Abstract
The chemokines use G protein-coupled receptors to regulate the migratory and proadhesive responses of leukocytes. Based on observations that G protein-coupled receptors undergo heterologous desensitization, we have examined the ability of chemokines to also influence the perception of pain by cross-desensitizing opioid G protein-coupled receptors function in vitro and in vivo. We find that the chemotactic activities of both μ- and δ-opioid receptors are desensitized following activation of the chemokine receptors CCR5, CCR2, CCR7, and CXCR4 but not of the CXCR1 or CXCR2 receptors. Furthermore, we also find that pretreatment with RANTES/CCL5, the ligand for CCR1, and CCR5 or SDF-1α/CXCL12, the ligand for CXCR4, followed by opioid administration into the periaqueductal gray matter of the brain results in an increased rat tail flick response to a painful stimulus. Because chemokine administration into the periaqueductal gray matter inhibits opioid-induced analgesia, we propose that the activation of proinflammatory chemokine receptors down-regulates the analgesic functions of opioid receptors, and this enhances the perception of pain at inflammatory sites.
Opioid and chemokine receptors are members of the Gi protein-linked seven-transmembrane receptor family. These receptors, as well as the chemokine and endogenous opioid peptide ligands, are widely distributed in brain tissue and the periphery. Chemokines have been classified into four families: C, CC, CXC, and CX3C based on the position of conserved cysteines, and they interact with receptors designated CR1, CCR1–11, CXCR1–5, or CX3CR1, respectively (1). Three classes of receptors have been identified for the opioids, designated μ, κ, and δ, and each of the opioid receptor genes expressed in brain tissue and immune cells has been cloned and sequenced (2–7).
The μ-, κ-, and δ-opioids are known to have inhibitory effects on both antibody and cellular immune responses (8, 9), natural killer cell activity (10), cytokine expression (11–13), and phagocytic activity (14), which may account for the decreased resistance to infections caused by morphine and heroin administration. Furthermore, pretreatment with opioids, including morphine, heroin, met-enkephalin, the selective μ-agonist [D-Ala2, N-Me-Phe-4, Gly-ol5]enkephalin (DAMGO), or the selective δ-agonist [D-Pen2, D-Pen5]enkephalin (DPDPE), leads to the inhibition of the chemotactic response of leukocytes to complement-derived chemotactic factors (15) and to the chemokines macrophage inflammatory protein (MIP-1α)/CCL3, regulated on activation normal T cell expressed and secreted (RANTES/CCL5), monocyte chemotactic protein-1 (MCP-1)/CCL2, and IL-8/CXCL8 (16). The latter results suggest that the activation of the μ- and δ-opioid receptors leads to the desensitization of the CC chemokine receptor 2 (CCR2) and CXC chemokine receptors CXCR1 and CXCR2. In fact, the latter two receptors are phosphorylated by prior administration of opioids. Moreover, the inhibition of CCL3 and CCL5 responses following opioid pretreatment is consistent with the desensitization of either CCR1 or CCR5, or both. This receptor crosstalk resulting in heterologous desensitization and phosphorylation of some of the chemokine receptors may contribute to the immunosuppressive effects of the opioids.
Conversely, we have considered the possibility that one or more of the chemokine receptors may desensitize the opioid receptors. We hypothesized that prior exposure to chemokines might result in heterologous desensitization of opioid receptors, a process with physiological relevance given the significant accumulation of chemokines in most inflammatory response states. For our analysis, we first examined the effect of chemokines on opioid receptor function by measuring opioid receptor-mediated chemotaxis. In experiments carried out with a number of diverse cell populations, including human monocytes and keratinocytes, and murine thymocytes, we found that the chemotactic activities of both the μ- and δ-opioid receptors were desensitized following activation of the chemokine receptors CCR2, CCR5, CCR7, and CXCR4 but not of the CXCR1 or CXCR2 receptors. In these studies, we have also evaluated the possibility that activation of chemokine receptors in vivo may desensitize brain μ-opioid receptor function and interfere with the perception of pain in the central nervous system.
Materials and Methods
Cells.
Human peripheral blood monocytes were isolated from leukopheresis packs (National Institutes of Health Clinical Center, Transfusion Medicine Department, Bethesda, MD) and enriched for monocytes by using isoosmotic Percoll gradient centrifugation (typically, >90% monocytes). The human keratinocyte cell line HaCaT was maintained in DMEM containing 10% FCS. Primary murine thymocytes were obtained from male BALB/c mice, 5–8 weeks of age (National Cancer Institute; Frederick, MD), and housed in the Temple University small animal barrier facility.
Chemotaxis Assay.
