Abstract
Neurological disorders including gliomas and neurodegenerative diseases are characterized by dysregulation of the central nerve system (CNS). Despite recent advances in disease-modifying treatments, pharmacological approaches for neurological disorders still face limitations due to the complexity of these diseases and the challenges in targeting the underlying mechanisms. Magnetic hyperthermia, an approach that utilizes magnetic nanoparticles (MNPs) to generate localized heat in target cells and tissues by responding to an alternating magnetic field (AMF), has been developed as a non-pharmacological treatment approach for targeting tumor cells or pathogens, primarily through thermal inactivation. Recently, beyond its traditional application in thermal therapies, magnetic hyperthermia has been increasingly explored for neurological diseases. Importantly, recent studies demonstrate the ability of magnetic hyperthermia in eliciting various biological effects by means of triggering heat shock protein (HSP) signaling, enhancing immune responses, and activating heat-sensitive ion channels in neurons. This review highlights the current understanding of magnetic hyperthermia in stimulating molecular and cellular effects on brain tissue and further discusses its potential in the treatment of neurological disorders including Glioblastoma Multiforme (GBM), Alzheimer’s Disease (AD), Parkinson’s Disease (PD). The studies discussed in this review were selected by using the search tool on PubMed with the suggested key words.
Keywords: Magnetic hyperthermia, magnetothermal stimulation, Glioblastoma Multiforme (GBM), Alzheimer’s disease (AD), Parkinson’s disease (PD), superparamagnetic iron oxide nanoparticles
1. Introduction
Neurological disorders including gliomas and neurodegenerative diseases are associated with central nerve system (CNS) dysregulation. In addition to having a poor prognosis, they significantly diminish the quality of life and impose a substantial economic burden on society, exceeding $1 trillion annually [1,2]. Gliomas are a type of brain tumor that develops from glial cells and can cause various neurological symptoms associated with the tumor’s growth and invasion on surrounding brain tissue. In particular, Glioblastoma Multiforme (GBM) is a malignant brain tumor of the CNS and affects the brain and spinal cord [3]. Neurodegenerative diseases such as Alzheimer’s Disease (AD) and Parkinson’s Disease (PD) are associated with the progressive loss of neurons. AD is a devastating neurodegenerative disease that is clinically characterized by progressive impairment of memory and cognitive functions. Many lines of genetic and biochemical evidence suggest a pathological role of beta-amyloid (Aβ) where extracellular deposition of Aβ plaques contributes to the loss of synapses and neurons, resulting in cognitive deficits and eventually dementia [4,5]. In the US alone, more than 7 million people and one-third of seniors aged 75 and above are estimated to suffer from dementia [6,7]. PD is the second most common neurodegenerative disease after AD and affects primarily motor movement and can also cause other non-motor symptoms such as olfactory dysfunction, cognitive impairment, and psychiatric symptoms. The pathological hallmark of PD is intracellular aggregates of α-synuclein (α-syn) in the form of Lewy bodies and Lewy neurites associated with the loss of dopaminergic neurons [8].
So far, the disease modifying treatments for neurological disorders have limited success due to the complexity of these diseases and the challenges in targeting the underlying mechanisms [9,10]. The current standard of care for GBM involves a combination of treatments including surgical resection, radiotherapy, and chemotherapy with temozolomide, which has shown limited success in significantly increasing long-term survival rates [11]. The treatment of AD has primarily been focused on anti-amyloid antibodies, associated with the pathological role of Aβ plaques in AD progression [12]. The recent Food and Drug Administration (FDA)-approved monoclonal antibodies showed an efficacy in slowing cognitive decline in patients in the early stages of AD. However, the treatments were associated with side effects in the form of Amyloid Related Imaging Abnormalities (ARIA) and the overall clinical benefit was found to be modest [13]. Currently, the best available treatments for PD are symptomatic, primarily aiming to restore dopamine levels or modulate dopamine pathways in the brain [14].
Along with the efforts in developing pharmacological treatments, non-pharmacological approaches have also been explored for managing neurological disorders. In particular, noninvasive treatments in the form of photo-activated methods have the potential to improve the outcome of current treatments. Tumor ablation in GBM using photothermal and photodynamic therapy has shown early promise in improving its prognosis [15,16]. The photothermal and photodynamic therapy have also shown potency in modulating Aβ aggregation in AD [17]. Similar approaches have been applied in PD models where photothermal therapy was found to improve blood–brain barrier (BBB) permeability and reduce neuroinflammation by scavenging reactive oxygen species (ROS) [18,19]. Another recent study in PD therapeutics employed gold-based dual functional nanoparticles to photothermally activate dopaminergic neurons and simultaneously eliminate preformed α-syn aggregates [20]. However, light-based approaches are limited by their poor targeting efficiency and biological permeation across the layers of the CNS [21]. Additionally, transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS) have also been explored as a means for noninvasive brain stimulation, which have proven beneficial in the improvement of neurological symptoms in AD and PD [22,23]. However, TMS, despite its ability to directly stimulate neurons, is often considered less versatile in stimulating multiple brain areas simultaneously. On the other hand, tDCS tends to have limitations in maintaining a field strength required to elicit long-lasting effects of neurostimulation.
Recently, nanoparticle-mediated magnetic hyperthermia, a minimally invasive approach that utilizes magnetic nanoparticles (MNPs) to generate localized heat in target cells and tissues by responding to an alternating magnetic field (AMF), has been increasingly explored for neurological diseases, particularly for brain tumors. Unlike photo-activated stimulation that relies on light exposure with limited penetration depth into biological tissues, magnetic hyperthermia allows for the stimulation of deep layers of the CNS without the need for invasive surgical procedures [24,25]. Additionally, magnetic hyperthermia offers potential advantages over TMS and tDCS by allowing for stimulation of multiple brain areas with tight control over the strength of stimulation. MNPs based on iron oxide and its derivatives are biocompatible and have been extensively studied for biomedical applications [26]. This is evident from the magnetite-based nanoparticles approved for clinical applications, most notable of them being ferumoxytol (Ferraheme) nanoparticles approved by the FDA for the treatment of iron deficiency anemia in adults with chronic kidney disease (CKD) [27]. Furthermore, iron oxide-based nanoparticles are modular and can easily be functionalized to increase stability and targeted delivery [28,29]. Another advantage of iron oxide-based nanoparticles is that they are retained by the target tissue for weeks, allowing for multiple rounds of magnetic hyperthermia stimulation for increased therapeutic efficacy [30].
As a rapidly developing field, magnetic hyperthermia stimulation holds promise for treating neurological diseases. Recent advancement in magnetic hyperthermia, especially in the context of thermal therapies, have been extensively discussed elsewhere [31–33]. This review focuses on discussing the current understanding of magnetic hyperthermia in stimulating molecular and cellular effects on brain tissue and further discusses its potential in the treatment of neurological disorders including GBM, AD, and PD. The primary research articles discussed in this review were selected by using the search tool on PubMed with search terms including “magnetothermal stimulation,” “magnetic hyperthermia,” “neuromodulation,” “Glioblastoma Multiforme,” “Parkinson’s Disease,” “Alzheimer’s Disease,” “beta-amyloid (Aβ)” without enabling the publication date filter.
2. Biological effects of magnetic hyperthermia
2.1. Heating mechanism of magnetic nanoparticles
MNPs can generate heat primarily through Néel and Brownian relaxations when subjected to AMF. Magnetic materials possess a magnetic anisotropy which keeps their moments aligned along specific, energetically favorable directions called “easy axes.” An external magnetic field provides the energy to force the alignments out of this easy axis. Upon removal of the magnetic field, the magnetic moments reorient toward the easy axis by Néel relaxation. Additionally, the magnetic material physically rotates in the medium by Brownian relaxation. When this process is repeated thousands of times per second (in the kilohertz range) in MNPs, the energy is dissipated as heat [34]. In a physiological context, it is crucial to control the intensity of magnetic hyperthermia toward maximizing its therapeutic effects on the target cell and tissue while minimizing off-target thermal damage to the surrounding tissue. The heating efficiency depends on several factors, including the size and chemical composition of MNPs, its concentration in the biological medium, and the frequency and field strength of AMF.
The choice of material in magnetic hyperthermia can also affect the heating efficiency, which is tied to the material’s hysteresis loop [35,36]. A material with a wide hysteresis loop and hence higher coercivity exhibits enhanced heating efficiency when exposed to an AMF with higher magnetic field amplitude and lower AMF frequency. Conversely, materials with a narrower hysteresis loop and lower magnetic coercivity exhibit improved heating efficiency under an AMF with lower field amplitude and higher frequency. This property of magnetic materials offers multiplexed control of neurological activities by altering the frequency and strength of AMF [37].
The readers may further refer to recently published review articles [25,32,35], which provide important insight into how different parameters of magnetic hyperthermia such as MNP size, shape, choice of material, AMF strength, and frequency affect heating efficiency of MNPs.
