov 8;293(6556):1186–1186. doi: 10.1136/bmj.293.6556.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher H., Zapf J., Torresani T., Prader A., Froesch E. R., Illig R. Insulin-like growth factors I and II, prolactin, and insulin in 19 growth hormone-deficient children with excessive, normal, or decreased longitudinal growth after operation for craniopharyngioma. N Engl J Med. 1983 Nov 10;309(19):1142–1146. doi: 10.1056/NEJM198311103091902. [DOI] [PubMed] [Google Scholar]
- Conway S., McCann S. M., Krulich L. On the mechanism of growth hormone autofeedback regulation: possible role of somatostatin and growth hormone-releasing factor. Endocrinology. 1985 Dec;117(6):2284–2292. doi: 10.1210/endo-117-6-2284. [DOI] [PubMed] [Google Scholar]
- Frasier S. D., Costin G., Lippe B. M., Aceto T., Jr, Bunger P. F. A dose-response curve for human growth hormone. J Clin Endocrinol Metab. 1981 Dec;53(6):1213–1217. doi: 10.1210/jcem-53-6-1213. [DOI] [PubMed] [Google Scholar]
- Gertner J. M., Genel M., Gianfredi S. P., Hintz R. L., Rosenfeld R. G., Tamborlane W. V., Wilson D. M. Prospective clinical trial of human growth hormone in short children without growth hormone deficiency. J Pediatr. 1984 Feb;104(2):172–176. doi: 10.1016/s0022-3476(84)80987-7. [DOI] [PubMed] [Google Scholar]
- Grunt J. A., Howard C. P., Daughaday W. H. Comparison of growth and somatomedin C responses following growth hormone treatment in children with small-for-date short stature, significant idiopathic short stature and hypopituitarism. Acta Endocrinol (Copenh) 1984 Jun;106(2):168–174. doi: 10.1530/acta.0.1060168. [DOI] [PubMed] [Google Scholar]
- Hindmarsh P. C., Stanhope R., Kendall B. E., Brook C. G. Tall stature: a clinical, endocrinological and radiological study. Clin Endocrinol (Oxf) 1986 Sep;25(3):223–231. doi: 10.1111/j.1365-2265.1986.tb01686.x. [DOI] [PubMed] [Google Scholar]
- Holder A. T., Aston R., Preece M. A., Ivanyi J. Monoclonal antibody-mediated enhancement of growth hormone activity in vivo. J Endocrinol. 1985 Dec;107(3):R9–12. doi: 10.1677/joe.0.107r009. [DOI] [PubMed] [Google Scholar]
- Lippe B. M., Van Herle A. J., LaFranchi S. H., Uller R. P., Lavin N., Kaplan S. A. Reversible hypothyroidism in growth hormone-deficient children treated with human growth hormone. J Clin Endocrinol Metab. 1975 Apr;40(4):612–618. doi: 10.1210/jcem-40-4-612. [DOI] [PubMed] [Google Scholar]
- Milner R. D. Clinical experience of somatrem: UK preliminary report. Acta Paediatr Scand Suppl. 1986;325:25–28. doi: 10.1111/j.1651-2227.1986.tb10359.x. [DOI] [PubMed] [Google Scholar]
- Milner R. D., Russell-Fraser T., Brook C. G., Cotes P. M., Farquhar J. W., Parkin J. M., Preece M. A., Snodgrass G. J., Mason A. S., Tanner J. M. Experience with human growth hormone in Great Britain: the report of the MRC Working Party. Clin Endocrinol (Oxf) 1979 Jul;11(1):15–38. doi: 10.1111/j.1365-2265.1979.tb03043.x. [DOI] [PubMed] [Google Scholar]
- Nakamoto J. M., Gertner J. M., Press C. M., Hintz R. L., Rosenfeld R. G., Genel M. Suppression of the growth hormone (GH) response to clonidine and GH-releasing hormone by exogenous GH. J Clin Endocrinol Metab. 1986 May;62(5):822–826. doi: 10.1210/jcem-62-5-822. [DOI] [PubMed] [Google Scholar]
- Plotnick L. P., Van Meter Q. L., Kowarski A. A. Human growth hormone treatment of children with growth failure and normal growth hormone levels by immunoassay: lack of correlation with somatomedin generation. Pediatrics. 1983 Mar;71(3):324–327. [PubMed] [Google Scholar]
- Porter B. A., Refetoff S., Rosenfeld R. L., De Groat L. J., Lang U. S., Stark O. Abnormal thyroxine metabolism in hyposomatotrophic dwarfism and inhibition of responsiveness to TRH during GH therapy. Pediatrics. 1973 Apr;51(4):668–674. [PubMed] [Google Scholar]
- Preece M. A., Tanner J. M., Whitehouse R. H., Cameron N. Dose dependence of growth response to human growth hormone in growth hormone deficiency. J Clin Endocrinol Metab. 1976 Mar;42(3):477–483. doi: 10.1210/jcem-42-3-477. [DOI] [PubMed] [Google Scholar]
- Root A. W., Snyder P. J., Rezvani I., DiGeorge A. M., Utiger R. D. Inhibition of thyrotropin releasing hormone-mediated secretion of thyrotropin by human growth hormone. J Clin Endocrinol Metab. 1973 Jan;36(1):103–107. doi: 10.1210/jcem-36-1-103. [DOI] [PubMed] [Google Scholar]