The migration of primary cells and cell lines was analyzed in a 48-well microchemotaxis chamber. Briefly, the lower compartments of the chamber were loaded with the chemoattractant diluted in a medium composed of RPMI-1640 containing 1% BSA and 25 mM Hepes. The upper compartments of the chamber were loaded with cells (1 to 2 × 106/ml), and the two compartments were separated by a fibronectin-coated (for thymocytes) or uncoated (for keratinocytes and monocytes) 5 μm (for thymocytes and monocytes)- or 12 μm (for keratinocytes)-pore size polycarbonate polyvinylpyrrolidone-free membrane. Following incubation for 45 min (keratinocytes), 1.5 h (monocytes), or 3 h (thymocyte), the filter was removed, the top of the membrane was wiped, and the membrane was fixed and stained with a Diff-Quick kit. Migrating cells were counted microscopically, and the average number of cells in four high-power fields (400×) was determined. The results are expressed as the fold increase in cells migrating in response to chemoattractant vs. the medium control (chemotaxis index). Data analysis was conducted by Biostatistical Services (Temple University School of Medicine) by using ANOVA.
Analysis of Analgesia.
The cold-water tail-flick test was used to assess the analgesic effects of DAMGO according to standard procedures used in our laboratories (17). Male Sprague–Dawley rats (Zivic–Miller) were anesthetized with an intramuscular injection of a mixture of ketamine hydrochloride (100–150 mg/kg) and acepromazine maleate (0.2 mg/kg) and stereotaxically (18) implanted unilaterally with a guide cannula (21-gauge stainless steel tubing, 14 mm long; Plastics One, Roanoke, VA) into the location above the periaqueductal gray (PAG; coordinates: AP, 0.6; R, 0.8; V, 1.0). Following surgery, animals were housed individually and were allowed to recover from the surgery at least 7 days. The cold-water tail-flick test was performed by placing the rat tail into a circulating cold water bath in a 1:1 mix of ethylene glycol:water maintained at −3°C. The nociceptive threshold was taken as the latency until the rat removed or flicked its tail. After the predrug latency measurements, designated concentrations of CXCL12 or CCL5, or saline were microinjected in 1 μl into the PAG at a rate of 1 μl/min. Thirty minutes later, DAMGO (0.4 μg/μl) in 1 μl (or saline) was microinjected into the PAG by the same method. The latency to tail-flick was tested between 15 and 120 min after DAMGO microinjection. If an animal did not respond within 60 s, the trial was terminated and a maximum latency of 60 s was recorded. The baseline latency (no drug treatment) ranged from 8 to 12 s. The analgesic effect of DAMGO treatment was calculated for each rat as follows: percent maximum possible analgesia (% MPA) = [(postdrug latency − baseline latency)/(60 − baseline latency)] × 100. Placement of the injection cannula was verified by bromophenol blue staining. Data from rats in which the cannula was not located within the PAG were not included in the results.
Receptor Phosphorylation.
The HaCaT keratinocyte cell line was treated with either medium, DAMGO (1 nM) or CCL5 (100 ng/ml); after 30 min, cells were lysed in RIPA buffer, and the membrane extracts were immunoprecipitated with a combination of anti-phosphoserine and anti-phosphothreonine (Sigma) by using immobilized Protein A/G (Pierce). Immunoprecipitates were subjected to Western blot analysis by using anti-μ-opioid receptor antibody as a probe and then subjected to the CDP-Star chemiluminescence reaction (Pierce) to develop the probed bands.
Results
Given the significant accumulation of chemokines in a variety of inflammatory response states, including a number of neuroinflammatory lesions (19–22), we hypothesized that prior exposure to chemokines might result in heterologous desensitization of opioid receptors. Like the chemokines, the μ-, κ-, and δ-opioids possess chemoattractant activity and induce the chemotaxis of both monocytes and neutrophils (16, 23, 24). Therefore, we examined the effect of chemokines on the ability of both μ- and δ-opioid receptors to mediate the chemotactic response of various cell types. We first analyzed the effect of preincubation with several chemokines on the chemotactic response of human peripheral blood monocytes to the δ-opioid selective agonist DPDPE. The data show that monocytes pretreated either with CCL5 (Fig. 1A) or CXCL12 (Fig. 1B) failed to respond to DPDPE. As expected, control experiments show that pretreatment with either CCL5 or CXCL12 induced homologous desensitization of the CCL5 or CXCL12 responses, respectively (Fig. 1, open symbols). Thus, both the CXCL12 and CCL5 chemokines cross-desensitized the δ-opioid receptor.
Fig 1.