For neurological applications, nanoparticle design must address several key factors such as bioavailability, toxicity, targeting efficiency, and blood–brain barrier (BBB) permeability to ensure effective and safe delivery to the brain. The BBB is the primary obstacle, severely restricting the passage of therapeutic agents to the CNS. Overcoming this barrier requires careful consideration of the nanoparticle’s physicochemical properties, surface modifications, and mechanism of delivery. The size and charge of nanoparticles is known to affect toxicity and delivery, where smaller, neutral, or negatively charged nanoparticles are generally well tolerated and delivered across biological membranes [32]. The shape of nanoparticles can affect biodistribution as shell-type structures are better absorbed compared to rod-like nanoparticles [30]. Equally important is the choice of material, which can not only affect the heating efficiency of the nanoparticle but also the neurotoxicity and retention in the neurological tissue. Together, an ideal choice of nanoparticles for neurological applications would comprise nontoxic material, functionalized with bioavailable and biocompatible ligands, which can permeate through the BBB and reach a target site of the brain.
The heating efficiency of MNP is measured in terms of specific absorption rate (SAR) or specific loss power (SLP) which is given by Equation (1):
| (1) |
Where C = specific heat capacity, gives the temperature-time slope, ms is the mass of the solvent, and mm is the mass of the magnetic nanoparticles.
The extent of thermal dose induced by the heating of MNPs is quantified as cumulative equivalent minutes at 43°C (CEM43), which indicates the potential for tissue damage during hyperthermia treatment [38]. CEM43 accounts for the various time-temperature exposures applied into an equivalent exposure time expressed as minutes at the reference temperature of 43°C and is calculated using the formula:
| (2) |
Where R = 0.25 when T < 43°C and 0.5 when T > 43°C, t is the time interval, and T is the average temperature during that time interval. Different organs and tissues have varying thresholds of hyperthermia tolerance as tissues have different structures, compositions, and metabolic rates, leading to differing responses to heat stress. In general, the brain is more vulnerable to heat stress compared to other tissues such as skin and muscles and the threshold thermal dose for brain tissue was reported to be in the range of 10 CEM43 minutes [38].
2.2. Biological effects of magnetic hyperthermia
Beyond its traditional application in thermal therapies, recent studies demonstrate the ability of magnetic hyperthermia in eliciting a variety of molecular and cellular effects on brain tissue (Figure 1). The elevated temperature induced by magnetic hyperthermia imposes heat stress on cells, leading to the increased expression of heat shock proteins (HSPs) [39]. HSPs act as molecular chaperones, helping misfolded proteins to refold and maintain cellular homeostasis by triggering autophagic responses to eliminate damaged or misfolded proteins [40]. Magnetic hyperthermia can also enhance immune response by promoting antigen generation and presentation. This is achieved by inducing immunogenic cell death in tumor cells, releasing tumor-associated antigens, and activating antigen-presenting cells (APCs), which in turn stimulates T-cell activation and other immune responses [41]. Additionally, magnetic hyperthermia can activate heat-sensitive ion channels such as the transient receptor potential cation channel subfamily V member (TRPV), leading to membrane depolarization, which plays a crucial role in supporting neuronal survival and function [35]. This section discusses the current understanding of the biological effects of magnetic hyperthermia in the context of neurological diseases.
Figure 1.

The biological effects of magnetic hyperthermia. Exposing MNPs such as iron oxide magnetic nanoparticles to a high frequency AMF causes heat generation, associated with the hysteresis loop of the magnetic material. When properly targeted, magnetic hyperthermia can elicit a multitude of biological effects on brain tissue by triggering HSPs signaling, enhancing immune responses, and activating heat-sensitive ion channels. Created in BioRender. Naveed, M. (2025) https://BioRender.com/75av13d.
2.2.1. Activation of heat shock protein (HSP) signaling
HSPs are expressed in response to heat and cellular stresses and play a crucial role in chaperone functions including clearance of misfolded proteins and refolding metastable proteins [42]. Among the HSP family, HSP70 plays a key role in immune responses as a carrier for antigenic peptides, which is cross presented by major histocompatibility complex (MHC) class I molecules, leading to the activation of CD8+ cytotoxic T cell response [43]. In a clinical study of magnetic hyperthermia in GBM patients, an upregulated expression of HSP70 was observed in the tissue surrounding the nanoparticles [44]. Importantly, magnetic hyperthermia-induced heat shock factor-1 (HSF-1), a key transcription factor that regulates HSP70 expression, was found to be beneficial in increasing sensitivity to chemotherapy by downregulating the expression of P-glycoprotein (P-gp) and mutant p53, both are known to expel chemotherapeutic drugs from the cell [45].
Impaired autophagy has been implicated in the progression of diseases characterized by the accumulation of misfolded and aggregated proteins in the brain, such as Aβ plaques in AD, and α-syn in PD. Recent studies indicate a synergistic link between HSP levels and autophagic response. Following acute heat shock in experimental models using C. elegans and human peripheral blood mononuclear cells (PMBCs), HSP70 and autophagic markers were upregulated [46], leading to the activation of chaperone-mediated autophagy [47]. In addition to HSP70, other members of the HSP family, including HSPB8, were also found to be involved in heat shock-mediated macro-autophagy [48].
Collectively, the elevated temperature induced by magnetic hyperthermia triggers a heat stress response in cells, leading to increased production of HSPs. This in turn can trigger a cascade of signaling pathways involving the activation of immune response for anti-tumor immunity, increased sensitivity to chemotherapy, and autophagy response for the degradation of misfolded protein aggregates.
2.2.2. Enhanced immune response
Magnetic hyperthermia can activate both innate and adaptive immune responses through the release of danger signals and tissue antigens. These responses trigger a range of immune responses, including skewing macrophages toward the pro-inflammatory phenotype, promoting antigen presentation, and stimulating T cell activation.
The iron oxide nanoparticles carry an intrinsic ability to generate ROS via the Fenton and Haber-Weiss reactions [49], which was shown to skew anti-inflammatory M2-like macrophages into pro-inflammatory M1-like phenotype, leading to innate anti-tumor activities [50]. Additionally, magnetic hyperthermia induced-HSPs act as danger signals, which also contributes to promoting the polarization of macrophages toward the M1-like phenotype [41].
In addition to the role of magnetic hyperthermia in triggering innate immune responses, magnetic hyperthermia can also activate the adaptive immune response, particularly T cell activation. Magnetic hyperthermia can induce the release of tissue antigens such as tumor-associated antigens from necrotic cells. These antigens are then presented by APCs such as macrophages and dendritic cells which lead to T cell activation and proliferation, culminating in an anti-tumor adaptive immune response [41]. Magnetic hyperthermia-induced macrophage polarization to pro-inflammatory M1 phenotypes could further contribute to promoting tumor-infiltrating T lymphocytes, associated with anti-tumor responses [51].
2.2.3. Activation of temperature-sensitive ion channel
The TRPV family consists of six known receptors, which are found mainly in the sensory neurons of the CNS and in the periphery [52]. Among the TRPV family, TRPV1–4 receptors are activated by a wide range of temperature thresholds and play a crucial role in detecting and communicating temperature changes [53]. In particular, TRPV1 and TRPV4 are activated in the ranges of mild hyperthermia (<43°C), making them ideal candidates for neural stimulation by magnetic hyperthermia [54]. Recently, several studies involving the overexpression of TRPV receptors via viral transfection in specific brain regions have demonstrated an increased neuronal activity in those affected areas following the stimulation by magnetic hyperthermia (Table 1).
Table 1.
Applications of magnetic hyperthermia for modulating neuronal activities by targeting heat-sensitive TRPV receptors.
| MNP material | Target | Molecular target | AMF | Exposure time | Biological validation | Target brain area | Method of delivery | Effect | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Manganese ferrite | Neurostimulation | TRPV1 | 40 MHz 8.4 G | 30 sec | C. elegans | Amphid sensory neurons | [55] | ||
| Magnetite | Neurostimulation | TRPV1 | 500 kHz, 15 kA/m | 10 sec pulses | Mice | Ventral tegmental area (VTA) | Direct Intracranial injection | [56] | |
| Cobalt-ferric-Manganese | Neurostimulation | TRPV1 | 570 kHz, 7.5 kA/m | 1 min | Mice | Motor cortex, striatum, ridge between Ventral & dorsal striatum | Direct intracranial injection | Locomotion, Rotation, freezing of gait | [57] |
| Overexpressed Ferritin coupled to TRPV1 | Bidirectional neural control | TRPV1 | 465 kHz, 0.2–1 T | 30 sec | Mice | Ventromedial hypothalamus | Direct intracranial injection | Increased or decrease glucose and insulin in plasma | [58] |
| Overexpressed Ferritin coupled to TRPV4 (Magneto2.0) | Neurostimulation | TRPV4 | 0.1 Hz 60–250 mT | 10 min | Mice | Striatum | Direct intracranial injection | Reward mechanism (place preference) | [59] |
In the first study of its kind, Huang et al. demonstrated the feasibility of magnetothermal stimulation in the opening of TRPV1. In the study, superparamagnetic nanoparticles were prepared to specifically bind to TRPV1 receptors on plasma membranes in mammalian cells and C. elegans. Subsequent application of AMF (40 MHz, 8.4 G) resulted in the opening of the channel and influx of Ca2+ ions [55]. Following this study, Chen et al. showed the feasibility of activating TRPV1 by magnetothermal stimulation in a mouse model with overexpressed TRPV1 in the ventral tegmental area (VTA) [56]. The mice were injected with MNPs into the VTA and were subsequently exposed to AMF (500 kHz, 15 kA/m). This resulted in a higher number of c-fos positive cells in the VTA and its downstream regions of medial prefrontal cortex (mPFC) and nucleus accumbens (NAc). Taking this further, Munshi et al. reported the first successful use of magnetothermal genetic stimulation to remotely activate distinct brain regions in awake and freely moving mice [57]. In the study, the TRPV1-overexpressing mice were injected with MNPs into the cortex area and applied with AMF (570 kHz, 7.5 kA/m). The mice stimulated in the motor cortex exhibited increased locomotion and running behavior, while those stimulated in the area between dorsal and ventral striatum exhibited a freezing of gait behavior.