- Rosenfeld R. G., Kemp S. F., Hintz R. L. Constancy of somatomedin response to growth hormone treatment of hypopituitary dwarfism, and lack of correlation with growth rate. J Clin Endocrinol Metab. 1981 Sep;53(3):611–617. doi: 10.1210/jcem-53-3-611. [DOI] [PubMed] [Google Scholar]
- Rosenfeld R. G., Wilson D. M., Dollar L. A., Bennett A., Hintz R. L. Both human pituitary growth hormone and recombinant DNA-derived human growth hormone cause insulin resistance at a postreceptor site. J Clin Endocrinol Metab. 1982 May;54(5):1033–1038. doi: 10.1210/jcem-54-5-1033. [DOI] [PubMed] [Google Scholar]
- Rosenthal S. M., Hulse J. A., Kaplan S. L., Grumbach M. M. Exogenous growth hormone inhibits growth hormone-releasing factor-induced growth hormone secretion in normal men. J Clin Invest. 1986 Jan;77(1):176–180. doi: 10.1172/JCI112273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. J., Borges F., Grossman A., Smith R., Ngahfoong L., Rees L. H., Savage M. O., Besser G. M. Growth hormone pretreatment in man blocks the response to growth hormone-releasing hormone; evidence for a direct effect of growth hormone. Clin Endocrinol (Oxf) 1987 Jan;26(1):117–123. doi: 10.1111/j.1365-2265.1987.tb03645.x. [DOI] [PubMed] [Google Scholar]
- Scheiwiller E., Guler H. P., Merryweather J., Scandella C., Maerki W., Zapf J., Froesch E. R. Growth restoration of insulin-deficient diabetic rats by recombinant human insulin-like growth factor I. Nature. 1986 Sep 11;323(6084):169–171. doi: 10.1038/323169a0. [DOI] [PubMed] [Google Scholar]
- Schlechter N. L., Russell S. M., Spencer E. M., Nicoll C. S. Evidence suggesting that the direct growth-promoting effect of growth hormone on cartilage in vivo is mediated by local production of somatomedin. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7932–7934. doi: 10.1073/pnas.83.20.7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiliotis B. E., August G. P., Hung W., Sonis W., Mendelson W., Bercu B. B. Growth hormone neurosecretory dysfunction. A treatable cause of short stature. JAMA. 1984 May 4;251(17):2223–2230. [PubMed] [Google Scholar]
- Takano K., Shizume K. Clinical experience with somatrem in Japan. Acta Paediatr Scand Suppl. 1986;325:19–24. doi: 10.1111/j.1651-2227.1986.tb10358.x. [DOI] [PubMed] [Google Scholar]
- Tanner J. M., Whitehouse R. H., Hughes P. C., Vince F. P. Effect of human growth hormone treatment for 1 to 7 years on growth of 100 children, with growth hormone deficiency, low birthweight, inherited smallness, Turner's syndrome, and other complaints. Arch Dis Child. 1971 Dec;46(250):745–782. doi: 10.1136/adc.46.250.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner J. M., Whitehouse R. H., Takaishi M. Standards from birth to maturity for height, weight, height velocity, and weight velocity: British children, 1965. II. Arch Dis Child. 1966 Dec;41(220):613–635. doi: 10.1136/adc.41.220.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trippel S. B., Van Wyk J. J., Mankin H. J. Localization of somatomedin-C binding to bovine growth-plate chondrocytes in situ. J Bone Joint Surg Am. 1986 Jul;68(6):897–903. [PubMed] [Google Scholar]
- Underwood L. E. Report of the conference on uses and possible abuses of biosynthetic human growth hormone. N Engl J Med. 1984 Aug 30;311(9):606–608. doi: 10.1056/NEJM198408303110925. [DOI] [PubMed] [Google Scholar]
- Van Vliet G., Styne D. M., Kaplan S. L., Grumbach M. M. Growth hormone treatment for short stature. N Engl J Med. 1983 Oct 27;309(17):1016–1022. doi: 10.1056/NEJM198310273091703. [DOI] [PubMed] [Google Scholar]
- Vicens-Calvet E., Vendrell J. M., Albisu M., Potau N., Audi L., Gusiñe M. The dosage dependency of growth and maturity in growth hormone deficiency treated with human growth hormone. Acta Paediatr Scand. 1984 Jan;73(1):120–126. doi: 10.1111/j.1651-2227.1984.tb09909.x. [DOI] [PubMed] [Google Scholar]
- Wit J. M., Faber J. A., Van den Brande J. L. Growth response to human growth hormone treatment in children with partial and total growth hormone deficiency. Acta Paediatr Scand. 1986 Sep;75(5):767–773. doi: 10.1111/j.1651-2227.1986.tb10288.x. [DOI] [PubMed] [Google Scholar]
- Zadik Z., Chalew S. A., McCarter R. J., Jr, Meistas M., Kowarski A. A. The influence of age on the 24-hour integrated concentration of growth hormone in normal individuals. J Clin Endocrinol Metab. 1985 Mar;60(3):513–516. doi: 10.1210/jcem-60-3-513. [DOI] [PubMed] [Google Scholar]