Cross-desensitization of the δ-opioid response of monocytes following CCL5 (A) or CXCL12 (B) pretreatment. Monocytes were either untreated (•) or pretreated with either CCL5 or CXCL12 (▾; 100 ng/ml) for 60 min, and the chemotactic response to the δ-opioid agonist DPDPE was determined. The responses CCL5-pretreated (▿) and untreated monocytes (○) to CCL5 (50 ng/ml; A) and CXCL12-pretreated monocytes to CXCL12 (100 ng/ml; open symbols, B) are also shown. *, P < 0.05. The data are representative of six independent experiments.
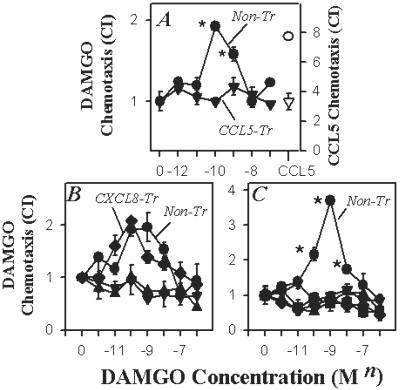
We also assessed the capacity of the chemokines to cross-desensitize the μ-opioid receptor. The results show that both primary human monocytes (Fig. 2A) and keratinocytes (Fig. 2B) pretreated with CCL5 failed to exhibit a detectable DAMGO response. Moreover, keratinocytes pretreated with the CCR7 ligand EBI1-ligand chemokine (ELC/CCL19) also failed to respond through the μ-opioid receptor (Fig. 2B). In contrast, keratinocytes preincubated with the CXCR1/CXCR2 ligand CXCL8/IL-8 exhibited normal μ-opioid responses. The keratinocyte cell line used for these studies expressed the receptor(s) for CXCL8, as these cells exhibit a significant chemotactic response to this CXC chemokine (data not shown). Therefore, the inability of CXCL8 to induce cross-desensitization was not because of an absence of the sensitizing (inducing) receptor. Finally, murine primary thymocytes pretreated with CCL5, CCL19, CXCL12, or the CCR2 ligand monocyte chemotactic protein-1 (MCP-1/CCL2) all failed to manifest a detectable DAMGO response (Fig. 2C). Taken together, these results document that activation of the chemokine receptors CCR2, CCR7, CCR1/5, and CXCR4, but not CXCR1 or CXCR2, functionally cross-desensitized both μ- and δ-opioid receptors expressed by a number of disparate cell populations.
Fig 2.
(A) Cross-desensitization of the μ-opioid response of monocytes after CCL5 pretreatment. Monocytes were either untreated (•) or pretreated with CCL5 (▾; 100 ng/ml) for 60 min, and the response to the μ-opioid agonist DAMGO was determined. The response of CCL5 pretreated cells to CCL5 (50 ng/ml) is also shown. (B) Cross-desensitization of the μ-opioid response of the HaCaT keratinocyte cell line after CCL5 (▴; 100 ng/ml), CCL19 (▵; 100 ng/ml), or CXCL12 (♦; 100 ng/ml) pretreatment. Nontreated cells (circles) and CXCL12-pretreated cells exhibit significant (P < 0.05) responses over the 0.1–10 nM range of DAMGO doses. (C) Cross-desensitization of the μ-opioid response of murine thymocytes without treatment (•) or following pretreatment (100 ng/ml) with CCL5 (▴), CCL2 (▾), CCL19 (⋄), and CXCL12 (▪). The data are representative of eight independent experiments.
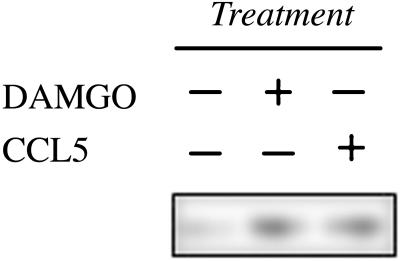
Cross-desensitization between G protein-linked seven-transmembrane receptors is often associated with phosphorylation of the target receptor (16, 25). We investigated whether the desensitization of the μ-opioid receptor following chemokine receptor activation is associated with an increase in the phosphorylation state of μ-receptor. Treatment with DAMGO induced homologous desensitization and an increase in phosphorylation of the μ-opioid receptor (Fig. 3), and treatment with CCL5 clearly induced a similar degree of phosphorylation of the μ-opioid receptor. Thus, the signal transduction events that occur as a part of the bidirectional crosstalk between chemokine and μ-opioid receptors includes the activation of a kinase(s) that phosphorylates this opioid receptor. The nature of these signaling events remains to be more completely characterized.
Fig 3.
Phosphorylation of the μ-opioid receptor after DAMGO or CCL5 treatment. HaCaT cells were treated with either DAMGO (1 nM) or CCL5 (100 ng/ml), and membrane extracts were obtained after 30 min in culture. The extracts were immunoprecipitated with a combination of anti-phosphoserine and anti-phosphothreonine by using protein A/G. The immunoprecipitates were subjected to Western blot analysis by using anti-μ-opioid receptor antibody as a probe, and the reaction was developed by chemiluminescence. The data are representative of four independent experiments.