In addition to exogenously administered MNPs, endogenously expressed protein ferritin and its magnetic properties have also been harnessed for magnetothermal activation of neurons. In 2016, two groups reported the successful use of genetic coupling of the endogenous iron-binding protein ferritin to heat-sensitive ion channels for neural stimulation. Stanley et al. demonstrated the remote activation of neuronal activity by applying magnetic fields in mice with Cre-dependent expression of a ferritin fusion protein tethered to the TRPV1 in glucose-sensing neurons [58]. When the mice were exposed to AMF at 465 kHz, a spike in plasma glucose was observed. In another study using the mice expressing ferritin fused to TRPV4 in the striatal dopamine receptor1 (SDR1), a reward behavior associated with increased neural firing rates was observed when the mice were exposed to a slowly varying magnetic field of 0.1 Hz at 50–250 mT [59].
Taken together, these findings demonstrate the feasibility of nanoparticle-mediated magnetic hyperthermia in modulating the activity of specific neural circuits, their downstream regions, and related behavioral and motor effects.
3. Applications of magnetic hyperthermia in the treatment of neurological diseases
Magnetic hyperthermia is being increasingly explored as a potential alternative to conventional pharmacological approaches for treating neurological diseases including GBM, PD, and AD by exerting various biological effects (Figure 2). In GBM, magnetic hyperthermia has shown promise for its ability to target tumors by conferring synergistic effects with standard treatments such as chemotherapy and radiation. For AD, magnetic hyperthermia has been explored to target Aβ plaques as well as to stimulate microglial activity toward the Aβ plaques. The application of magnetic hyperthermia in PD has primarily focused on modulating neuronal activity. This section highlights current progress of the application of magnetic hyperthermia in the treatment of GBM, PD, and AD. The advantages and limitations of magnetic hyperthermia are compared with different noninvasive neuromodulation techniques in Table 2.
Figure 2.
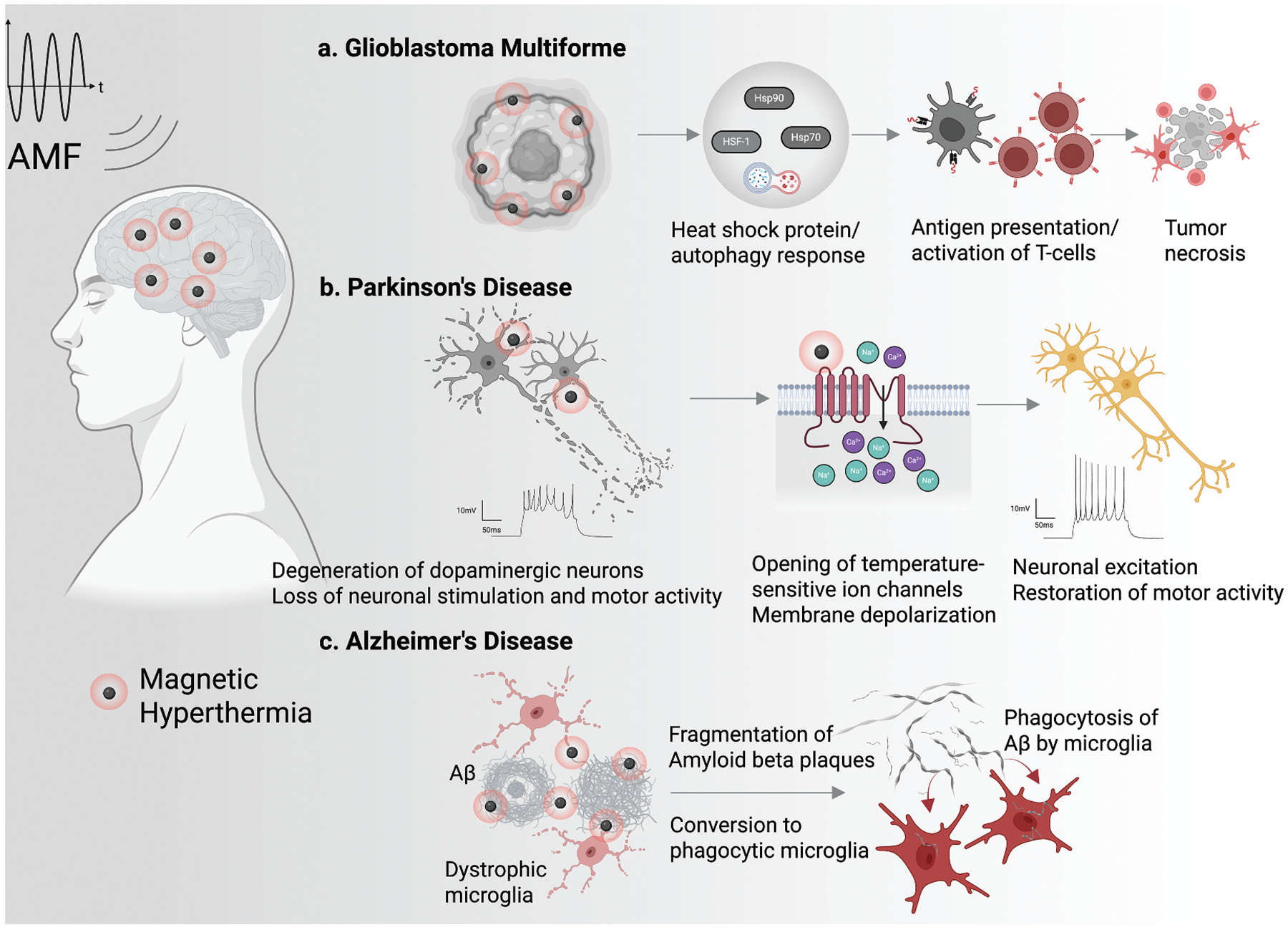
Applications of magnetic hyperthermia in the treatment of neurological disorders including GBM, PD, and AD. Magnetic hyperthermia has translational implications for the treatment of GBM, PD, and AD by eliciting various biological responses. a. in GBM, magnetic hyperthermia treatment confers therapeutic effects by sensitizing the effectiveness of standard treatments such as chemotherapy and radiation by triggering anti-tumor immune responses. b. Magnetic hyperthermia has potential for remotely modulating neuronal activities through the targeted activation of heat-sensitive ion channels such as TRPV1, which can be developed for the treatment of PD by achieving DBS noninvasively. c. Magnetic hyperthermia confers Aβ clearance effects through dual functions involving the direct fragmentation of Aβ plaques as well as enhancement of microglial phagocytic activity, potentially mediated by HSP signaling, toward facilitating the clearance of Aβ plaques. This can be developed as a potential non-pharmacological anti-Aβ therapy for treating AD. Created in BioRender. Naveed, M. (2025) https://biorender.com/bgssimq.
Table 2.
Comparison of different noninvasive neuromodulation techniques.
| Transcranial magnetic stimulation (TMS) | Transcranial direct current stimulation (tDCS) | Focused ultrasound (fUS) | Optogenetics | Magnetic hyperthermia/Magnetothermal Stimulation | References | |
|---|---|---|---|---|---|---|
| Spatial resolution | Low | Low | High (sub-millimeter) | High | High | [60,61] |
| Reversibility | Reversible | Reversible | Reversible | Limited | Reversible (based on treatment length and intensity) | [60–63] |
| Safety | Safe | Safe | Safe | Undetermined | Safe for short and mild hyperthermia treatment, but the long-term effect remains to be determined | [63,64] |
| Status of clinical translation | Approved for psychological disorders (depression, OCD) | Not yet approved | Approved for multiple applications, including tremors in PD | Not yet approved | Approved for GBM | [33,61] |
| Penetration across biological tissue | Low (2–4 cm) | Low | High (up to 20 cm, depending on frequency) | Low (order of millimeters) | High (depending on frequency) | [60,61] |
| Direct disease modifying effect | Undetermined (offer symptomatic relief) | Undetermined | Tumor ablation, transient opening of BBB | Undetermined | Tumor ablation, amyloid disruption | [44,65–70] |
| Adverse/off-target effects | Moderate (discomfort, headaches) | Low (itching, tingling) | Mild to moderate (nausea, visual and locomotor disturbances, facial weakness) | Moderate | Undetermined | [61,63,64] |
3.1. Glioblastoma Multiforme
3.1.1. Preclinical studies
GBM is classified as a level IV glioma by the World Health Organization. Owing to its invasive nature, it is usually not possible to surgically resect 100% of the tumor mass. Therefore, the current treatment of GBM relies primarily on adjuvant chemoradiation therapy (CRT). However, the current standard-of-care treatment of GBM is largely resistant to CRT and therefore has limited success in improving patient prognosis.
Magnetic hyperthermia therapy was shown to be effective as an adjuvant to CRT against GBM in preclinical studies. The application of magnetic hyperthermia could successfully sensitize GBM to CRT in a study using athymic nude mice [71]. In the study, MNPs were injected into the tumors using convection-enhanced delivery (CED) and the mice were exposed to AMF (383.5 kHz, 8.28 kA/m) for 20 min. This was associated with increased DNA damage, elevated HSP90, and infiltration of microglia and CD4+ T-cells in tumors.
Magnetic hyperthermia can exhibit an anti-tumor effect linked to the thermal bystander effect [72]. In a recent study using GBM mouse model by Alphandery et al. complete disappearance of intracranial U87-Luc glioblastoma was observed after 27 AMF sessions over 68 days, where magnetosomes coated with Poly-L lysine (PLL) were used as MNP [73]. In addition to the anti-tumor activity in the area directly heated by magnetic hyperthermia, tumor destruction was also observed outside the heated region, which was attributed to an increased infiltration of polynuclear neutrophils (PMNs).
While most magnetic hyperthermia therapies utilize high-frequency AMF to elicit a hyperthermia response in GBM, it is also possible to induce anti-tumor activity by applying slowly varying magnetic fields which can exert mechanical force onto the cells. In a first-of-its-kind study, Cheng et al. showed that low-frequency AMF could damage the structure of cancer cells in a mouse model of GBM with U87 glioma [74]. In the study, MNPs were intracranially injected into the tumor area and the mice were then exposed to slowly varying magnetic field (20 Hz, 1 T) for 1 h daily for 7 days. The treatment resulted in the reduction of brain tumor size and increase of the survival rate of mice bearing intracranial glioma xenografts.
Taken together, these preclinical studies using nanoparticle-mediated hyperthermia for the treatment of GBM uncover dual mechanisms of direct and indirect anti-tumor activity. Magnetic hyperthermia can exhibit potent anti-tumor activity in GBM by not only exerting direct effects involving the thermo-mechanical destruction of cancer cells but also triggering immune responses that alter the tumor microenvironment to inhibit growth and recurrence.
3.1.2. Clinical studies of magnetic hyperthermia in GBM patients
Clinical trials of magnetic hyperthermia have been conducted in combination with traditional therapies such as radiation therapy and chemotherapy, revealing promising results in improving the prognosis of GBM. In 2007, Maier-Hauff et al. evaluated the feasibility and tolerability of the thermotherapy using MNPs on recurrent Glioblastoma Multiforme, where 14 patients received intratumoral injection of aminosilane coated iron oxide nanoparticles and received 4 to 10 of AMF sessions (100 kHz, 2.5–18 kA/m) for 1 h for each session [65]. The median maximum intratumoral temperature was reported to be 44.6°C with a median CEM43 of 7.7 equivalent minutes. This study demonstrated that deep cranial thermotherapy using MNPs could be safely applied to GBM patients. The follow-up efficacy study in 2011 revealed an increase in survival outcome in GBM patients treated with magnetic hyperthermia in combination with stereotactic radiotherapy [66]. Biocompatible iron oxide nanoparticles were directly injected into the tumor and the patients were treated with six sessions of 1-h AMF exposure (100 kHz, 2–15 kA/m). The side effects of the treatment were observed to be moderate without serious complications.
The latest clinical study using magnetic hyperthermia in GBM was done in 2019, in which six patients with recurrent GBM were treated with magnetic hyperthermia in combination with radiotherapy [44]. In the study, the patients were implanted with a NanoPaste (consisting of iron oxide nanoparticles with a hydroxycellulose mesh and fibrin glue) and then underwent six sessions of 1-h AMF exposure (100 kHz, 2.5–15 kA/m). Histopathology revealed sustained tumor necrosis directly adjacent to aggregated nanoparticles, which was associated with upregulation of HSP70, increased infiltration of macrophages with ingested nanoparticles, increased intracellular ratios of IFN-γ to IL-4 in CD4+ and CD8+ memory T cells, and activation of tumor-associated myeloid cells and microglia. Although there were no immediate side effects during active treatment, patients were observed to develop cerebral edema around nanoparticle deposits after 2–5 months. As of August 2025, there is one ongoing clinical trial using magnetic hyperthermia against recurrent GBM (NCT06271421). The trial is focused toward determining the tolerance and efficacy of NanoTherm system comprising a sterile suspension of superparamagnetic iron oxide nanoparticles and is expected to conclude in February 2027.
Collectively, preclinical and clinical studies suggest that magnetic thermotherapy has the potential to help reprogram the immunosuppressive microenvironment of GBM toward an anti-tumor immune environment. However, given the observed side effects of magnetic thermotherapy, it is critical to carefully determine the optimal thermal dose as well as nanoparticle dose toward maximizing potent anti-tumor immune responses while minimizing unwanted side effects.
3.2. Parkinson’s disease
PD typically affects dopaminergic neurons in the substantia nigra, which results in impaired executive function and language abilities [75]. In view of this, studies on applying magnetic hyperthermia for PD treatment have focused on stimulating target neurons within deep brain structures, especially in the subthalamic nucleus (STN), which is the clinical target of conventional deep brain stimulation (DBS) for treating patients with PD.
The first study of its kind was reported by Hescham et al., in which they demonstrated the feasibility of magnetic hyperthermia as a means for DBS to provide therapeutic benefits in mouse models of PD, induced by injecting 6-OHDA and MPTP in one or both brain hemispheres [76]. In this study, neurons in the STN of the PD mice were first heat-sensitized through lentiviral delivery of TRPV1. The mice were injected with MNPs intracranially into the STN and subsequently exposed to 3 min of AMF (160 kHz, 30 kA/m). The AMF exposure triggered reversible firing of TRPV1-expressing neurons, resulting in the influx of calcium ions, membrane depolarization, and subsequent neural excitation. Indeed, magnetothermal stimulation could reverse the motor deficits in a mild and severe PD model, demonstrating the feasibility of magnetothermal neurostimulation in activating deep-brain circuits without the need for permanently implanted hardware and connectors.
Recently, a magnetogenetic system utilizing a single anti-ferritin nanobody-TRPV1 receptor fusion protein was demonstrated to regulate neuronal activity in a mouse model of PD [77]. The nanobody was designed to specifically bind to ferritin that is responsive to AMF. In the study, lesions were introduced to mimic Parkinsonian phenotypes in mice using 6-OHDA which led to contralateral rotations induced by the dopaminergic agonist, apomorphine. These contralateral rotations were significantly reduced after intracranial injection with the nanobody-TRPV1 receptor construct, followed by the exposure of direct magnetic fields (500 mT to 1.3 T). This was associated with the reduction in neuronal activity in STN and a consequent improvement in motor function.
Collectively, these studies implicate the potential for bidirectional regulation of neuronal activity by targeted magnetic hyperthermia stimulation. It is possible to create a system that can either activate or inhibit neuronal activity by modifying heat-sensitive ion channels including TRPV1 receptor, showing the potential of magnetic hyperthermia for therapeutic applications for PD. However, PD mouse models that rely on the overexpression of TRPV channels or magnetogenetic models of PD are not fully physiologically relevant for therapeutic development, though they can be useful for studying specific disease mechanisms or proof-of-concept studies. The main limitation is that forced, non-endogenous expression does not accurately mimic the complex, progressive nature of human PD. It also remains to be elucidated about the risk of off-target activation of other temperature-sensitive or mechanical-sensitive proteins.
3.3. Alzheimer’s disease
While traditional treatments for PD have focused on localized stimulation of target neurons, such as with DBS, AD treatment has largely focused on developing disease modifying therapies that target the underlying disease processes, given that AD affects multiple brain regions through the deposition of extracellular Aβ plaques. This makes targeted neurostimulation less impactful for AD treatment. It is, therefore, no surprise that magnetic hyperthermia has found application in AD treatment focusing on reducing Aβ burdens.