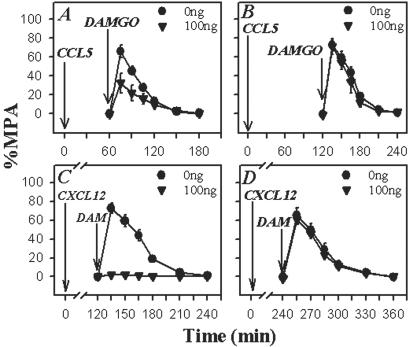
To be physiologically relevant, heterologous desensitization should lead to a decrease in opioid receptor function in vivo. To determine if this is so, we investigated the impact of chemokines on opioid-mediated analgesic activity in the brain. Cannulas were placed into the PAG matter of rats (the brain area that is the focus of opioid analgesic actions), and the chemokine CXCL12 or CCL5 was administered over a range of doses from 0 to 100 ng to induce cross-desensitization. After 30 min, 400 ng of DAMGO was administered, and the degree of analgesia was measured by using the cold-water tail-flick assay. This dose of DAMGO produces marked analgesia when administered into the PAG. In this test, all opioid agonists can produce a full analgesic response, whereas partial agonists or mixed agonists/antagonists produce a lesser degree of analgesia (26). Moreover, drugs such as aspirin fail to exhibit an effect. As expected, rats receiving an initial injection of saline followed by DAMGO manifested a significant increase in tail-flick latency (analgesia), beginning at 15 min post-DAMGO administration (Fig. 4). However, rats receiving an initial injection of CXCL12 (Fig. 4A) exhibited a dose-dependent reduction in analgesic responses over the entire 120-min duration of the experiment. Importantly, control experiments in which animals received an initial injection of SDF-1α/CXCL12 (Fig. 4B), followed after 30 min with an injection of saline (open symbols), show that the SDF-1α/CXCL12 preparation by itself failed to exhibit any evidence of either analgesia or hyperalgesia.
Fig 4.
The effect of CXCL12, CCL5, and CCL2 on the DAMGO-induced analgesic response in the rat PAG. (A) Rats were cannulated into the PAG, and SDF-1α was administered at the designated concentrations. After 30 min, DAMGO (400 ng) was administered, and the analgesic response, expressed as the percent maximum possible analgesia, in the cold-water tail-flick assay was determined. (B) Administration of designated concentrations of CXCL12, followed 30 min later with saline, did not show any evidence of detectable analgesia or hyperalgesia. Control experiments showed that the administration of saline, followed 30 min later with saline, also failed to show any evidence of analgesia (data not shown). CCL5 (C) or CCL2 (D) also was administered into the PAG at the designated concentrations. After 30 min, DAMGO (400 ng; solid symbols) or saline (□) was administered, and the analgesic response was determined. Results are presented as the mean ± SD and are representative of four independent experiments.
Similar results were obtained from experiments carried out with CCL5 pretreatment. The data show (Fig. 4C) that administration of as little as 1 ng of CCL5 induced a dramatic decrease in the analgesic activity of DAMGO, and administration of 100 ng eliminated virtually all detectable analgesic function. Interestingly, pretreatment with CCL2 (Fig. 4D) failed to alter the analgesic activity of DAMGO. These results are not unexpected as it seems that the CCL2 receptor CCR2 is not expressed, or is expressed at very low levels, by neurons (27, 28). Recent studies suggest, however, that CCR2 may be expressed by astrocytes or microglial cells, as well as blood-derived monocytes that may be present during neuroinflammatory responses (27).
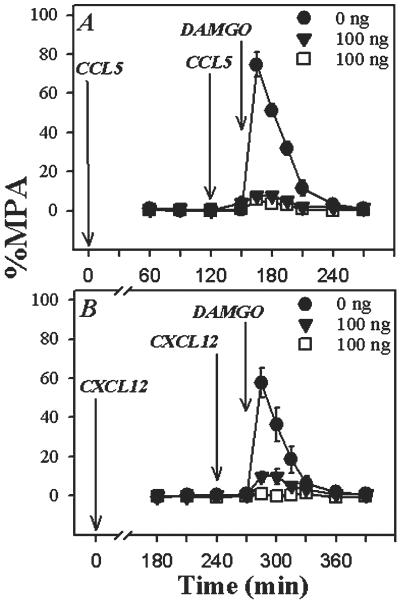
We attempted to determine the duration of the desensitization response induced by CCL5 and CXCL12. First, experiments were carried out in which CCL5 was administered, and the analgesic response to DAMGO was tested at either 60 or 120 min. The results show (Fig. 5) that the CCL5 treatment significantly inhibited the analgesic activity of DAMGO at 60 min. It should be noted that the degree of CCL5-induced inhibition was less than we observed at 30 min (compare with Fig. 4). However, the CCL5 administration failed to alter the DAMGO-induced analgesia at 120 min (Fig. 5B). This suggests that the desensitization mediated by CCL5 is lost by 2 h. On the other hand, our results show that DAMGO-induced analgesia is still completely inhibited 120 min following CXCL12 administration (Fig. 5C). The data from these experiments, however, show that the desensitization induced by CXCL12 is lost by 4 h (Fig. 5D).