Loynachan et al. firstly reported the application of magnetic hyperthermia as a means for disaggregating Aβ aggregates [67]. Aβ fibrils were incubated with iron oxide nanoparticles functionalized with Aβ-targeting peptide and applied with AMF (100 kHz, 28 kA/m) for 3 h, resulting in the disaggregation of Aβ. Cytotoxicity studies in primary hippocampal neurons revealed lowered toxicity in the fragmented-Aβ compared to non-disaggregated Aβ, suggesting that magnetic hyperthermia-induced fragmentation of Aβ fibrils could ameliorate Aβ induced toxicity. In a subsequent study, Du et al. reported an Aβ oligomer-specific “sense-and-treat” MNP system that not only recognizes Aβ oligomers but also disassembles them under AMF exposure [68]. The MNPs were decorated with a fluorescent probe Naphthalimide (NFP) and functionalized with an Aβ-targeting peptide. Upon 2 h of exposure to AMF (150 kHz, 30 kA/m), the Aβ aggregates were shown to be disaggregated, along with their reduced cytotoxicity against neuronal cell lines. In contrast to the earlier studies that applied magnetic hyperthermia at higher thermal doses, Dyne et al. reported the feasibility of mild magnetic hyperthermia, applied within the safe thermal dose for brain tissue, in disaggregating Aβ aggregates [69]. This study demonstrated that the application of magnetic hyperthermia at a mild thermal dose (CEM43 thermal dose of 0.55 min) was sufficient to disrupt Aβ plaques into smaller fragments via CEM43 thermal dose-dependent manner (Figure 3).
Figure 3.

Effects of magnetic hyperthermia on the fragmentation of Aβ plaques. Representative scanning transmission electron microscope (STEM) images of Aβ for varying CEM43 thermal dose of magnetic hyperthermia. The application of magnetic hyperthermia resulted in the fragmentation of Aβ aggregates even at a lower thermal dose (CEM43 = 0.55 min) and the extent of Aβ fragmentation increased with an increase of thermal dose. This implicates the feasibility of mild magnetic hyperthermia as a strategy for anti-Aβ therapy. Adapted from [69] with permission. Copyright 2021, Elsevier.
Despite the potency of magnetic hyperthermia in disaggregating Aβ aggregates, one important consideration is to characterize the fate of Aβ plaque fragmented by magnetic hyperthermia. Small, soluble oligomers of Aβ are known to be highly toxic and are closely associated with AD pathology [70]. It is therefore important to investigate the structural and functional nature of fragmented plaques whether the fragmented Aβ turns into toxic intermediates and thereby exacerbates amyloid burden by giving rise to diffusible amyloidogenic seeds.
Importantly, in addition to the ability of magnetic hyperthermia in directly fragmenting Aβ plaques, the application of magnetic hyperthermia could also skew microglia toward enhanced phagocytic phenotype associated with the increased expression of HSPs in the cell culture model using human microglia cell line [69]. The effect of magnetic hyperthermia on modulating microglial activities was further validated in vivo [78]. In this study, C57BL/6 J mice were injected with MNPs via stereotaxic injection into the dentate gyrus of the hippocampus and subsequently stimulated with AMF (375 kHz, 68 kA/m) for 10 min, in which the thermal dose applied in the hippocampus was tuned by altering the amount of injected MNPs. The findings showed the thermal dose-dependent increase in the infiltration of microglia as well as their morphological changes from resting and ramified into activated and amoeboid morphology (Figure 4). This was associated with increased expression of HSP70 and autophagic marker LC3II. These studies implicate the feasibility of developing mild magnetic hyperthermia as a strategy for localized stimulation of brain. Particularly, the ability of magnetic hyperthermia in activating autophagic signaling can be harnessed as a means for facilitating microglia-dependent Aβ clearance given that AD is associated with impaired autophagic response for Aβ clearance, leading to AD pathology [46].
Figure 4.

Effects of magnetic hyperthermia on modulating microglial activation in the hippocampus of mice. (a) Representative images showing the infiltration of microglia (iba-1 positive cells, green) in the hippocampus of C57BL/6 mice stimulated with magnetic hyperthermia for varying thermal doses. (b) Representative images showing the morphological change of microglia in the hippocampus of C57BL/6 mice stimulated with magnetic hyperthermia for varying thermal doses. The extent of magnetic hyperthermia-induced thermal dose was increased by increasing the dose of MNPs injected into the hippocampus from 1 to 10 μg, followed by AMF stimulation. PBS injection was used as a vehicle control. The findings show the potential for using mild magnetic hyperthermia as a strategy for localized stimulation of brain for modulating microglia functions. Adapted from [78] with permission. Copyright 2025, Springer Nature.
4. Future perspectives
Beyond its traditional application in thermal therapies, primarily for cancer treatment, the use of magnetic hyperthermia treatment as a new strategy for neurostimulation has recently received considerable attention. However, this approach, while holding promise for potential for neurological disease treatment, still faces several challenges in clinical translation.
Firstly, the delivery of MNPs to specific brain regions for magnetic hyperthermia therapy currently relies on invasive methods such as burr hole surgery or craniotomies since this approach enables precise delivery of MNPs to the target brain regions. However, these methods carry the risk of neurovascular injury, CSF leaks, hemorrhages, and bacterial infections [79,80]. As a result, low patient compliance with intracranial injections has been shown to be a significant obstacle in treating various neurological conditions [81]. Developing minimally invasive techniques for MNP delivery is crucial to minimizing risks and improving patient outcomes. The local delivery of MNPs is still essential for magnetic hyperthermia therapy for GBM and PD since this approach allows for focused heating of the target areas such as tumor in GBM or dopaminergic neurons in the STN of the brain’s midbrain region in PD, without affecting surrounding healthy tissues. AD, on the other hand, is characterized by Aβ plaques and hyperphosphorylated tau neurofibrillary tangles that are widely distributed across the brain. Delivering MNPs precisely to these areas while avoiding off-target effects remains a significant challenge. The clinical translation of magnetic hyperthermia therefore necessitates successful delivery of MNPs systemically through the bloodstream to reach areas where the Aβ plaques are deposited. However, the BBB presents a major challenge in delivering therapeutic agents, including MNPs, to the brain. To overcome this challenge, MNPs can be modified to enhance their ability to cross the BBB by functionalizing them with molecules that utilize receptor-mediated BBB transport. For instance, in a recent study, Barker et al. demonstrated that the engineered transport vehicles with a human transferrin receptor binding molecule could effectively deliver payload across the BBB after intravenous administration in rodent and primate models [82]. Because receptor-mediated transport can enable nanoparticles to cross the BBB and potentially access the deeper layers of the CNS, and AMF is virtually unobstructed by biological tissue in penetration depth, the combination of both technologies can be harnessed toward overcoming the challenge of targeted delivery of MNPs, especially in disease like AD.
Secondly, the possibility of off-target heating of MNPs in locations other than the target area poses a risk of damaging healthy brain tissue, which necessitates precise monitoring of temperature during magnetic hyperthermia treatment. However, the lack of real-time, in vivo monitoring of hyperthermia in the target tissue is a significant limitation in current MNP-based hyperthermia approaches. To optimize therapeutic outcomes, real-time monitoring is crucial to ensure appropriate temperature elevations within the threshold of a safe thermal dose and to minimize heat-related damage to surrounding healthy tissue. While magnetic resonance imaging (MRI) thermometry allows real-time temperature monitoring for many thermal therapy techniques, the high static magnetic field of an MRI scanner makes it incompatible with magnetic hyperthermia by interfering with heat generation by MNPs upon exposure to AMF [83]. New temperature monitoring techniques such as magnetic particle imaging (MPI), ultrasound thermometry (UST), and luminescent nanothermometry (LNT) are actively being investigated, which can be further developed to be coupled with magnetic hyperthermia system to enable real-time temperature monitoring during the application of magnetic hyperthermia [84–86].
Thirdly, while promising results have been reported in GBM through numerous preclinical and clinical studies, the application of magnetic hyperthermia in AD and PD is still in early stages, facing several challenges for clinical translation. Although recent studies have contributed to elucidating the biological effects of magnetic hyperthermia at the molecular and cellular levels, the precise mechanisms by which magnetic hyperthermia can exert its effects on AD and PD are not yet fully understood and more extensive research is needed.