Fig 5.
Duration of cross-desensitization induced by CCL5 or CXCL12. (A and B) CCL5 was administered into the PAG at the designated concentrations. After 60 min (A) or 120 min (B), DAMGO (400 ng; ▾) was administered, and the analgesic response was determined. (C and D) Alternatively, CXCL12 was administered into the PAG at the designated concentrations, and after 120 min (C) or 240 min (D), DAMGO (400 ng; ▾) was administered, and the analgesic response was determined. Results are presented as the mean ± SD and are representative of four independent experiments.
We considered the possibility that the loss of chemokine-induced desensitization may be the result of catabolism of the chemokine ligand in the PAG. The presence of protease activity is readily detectable in the PAG (29, 30). Experiments were conducted to determine whether the re-administration of either CCL5 or CXCL12 would restore the cross-desensitization effect. In the first set of experiments, CCL5 was administered as described above, and after 120 min, CCL5 was administered again. The analgesic activity of DAMGO was then tested after an additional 30 min. Our results show (Fig. 6A) that the re-administration of CCL5 again completely inhibited the analgesic activity of DAMGO. In the second set of experiments, CXCL12 was administered, and a second treatment with CXCL12 was given 240 min later. The analgesic activity of DAMGO was then tested after an additional 30 min, and the results show (Fig. 6B) that the combined CXCL12 treatments significantly reduced the DAMGO-induced analgesia. These results suggest that chemokine-induced cross-desensitization is directly related to the persistent presence of intact chemokine ligand.
Fig 6.
Restoration of cross-desensitization by readministration of CCL5 or CXCL12. (A) CCL5 was administered into the PAG at a concentration of 100 ng, followed by a second administration of CCL5 at the designated concentrations at 120 min. (B) Alternatively, CXCL12 was administered into the PAG at a concentration of 100 ng, followed by a second administration of CXCL12 at the designated concentrations at 240 min. After an additional 30 min, DAMGO (400 ng; solid symbols) was administered, and the analgesic response was determined. The analgesic activity of CCL5- or CXCL12-treated mice in the absence of DAMGO is also shown (open symbols). Results are presented as the mean ± SD and are representative of four independent experiments.
Discussion
Our results provide a report of the in vitro and apparent in vivo inactivation of opioid receptors by chemoattractant factors. The results of the current study, together with the previous observation that μ- and δ-opioid receptor activation led to the desensitization of CCR1 and CCR2 (16), suggest the desensitization is a bidirectional process. Moreover, these results represent evidence that the process of heterologous desensitization among chemoattractant receptors may have significant consequences in vivo. The analgesic activity of the opioids in the brain presumably is overcome under conditions where there are elevated levels of the CCR5 and CXCR4 chemokine ligands. Thus, the cross-desensitization of the μ-opioid receptor by CCL5 and CXCL12 seems to change the balance between analgesia and hyperalgesia.
Under the conditions of our analysis, the duration of the chemokine-induced cross-desensitization is limited to less than 2 h for CCL5 and to less than 4 h for CXCL12. Re-administration studies suggest that the limited duration of the desensitization effect is most likely because of the degradation of these chemokine ligands. Our data show that cross-desensitization can be restored by the addition of fresh chemokine to the PAG. Proteolysis of the chemokine proteins is a likely possibility, given the well established presence of protease activity at this anatomic site (29, 30). Although little is known about the normal catabolism of the chemokines under physiological conditions, it is now clear that membrane-associated CD26/dipeptidyl peptidase rapidly degrades both of these chemokines and several others (31). However, it is uncertain whether CD26 is expressed in the PAG. It is also possible that CCL5 and CXCL12 are internalized by their respective cognate receptors. Finally, it is also possible that the effective concentration of these chemokines may be reduced by diffusion away from the PAG into the surrounding tissues. In any case, we suggest that conditions which result in the persistent production of either CCL5 or CXCL12 will sustain the cross-desensitization process until the expression of these chemokines declines.
There is growing evidence that chemokines participate in both normal physiological processes and induced pathological responses in the brain. Not only are opioid receptors widely expressed in the central nervous system, but there are recent reports showing that CXCR2, CXCR4, CCR1, CCR4, CCR5, CCR9, and CX3CR1 are also broadly expressed in the brain, including on neurons in the hippocampus, regions of the cerebral cortex, amygdala, thalamus, and basal ganglia (27, 28, 32). First, CXCR4 is particularly well expressed in the brain and has been detected on neurons, glial cells, astrocytes, microglial cells, endothelial cells, and blood-derived leukocytes (33–38). In contrast, CCR5 is less widely distributed, and more weakly expressed, by neurons in the brain (28, 32, 39). Both CXCL12 and CXCR4 are critical developmental factors, and mutant mice with defects in either CXCL12 or CXCR4 exhibit severely impaired lymphogenesis, abnormal angiogenesis, and defects in the formation of the central nervous system (40–43). The expression of the chemokines is not limited to cells of the leukocyte lineage. For example, CXCL12 transcripts have been detected in the spleen, ovary, pancreas, colon, small intestine, placenta, and brain (44). Several chemokines are produced under normal conditions in the brain, including CCL2, CCL3, CCL5, CXCL1, CXCL8, CXCL12, and CX3CL1/fractalkine (45, 46). Second, evidence suggests that certain chemokines have the potential to provide trophic support for brain cells. The CXCR2 ligand growth-regulated oncogene-α (GROα/CXCL1) synergizes with platelet-derived growth factor to promote the growth of immature oligodendrocytes (47). In addition, several chemokines, including both RANTES/CCL5 and monocyte-derived chemokine (MDC/CCL22), have been shown to prevent HIV-1 gp120-induced apoptosis of hippocampal neurons (32). Third, chemokine expression in the brain induces the migration of leukocytes and promotes the activation of blood-derived leukocytes, microglial cells, and astrocytes. For example, the injection of MCP-1/CCL2, RANTES/CCL5, or IP-10/CXCL10 into the hippocampus has been shown to induce the transmigration of monocytes to the brain parenchyma (48). Thus, chemokines can apparently overcome the intrinsic resistance to leukocytic recruitment at the blood–brain barrier. The levels of the CCR1/5 ligands (CCL3 and CCL5), CXCR4 ligands (CXCC12), as well as other critical chemokines (CCL2 and CX3CL1) are increased in a variety of pathological states, including bacterial meningitis (49), lymphocytic choriomeningitis (50), mouse adenovirus (51), herpes simplex virus encephalitis (52), multiple sclerosis (20), cancer (53), and HIV dementia (54) and may account for the increased sensitivity to pain associated with the “sickness syndrome” seen in most of these conditions. It should be noted that infection by either R5 or X4 strains of HIV induce the expression of several chemokines, including the chemokine ligands for CCR5 (55, 56), and the infection of microglial cells in the brain may provide a source of desensitizing chemokine ligands.
Based on the process of heterologous desensitization, chemokine ligands for CXCR4 and CCR1/5 can apparently inactivate the normal neuronal signaling pathway involved in reducing the sensation of pain. Additional evidence that chemokines participate directly in the regulation of neuronal transmission is shown by in vitro studies, which report that exposure to the CXC chemokines CXCL8 and CXCL1 enhances postsynaptic currents and reduces the magnitude of neurotransmitter release from Purkinje neurons (57). The circumstances under which the chemokines participate in the function of the central nervous system at this level remain uncertain, as some chemokines seem to be expressed in the brain under normal physiological conditions. However, the implication of our findings is that the elevated level of chemokines associated with episodes of inflammation and tissue injury in the brain would result in altered neuronal function and, specifically, in reduced μ-opioid receptor-mediated analgesia. Recent studies in healthy human subjects have confirmed a large volume of experimental animal data which show that μ-opioid receptors in the brain are critical for the sensation of sustained peripheral pain (58). It is well established that exaggerated pain (hyperalgesia) occurs as a part of inflammatory stress reactions (59, 60). This pain response is a condition that often occurs with systemic inflammatory “flu-like” reactions, with symptoms of joint and muscle aches, fever, malaise, somnolence, and decreased locomotion. The possibility that a reduction in analgesia may contribute to the pain in the periphery associated with a variety of inflammatory disease states including rheumatoid arthritis, dental caries, and certain infectious diseases should be investigated further. Our studies support the hypothesis that the cross-desensitization of the μ-opioid receptor induced by chemokines may provide a basis for the hyperalgesia associated with inflammatory reactions in general.
Acknowledgments
We thank Drs. Dan Mellon and Scott Durham, Laboratory of Molecular Immunoregulation, National Cancer Institute, Frederick, MD 21702 for the critical reviews of the manuscript. This work was supported in part by National Institutes of Health Grants DA-06650, DA-11130, DA-00376, DA-14230, R24 CA-88261, and P30 DA-13429.