Lastly, regulatory issues concerning the safety of magnetic hyperthermia need to be addressed. CNS applications of hyperthermia therapies face a translational bottleneck owing to the brain’s high sensitivity to thermal damage that can cause the risk of damaging surrounding healthy neural tissue, warranting increased regulatory scrutiny for clinical approval. The FDA guidelines recommend tissue heating at 43°C for a short duration alongside real-time monitoring of the time-temperature history using probes or imaging (FDA-2024-D-0664). The EU has similar but less clear requirements, including that the target and surrounding tissue should not reach dangerous temperatures (EU 2017–745). It should be noted that these guidelines and recommendations are for biological tissue in general and the criteria are expected to be more stringent for CNS applications due to the high vulnerability of brain tissue to thermal damage. In addition to the short-term effects involving acute tissue damage and heat distribution, the long-term efficacy and risks of magnetic hyperthermia therapy on the brain tissue need to be understood. Additionally, the lack of standardized protocols for magnetic hyperthermia application in neurodegenerative diseases poses challenges in translating research findings to clinical practice. The parameters of magnetic hyperthermia treatment (e.g., nanoparticle dosage, field strength and frequency, treatment duration) need to be standardized to compare results across different preclinical and clinical studies [87,88].
5. Conclusion
Despite recent advances in disease-modifying treatments, pharmacological approaches for neurological disorders still face limitations due to the complexity of these diseases and the challenges in targeting the underlying mechanisms. Nanoparticle-mediated magnetic hyperthermia holds great promise in the treatment of neurological diseases due to its ability to exert multi-faceted effects not only through the direct destruction of tumors and protein aggregates such as toxic amyloid plaques but also through the modulation of immune responses and neuronal activities. However, its translation into clinical practice is still hindered by several hurdles, which include the challenge of delivering MNPs specifically to target brain regions, the need for accurate and real-time temperature monitoring during magnetic hyperthermia, and the need for deeper understanding of how magnetic hyperthermia exerts its therapeutic effects on the pathological conditions of AD and PD. Overcoming these hurdles is crucial for successful implementation of magnetic hyperthermia therapy for treating neurological disorders.
Article highlights.
This review highlights the current understanding of magnetic hyperthermia in stimulating molecular and cellular effects on brain tissue and further discusses its potential in the treatment of neurological disorders.
Nanoparticle-mediated magnetic hyperthermia is a minimally invasive treatment approach that utilizes magnetic nanoparticles (MNPs) to generate localized heat in target cells and tissues by responding to an alternating magnetic field (AMF).
Magnetic hyperthermia exerts biological effects on the brain tissue by increasing heat shock protein expression, activating innate and adaptive immune responses, and modulating neural activities by activating heat-sensitive ion channels
Magnetic hyperthermia promotes antitumor activity against GBM by reprograming the immunosuppressive microenvironment of GBM toward an antitumor immune environment.
Magnetic hyperthermia can exert therapeutic effects on PD by selectively activating heat-sensitive ion channels in neurons, particularly TRPV1. This activation leads to calcium influx, membrane depolarization, and ultimately, neural excitation, offering a potential therapeutic approach for PD.
Magnetic hyperthermia can exert therapeutic effects on AD by not only fragmenting Aβ plaques but also skewing microglia toward enhanced phagocytic phenotype associated with the increased expression of HSPs.
The translation of magnetic hyperthermia into clinical practice is still hindered by several hurdles, which include the challenge of delivering MNPs specifically to target brain regions, the need for accurate and real-time temperature monitoring during magnetic hyperthermia, and a deeper understanding of how magnetic hyperthermia exerts its therapeutic effects on the pathological conditions of AD and PD.
Funding
This manuscript was funded by the National Institutes of Health under [R01 AG076699] grant. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Disclosure statement
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Gooch CL, Pracht E, Borenstein AR. The burden of neurological disease in the United States: a summary report and call to action. Ann Neurol. 2017;81(4):479–484. doi: 10.1002/ana.24897 [DOI] [PubMed] [Google Scholar]
- 2.Ostrom QT, Gittleman H, Farah P, et al. Cbtrus statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 2013;15(suppl 2):ii1–ii56. doi: 10.1093/neuonc/not151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Price M, Ballard C, Benedetti J, et al. Cbtrus statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2017–2021. Neuro Oncol. 2024;26 (Supplement_6):vi1–vi85. doi: 10.1093/neuonc/noae145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med. 2016;8(6):595–608. doi: 10.15252/emmm.201606210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256(5054):184–185. doi: 10.1126/science.1566067 [DOI] [PubMed] [Google Scholar]
- 6.Rajan KB, Weuve J, Barnes LL, et al. Population estimate of people with clinical Alzheimer’s disease and mild cognitive impairment in the United States (2020–2060). Alzheimer’s Dementia. 2021;17 (12):1966–1975. doi: 10.1002/alz.12362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alzheimer’s Association. 2024 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia. 2024;20(5):3708–3821. doi: 10.1002/alz.13809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386 (9996):896–912. doi: 10.1016/S0140-6736(14)61393-3 [DOI] [PubMed] [Google Scholar]
- 9.Szeto JYY, Lewis SJG. Current treatment options for Alzheimer’s disease and Parkinson’s disease dementia. CN. 2016;14(4):326–338. doi: 10.2174/1570159X14666151208112754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao X Editorial: disease-modifying targets and strategies for Alzheimer’s disease and Parkinson’s disease. Front Aging Neurosci. 2023;15:1247256. doi: 10.3389/fnagi.2023.1247256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandes C, Costa A, Osório L, et al. Current standards of care in glioblastoma therapy. In: De Vleeschouwer S, editor. Glioblastoma. Brisbane (AU): Codon Publications; 2017. p. 197–241. doi: 10.15586/codon.glioblastoma.2017 [DOI] [PubMed] [Google Scholar]
- 12.Cummings J, Zhou Y, Lee G, et al. Alzheimer’s disease drug development pipeline: 2023. A&D Transl Res Clin Interv. 2023;9(2): e12385. doi: 10.1002/trc2.12385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim B-H, Kim S, Nam Y, et al. Second-generation anti-amyloid monoclonal antibodies for Alzheimer’s disease: current landscape and future perspectives. Transl Neurodegener. 2025;14(1):6. doi: 10.1186/s40035-025-00465-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foltynie T, Bruno V, Fox S, et al. Medical, surgical, and physical treatments for Parkinson’s disease. Lancet. 2024;403 (10423):305–324. doi: 10.1016/S0140-6736(23)01429-0 [DOI] [PubMed] [Google Scholar]
- 15.Bastiancich C, Da Silva A, Estève M-A. Photothermal therapy for the treatment of glioblastoma: potential and preclinical challenges. Front Oncol. 2021;10:610356. doi: 10.3389/fonc.2020.610356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cramer SW, Chen CC. Photodynamic therapy for the treatment of glioblastoma. Front Surg. 2020;6:81. doi: 10.3389/fsurg.2019.00081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Öztürk B, Demir H, Günay MS, et al. Photodynamic and photothermal therapies using nanotechnology approach in Alzheimer’s disease. Curr Neuropharmacol. 2025;23(14):1985–2003. doi: 10.2174/011570159X370790250317045223 [DOI] [PubMed] [Google Scholar]
- 18.Liang K, Yang L, Kang J, et al. Improving treatment for Parkinson’s disease: harnessing photothermal and phagocytosis-driven delivery of levodopa nanocarriers across the blood-brain barrier. Asian J Pharm Sci. 2024;19(6):100963. doi: 10.1016/j.ajps.2024.100963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li T, Liu Y, Bao W, et al. Synergistic photothermal and chemical therapy by smart dual-functional graphdiyne nanosheets for treatment of Parkinson’s disease. Adv Ther. 2021;4(7):2100082. doi: 10.1002/adtp.202100082 [DOI] [Google Scholar]
- 20.Wu J, Cui X, Bao L, et al. A nanoparticle-based wireless deep brain stimulation system that reverses Parkinson’s disease. Sci Adv. 2025;11(3):eado4927. doi: 10.1126/sciadv.ado4927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meglinski IV, Matcher SJ. Quantitative assessment of skin layers absorption and skin reflectance spectra simulation in the visible and near-infrared spectral regions. Physiol Meas. 2002;23 (4):741–753. doi: 10.1088/0967-3334/23/4/312 [DOI] [PubMed] [Google Scholar]
- 22.Ni Z, Chen R. Transcranial magnetic stimulation to understand pathophysiology and as potential treatment for neurodegenerative diseases. Transl Neurodegener. 2015;4(1):22. doi: 10.1186/s40035-015-0045-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cammisuli DM, Cignoni F, Ceravolo R, et al. Transcranial direct current stimulation (tDCS) as a useful rehabilitation strategy to improve cognition in patients with Alzheimer’s disease and Parkinson’s disease: an updated systematic review of randomized controlled trials. Front Neurol. 2022;12:798191. doi: 10.3389/fneur.2021.798191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Périgo EA, Hemery G, Sandre O, et al. Fundamentals and advances in magnetic hyperthermia. Appl Phys Rev. 2015;2(4):041302. doi: 10.1063/1.4935688 [DOI] [Google Scholar]
- 25.Christiansen MG, Senko AW, Anikeeva P. Magnetic strategies for nervous system control. Annu Rev Neurosci. 2019;42(1):271–293. doi: 10.1146/annurev-neuro-070918-050241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meng YQ, Shi YN, Zhu YP, et al. Recent trends in preparation and biomedical applications of iron oxide nanoparticles. J Nanobiotechnol. 2024;22(1):24. doi: 10.1186/s12951-023-02235-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu M, Cohen MH, Rieves D, et al. Fda report: ferumoxytol for intravenous iron therapy in adult patients with chronic kidney disease. Am J Hematol. 2010;85(5):315–319. doi: 10.1002/ajh.21656 [DOI] [PubMed] [Google Scholar]
- 28.Amstad E, Textor M, Reimhult E. Stabilization and functionalization of iron oxide nanoparticles for biomedical applications. Nanoscale. 2011;3(7):2819. doi: 10.1039/c1nr10173k [DOI] [PubMed] [Google Scholar]
- 29.Albukhaty S, Sulaiman GM, Al-Karagoly H, et al. Iron oxide nanoparticles: the versatility of the magnetic and functionalized nanomaterials in targeting drugs, and gene deliveries with effectual magnetofection. J Drug Delivery Sci Technol. 2024;99:105838. doi: 10.1016/j.jddst.2024.105838 [DOI] [Google Scholar]
- 30.Feng Q, Liu Y, Huang J, et al. Uptake, distribution, clearance, and toxicity of iron oxide nanoparticles with different sizes and coatings. Sci Rep. 2018;8(1):2082. doi: 10.1038/s41598-018-19628-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodriguez B, Rivera D, Zhang JY, et al. Magnetic hyperthermia therapy for high-grade glioma: a state-of-the-art review. Pharmaceuticals. 2024;17(3):300. doi: 10.3390/ph17030300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang X, Kubican SE, Yi Z, et al. Advances in magnetic nanoparticles for molecular medicine. Chem Commun. 2025;61(15):3093–3108. doi: 10.1039/D4CC05167J [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahmoudi K, Bouras A, Bozec D, et al. Magnetic hyperthermia therapy for the treatment of glioblastoma: a review of the therapy’s history, efficacy and application in humans. Int J Hyperthermia. 2018;34(8):1316–1328. doi: 10.1080/02656736.2018.1430867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Obaidat IM, Narayanaswamy V, Alaabed S, et al. Principles of magnetic hyperthermia: a focus on using multifunctional hybrid magnetic nanoparticles. Magnetochemistry. 2019;5(4):67. doi: 10.3390/magnetochemistry5040067 [DOI] [Google Scholar]
- 35.Romero G, Park J, Koehler F, et al. Modulating cell signalling in vivo with magnetic nanotransducers. Nat Rev Methods Primers. 2022;2 (1):92. doi: 10.1038/s43586-022-00170-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moon J, Christiansen MG, Rao S, et al. Magnetothermal multiplexing for selective remote control of cell signaling. Adv Funct Mater [Internet]. 2020. [cited 2025 Apr 30];30(36). doi: 10.1002/adfm.202000577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sebesta C, Torres Hinojosa D, Wang B, et al. Subsecond multichannel magnetic control of select neural circuits in freely moving flies. Nat Mater. 2022;21(8):951–958. doi: 10.1038/s41563-022-01281-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Rhoon GC, Samaras T, Yarmolenko PS, et al. CEM43°C thermal dose thresholds: a potential guide for magnetic resonance radio-frequency exposure levels? Eur Radiol. 2013;23(8):2215–2227. doi: 10.1007/s00330-013-2825-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carter TJ, Agliardi G, Lin F, et al. Potential of magnetic hyperthermia to stimulate localized immune activation. Small. 2021;17 (14):2005241. doi: 10.1002/smll.202005241 [DOI] [PubMed] [Google Scholar]
- 40.Vabulas RM, Raychaudhuri S, Hayer-Hartl M, et al. Protein folding in the cytoplasm and the heat shock response. Cold Spring Harb Perspect Biol. 2010;2(12):a004390–a004390. doi: 10.1101/cshperspect.a004390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiao W, Dai L, Yan B, et al. Heating up the immune battle: magnetic hyperthermia against cancer. Fundamental Res. 2024: S2667325824003480. doi: 10.1016/j.fmre.2024.08.006 [DOI] [Google Scholar]
- 42.Hu C, Yang J, Qi Z, et al. Heat shock proteins: biological functions, pathological roles, and therapeutic opportunities. MedComm. 2022;3(3):e161. doi: 10.1002/mco2.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shevtsov M, Multhoff G. Heat shock protein–peptide and HSP-based immunotherapies for the treatment of cancer. Front Immunol [Internet]. 2016. [cited 2025 May 14];7. doi: 10.3389/fimmu.2016.00171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grauer O, Jaber M, Hess K, et al. Combined intracavitary thermotherapy with iron oxide nanoparticles and radiotherapy as local treatment modality in recurrent glioblastoma patients. J Neurooncol. 2019;141(1):83–94. doi: 10.1007/s11060-018-03005-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krishnamurthy K, Vedam K, Kanagasabai R, et al. Heat shock factor-1 knockout induces multidrug resistance gene, MDR1b, and enhances P-glycoprotein (ABCB1)-based drug extrusion in the heart. Proc Natl Acad Sci USA. 2012;109(23):9023–9028. doi: 10.1073/pnas.1200731109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCormick JJ, Dokladny K, Moseley PL, et al. Autophagy and heat: a potential role for heat therapy to improve autophagic function in health and disease. J Appl Physiol. 2021;130(1):1–9. doi: 10.1152/japplphysiol.00542.2020 [DOI] [PubMed] [Google Scholar]
- 47.Zhang B, Qi R. The dual-function of HSP70 in immune response and tumor immunity: from molecular regulation to therapeutic innovations. Front Immunol. 2025;16:1587414. doi: 10.3389/fimmu.2025.1587414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y, Calderwood SK. Autophagy, protein aggregation and hyperthermia: a mini-review. Int J Hyperthermia. 2011;27 (5):409–414. doi: 10.3109/02656736.2011.552087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vangijzegem T, Lecomte V, Ternad I, et al. Superparamagnetic iron oxide nanoparticles (SPION): from fundamentals to state-of-the-art innovative applications for cancer therapy. Pharmaceutics. 2023;15 (1):236. doi: 10.3390/pharmaceutics15010236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nascimento CS, Alves ÉAR, De Melo CP, et al. Immunotherapy for cancer: effects of iron oxide nanoparticles on polarization of tumor-associated macrophages. Nanomed (Lond). 2021;16 (29):2633–2650. doi: 10.2217/nnm-2021-0255 [DOI] [PubMed] [Google Scholar]
- 51.Liu X, Yan B, Li Y, et al. Graphene oxide-grafted magnetic nanorings mediated magnetothermodynamic therapy favoring reactive oxygen species-related immune response for enhanced antitumor efficacy. ACS Nano. 2020;14(2):1936–1950. doi: 10.1021/acsnano.9b08320 [DOI] [PubMed] [Google Scholar]
- 52.Huynh KW, Cohen MR, Chakrapani S, et al. Structural insight into the assembly of TRPV channels. Structure. 2014;22(2):260–268. doi: 10.1016/j.str.2013.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang M, Ma Y, Ye X, et al. Trp (transient receptor potential) ion channel family: structures, biological functions and therapeutic interventions for diseases. Sig Transduct Target Ther. 2023;8 (1):261. doi: 10.1038/s41392-023-01464-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Poletini MO, Moraes MN, Ramos BC, et al. Trp channels: a missing bond in the entrainment mechanism of peripheral clocks throughout evolution. Temperature. 2015;2(4):522–534. doi: 10.1080/23328940.2015.1115803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang H, Delikanli S, Zeng H, et al. Remote control of ion channels and neurons through magnetic-field heating of nanoparticles. Nat Nanotechnol. 2010;5(8):602–606. doi: 10.1038/nnano.2010.125 [DOI] [PubMed] [Google Scholar]; •• This is the first study reporting the application of magnetothermal stimulation in neurons in vivo.
- 56.Chen R, Romero G, Christiansen MG, et al. Wireless magnetothermal deep brain stimulation. Science. 2015;347(6229):1477–1480. doi: 10.1126/science.1261821 [DOI] [PubMed] [Google Scholar]
- 57.Munshi R, Qadri SM, Zhang Q, et al. Magnetothermal genetic deep brain stimulation of motor behaviors in awake, freely moving mice. Svoboda K, editor. Elife. 2017;6:e27069. doi: 10.7554/eLife.27069 [DOI] [PMC free article] [PubMed] [Google Scholar]; • This study reported the feasibility of magnetothermal stimulation in modulating behavioral and locomotor changes in freely moving rodents.