Abbreviations
DAMGO, [d-ala2, N-Me-Phe4, Gly-ol5]enkephalin
DPDPE, [d-Pen2, d-Pen5]enkephalin
PAG, periaqueductal gray
References
- 1.Murphy P. M., Baggiolini, M., Charo, I. F., Hebert, C. A., Horuk, R., Matsushima, K., Miller, L. H., Oppenheim, J. J. & Power, C. (2000) Pharmacol. Rev. 52, 145-176. [PubMed] [Google Scholar]
- 2.Chen Y., Mestek, A., Liu, J. & Yu, L. (1993) Biochem. J. 295, 625-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li S., Zhu, J., Chen, C., Chen, Y. W., Deriel, J. K., Ashby, B. & Liu-Chen, L. Y. (1993) Biochem. J. 295, 629-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans C. J., Keith, D., Jr., Morrison, H., Magendzo, K. & Edwards, R. H. (1992) Science 258, 1952-1956. [DOI] [PubMed] [Google Scholar]
- 5.Chuang L. F., Chuang, T. K., Killam, K. F., Jr., Chuang, A. J., Kung, H. F., Yu, L. & Chuang, R. Y. (1994) Biochem. Biophys. Res. Commun. 202, 1291-1299. [DOI] [PubMed] [Google Scholar]
- 6.Belkowski S. M., Zhu, J., Liu-Chen, L. Y., Eisenstein, T. K., Adler, M. W. & Rogers, T. J. (1995) J. Neuroimmunol. 62, 113-117. [DOI] [PubMed] [Google Scholar]
- 7.Sedqi M., Roy, S., Ramakrishnan, S., Elde, R. & Loh, H. H. (1995) Biochem. Biophys. Res. Commun. 209, 563-574. [DOI] [PubMed] [Google Scholar]
- 8.Taub D. D., Eisenstein, T. K., Geller, B. E., Adler, M. W. & Rogers, T. J. (1991) Proc. Natl. Acad. Sci. USA 88, 360-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pellis N. R., Harper, C. & Dafny, N. (1986) Exp. Neurol. 93, 92-97. [DOI] [PubMed] [Google Scholar]
- 10.Weber R. J. & Pert, A. (1989) Science 245, 188-190. [DOI] [PubMed] [Google Scholar]
- 11.Chao C. C., Molitor, T. W., Close, K., Hu, S. & Peterson, P. K. (1993) Int. J. Immunopharmacol. 15, 447-453. [DOI] [PubMed] [Google Scholar]
- 12.Peterson P. K., Sharp, B., Gekker, G., Brummitt, C. & Keane, W. F. (1987) J. Clin. Invest. 80, 824-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belkowski S. M., Alicea, C., Eisenstein, T. K., Adler, M. W. & Rogers, T. J. (1995) J. Pharmacol. Exp. Ther. 273, 1491-1496. [PubMed] [Google Scholar]
- 14.Szabo I., Rojavin, M., Bussiere, J. L., Eisenstein, T. K., Adler, M. W. & Rogers, T. J. (1993) J. Pharmacol. Exp. Ther. 267, 703-706. [PubMed] [Google Scholar]
- 15.Liu Y., Blackbourn, D. J., Chuang, L. F., Killam, K. F., Jr. & Chuang, R. Y. (1992) J. Pharmacol. Exp. Ther. 263, 533-539. [PubMed] [Google Scholar]
- 16.Grimm M. C., Ben-Baruch, A., Taub, D. D., Howard, O. M., Resau, J. H., Wang, J. M., Ali, H., Richardson, R., Snyderman, R. & Oppenheim, J. J. (1998) J. Exp. Med. 188, 317-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tiseo P. J., Geller, E. B. & Adler, M. W. (1988) J. Pharmacol. Exp. Ther. 246, 449-453. [PubMed] [Google Scholar]
- 18.Pellegrino L. J. & Cushman, A. J., (1967) A Stereotaxic Atlas of the Rat Brain (Appleton, New York).