- 58.Stanley SA, Kelly L, Latcha KN, et al. Bidirectional electromagnetic control of the hypothalamus regulates feeding and metabolism. Nature. 2016;531(7596):647–650. doi: 10.1038/nature17183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wheeler MA, Smith CJ, Ottolini M, et al. Genetically targeted magnetic control of the nervous system. Nat Neurosci. 2016;19 (5):756–761. doi: 10.1038/nn.4265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu X, Qiu F, Hou L, et al. Review of noninvasive or minimally invasive deep brain stimulation. Front Behav Neurosci. 2022;15:820017. doi: 10.3389/fnbeh.2021.820017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mahoney JJ, Hanlon CA, Marshalek PJ, et al. Transcranial magnetic stimulation, deep brain stimulation, and other forms of neuromodulation for substance use disorders: review of modalities and implications for treatment. J Neurol Sci. 2020;418:117149. doi: 10.1016/j.jns.2020.117149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou D, Li X, Wei S, et al. Transcranial direct current stimulation combined with repetitive transcranial magnetic stimulation for depression: a randomized clinical trial. JAMA Netw Open. 2024;7 (11):e2444306. doi: 10.1001/jamanetworkopen.2024.44306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roet M, Hescham S-A, Jahanshahi A, et al. Progress in neuromodulation of the brain: a role for magnetic nanoparticles? Prog NeuroBiol. 2019;177:1–14. doi: 10.1016/j.pneurobio.2019.03.002 [DOI] [PubMed] [Google Scholar]
- 64.Zewdie E, Ciechanski P, Kuo HC, et al. Safety and tolerability of transcranial magnetic and direct current stimulation in children: prospective single center evidence from 3.5 million stimulations. Brain Stimul. 2020;13(3):565–575. doi: 10.1016/j.brs.2019.12.025 [DOI] [PubMed] [Google Scholar]
- 65.Maier-Hauff K, Rothe R, Scholz R, et al. Intracranial thermotherapy using magnetic nanoparticles combined with external beam radiotherapy: results of a feasibility study on patients with glioblastoma multiforme. J Neurooncol. 2007;81(1):53–60. doi: 10.1007/s11060-006-9195-0 [DOI] [PubMed] [Google Scholar]; •• This is the first clinical study using magnetic hyperthermia for the treatment of Glioblastoma patients.
- 66.Maier-Hauff K, Ulrich F, Nestler D, et al. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J Neurooncol. 2011;103 (2):317–324. doi: 10.1007/s11060-010-0389-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Loynachan CN, Romero G, Christiansen MG, et al. Targeted magnetic nanoparticles for remote magnetothermal disruption of amyloid-β aggregates. Adv Healthcare Mater. 2015;4(14):2100–2109. doi: 10.1002/adhm.201500487 [DOI] [PubMed] [Google Scholar]; • This is the first study that demonstrated the feasibility of magnetic hyperthermia as a means to disaggregate amyloid-beta plaques.
- 68.Du Z, Gao N, Guan Y, et al. Rational design of a “sense and treat” system to target amyloid aggregates related to Alzheimer’s disease. Nano Res. 2018;11(4):1987–1997. doi: 10.1007/s12274-017-1815-9 [DOI] [Google Scholar]
- 69.Dyne E, Prakash PS, Li J, et al. Mild magnetic nanoparticle hyperthermia promotes the disaggregation and microglia-mediated clearance of beta-amyloid plaques. Nanomed: Nanotechnol Biol Med. 2021;34:102397. doi: 10.1016/j.nano.2021.102397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sengupta U, Nilson AN, Kayed R. The role of amyloid-β oligomers in toxicity, propagation, and immunotherapy. EBioMedicine. 2016;6:42–49. doi: 10.1016/j.ebiom.2016.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rivera D, Bouras A, Mattioli M, et al. Magnetic hyperthermia therapy enhances the chemoradiosensitivity of glioblastoma. Sci Rep. 2025;15(1):10532. doi: 10.1038/s41598-025-95544-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang R, Zhou T, Liu W, et al. Molecular mechanism of bystander effects and related abscopal/cohort effects in cancer therapy. Oncotarget. 2018;9(26):18637–18647. doi: 10.18632/oncotarget.24746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alphandéry E, Idbaih A, Adam C, et al. Development of non-pyrogenic magnetosome minerals coated with poly-l-lysine leading to full disappearance of intracranial U87-luc glioblastoma in 100% of treated mice using magnetic hyperthermia. Biomaterials. 2017;141:210–222. doi: 10.1016/j.biomaterials.2017.06.026 [DOI] [PubMed] [Google Scholar]
- 74.Cheng Y, Muroski ME, Petit DCMC, et al. Rotating magnetic field induced oscillation of magnetic particles for in vivo mechanical destruction of malignant glioma. J Control Release. 2016;223:75–84. doi: 10.1016/j.jconrel.2015.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sanches C, Stengel C, Godard J, et al. Past, present, and future of non-invasive brain stimulation approaches to treat cognitive impairment in neurodegenerative diseases: time for a comprehensive critical review. Front Aging Neurosci [Internet]. 2021;12:12–2020. doi: 10.3389/fnagi.2020.578339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hescham S-A, Chiang P-H, Gregurec D, et al. Magnetothermal nanoparticle technology alleviates parkinsonian-like symptoms in mice. Nat Commun [Internet]. 2021. [cited 2025 May 1];12(1). doi: 10.1038/s41467-021-25837-4 [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This is the first study that applied magnetothermal stimulation to stimulate neurons in a rodent model of Parkinson’s Disease.
- 77.Unda SR, Pomeranz LE, Marongiu R, et al. Bidirectional regulation of motor circuits using magnetogenetic gene therapy. Sci Adv [Internet]. 2024. [cited 2025 May 1];10(41). doi: 10.1126/sciadv.adp9150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jeon BT, Naveed M, Puleo M, et al. Localized brain stimulation with mild magnetic hyperthermia promotes microglia activity towards reactive and autophagic phenotypes in vivo. Sci Rep. 2025;15 (1):24425. doi: 10.1038/s41598-025-10441-z [DOI] [PMC free article] [PubMed] [Google Scholar]; • This study demonstrated the feasibility of localized magnetic hyperthermia in modulating microglial activities in vivo in mouse brain.
- 79.Cohen-Pfeffer JL, Gururangan S, Lester T, et al. Intracerebroventricular delivery as a safe, long-term route of drug administration. Pediatr Neurol. 2017;67:23–35. doi: 10.1016/j.pediatrneurol.2016.10.022 [DOI] [PubMed] [Google Scholar]
- 80.Anton S, Margold M, Kowalski T, et al. Complications of intracerebroventricular chemotherapy via subgaleal reservoir in primary central nervous system lymphoma: a single-institution experience on 1247 installations in 94 consecutive patients. Hematol Oncol. 2021;39(2):176–184. doi: 10.1002/hon.2833 [DOI] [PubMed] [Google Scholar]
- 81.Cook AM, Mieure KD, Owen RD, et al. Intracerebroventricular administration of drugs. Pharmacotherapy. 2009;29(7):832–845. doi: 10.1592/phco.29.7.832 [DOI] [PubMed] [Google Scholar]
- 82.Barker SJ, Thayer MB, Kim C, et al. Targeting the transferrin receptor to transport antisense oligonucleotides across the mammalian blood-brain barrier. Sci Transl Med. 2024;16(760):eadi2245. doi: 10.1126/scitranslmed.adi2245 [DOI] [PubMed] [Google Scholar]
- 83.Rodrigues HF, Capistrano G, Bakuzis AF. In vivo magnetic nanoparticle hyperthermia: a review on preclinical studies, low-field nano-heaters, noninvasive thermometry and computer simulations for treatment planning. Int J Hyperthermia. 2020;37(3):76–99. doi: 10.1080/02656736.2020.1800831 [DOI] [PubMed] [Google Scholar]
- 84.Chandrasekharan P, Tay ZW, Hensley D, et al. Using magnetic particle imaging systems to localize and guide magnetic hyperthermia treatment: tracers, hardware, and future medical applications. Theranostics. 2020;10(7):2965–2981. doi: 10.7150/thno.40858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hadadian Y, Uliana JH, Carneiro AAO, et al. A novel theranostic platform: integration of magnetomotive and thermal ultrasound imaging with magnetic hyperthermia. IEEE Trans Biomed Eng. 2021;68(1):68–77. doi: 10.1109/TBME.2020.2990873 [DOI] [PubMed] [Google Scholar]
- 86.Horcajo M, Arranz D, Weigand R, et al. Multifunctional platform for photothermal therapy combined with luminescence nanothermometry probes. AIP Adv. 2024;14(2):025101. doi: 10.1063/9.0000729 [DOI] [Google Scholar]
- 87.Healy S, Bakuzis AF, Goodwill PW, et al. Clinical magnetic hyperthermia requires integrated magnetic particle imaging. WIREs Nanomed Nanobiotechnol. 2022;14(3):e1779. doi: 10.1002/wnan.1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xiong L, Liang B, Yu K. Magnetic hyperthermia in oncology: nanomaterials-driven combinatorial strategies for synergistic therapeutic gains. Mater Today Bio. 2025;33:102070. doi: 10.1016/j.mtbio.2025.102070 [DOI] [PMC free article] [PubMed] [Google Scholar]