- 19.Mennicken F., Maki, R., de Souza, E. B. & Quirion, R. (1999) Trends Pharmacol. Sci. 20, 73-78. [DOI] [PubMed] [Google Scholar]
- 20.Sorensen T. L., Tani, M., Jensen, J., Pierce, V., Lucchinetti, C., Folcik, V. A., Qin, S., Rottman, J., Sellebjerg, F., Strieter, R. M., et al. (1999) J. Clin. Invest. 103, 807-815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glabinski A. R. & Ransohoff, R. M. (1999) J. Neurovirol. 5, 3-12. [DOI] [PubMed] [Google Scholar]
- 22.Ransohoff R. M. & Tani, M. (1998) Trends Neurosci. 21, 154-159. [DOI] [PubMed] [Google Scholar]
- 23.Ruff M. R., Wahl, S. M., Mergenhagen, S. & Pert, C. B. (1985) Neuropeptides 5, 363-366. [DOI] [PubMed] [Google Scholar]
- 24.Van Epps D. E. & Saland, L. (1984) J. Immunol. 132, 3046-3053. [PubMed] [Google Scholar]
- 25.Ali H., Richardson, R. M., Haribabu, B. & Snyderman, R. (1999) J. Biol. Chem. 274, 6027-6030. [DOI] [PubMed] [Google Scholar]
- 26.Pizziketti R. J., Pressman, N. S., Geller, E. B., Cowan, A. & Adler, M. W. (1985) Eur. J. Pharmacol. 119, 23. [DOI] [PubMed] [Google Scholar]
- 27.Gabuzda D., He, J., Ohagen, A. & Vallat, A. V. (1998) Semin. Immunol. 10, 203-213. [DOI] [PubMed] [Google Scholar]
- 28.Meucci O., Fatatis, A., Simen, A. A., Bushell, T. J., Gray, P. W. & Miller, R. J. (1998) Proc. Natl. Acad. Sci. USA 95, 14500-14505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Young E. A., Walker, J. M., Houghten, R. & Akil, H. (1987) Peptides 8, 701-707. [DOI] [PubMed] [Google Scholar]
- 30.al Rodhan N., Chipkin, R. & Yaksh, T. L. (1990) Brain Res. 520, 123-130. [DOI] [PubMed] [Google Scholar]
- 31.Lambeir A. M., Proost, P., Durinx, C., Bal, G., Senten, K., Augustyns, K., Scharpe, S., Van Damme, J. & De Meester, I. (2001) J. Biol. Chem. 276, 29839-29845. [DOI] [PubMed] [Google Scholar]
- 32.Bajetto A., Bonavia, R., Barbero, S., Florio, T., Costa, A. & Schettini, G. (1999) Ann. NY Acad. Sci. 876, 201-209. [DOI] [PubMed] [Google Scholar]
- 33.Hesselgesser J., Halks-Miller, M., DelVecchio, V., Peiper, S. C., Hoxie, J., Kolson, D. L., Taub, D. & Horuk, R. (1997) Curr. Biol. 7, 112-121. [DOI] [PubMed] [Google Scholar]
- 34.Ohtani Y., Minami, M., Kawaguchi, N., Nishiyori, A., Yamamoto, J., Takami, S. & Satoh, M. (1998) Neurosci. Lett. 249, 163-166. [DOI] [PubMed] [Google Scholar]
- 35.Tanabe S., Heesen, M., Yoshizawa, I., Berman, M. A., Luo, Y., Bleul, C. C., Springer, T. A., Okuda, K., Gerard, N. & Dorf, M. E. (1997) J. Immunol. 159, 905-911. [PubMed] [Google Scholar]
- 36.Volin M. V., Joseph, L., Shockley, M. S. & Davies, P. F. (1998) Biochem. Biophys. Res. Commun. 242, 46-53. [DOI] [PubMed] [Google Scholar]
- 37.Feil C. & Augustin, H. G. (1998) Biochem. Biophys. Res. Commun. 247, 38-45. [DOI] [PubMed] [Google Scholar]
- 38.Baggiolini M., Dewald, B. & Moser, B. (1997) Annu. Rev. Immunol. 15, 675-705. [DOI] [PubMed] [Google Scholar]
- 39.Miller R. J. & Meucci, O. (1999) Trends Neurosci. 22, 471-479. [DOI] [PubMed] [Google Scholar]
- 40.Nagasawa T., Hirota, S., Tachibana, K., Takakura, N., Nishikawa, S., Kitamura, Y., Yoshida, N., Kikutani, H. & Kishimoto, T. (1996) Nature (London) 382, 635-638. [DOI] [PubMed] [Google Scholar]
- 41.Ma Q., Jones, D., Borghesani, P. R., Segal, R. A., Nagasawa, T., Kishimoto, T., Bronson, R. T. & Springer, T. A. (1998) Proc. Natl. Acad. Sci. USA 95, 9448-9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tachibana K., Hirota, S., Iizasa, H., Yoshida, H., Kawabata, K., Kataoka, Y., Kitamura, Y., Matsushima, K., Yoshida, N., Nishikawa, S., et al. (1998) Nature (London) 393, 591-594. [DOI] [PubMed] [Google Scholar]
- 43.Zou Y. R., Kottmann, A. H., Kuroda, M., Taniuchi, I. & Littman, D. R. (1998) Nature (London) 393, 595-599. [DOI] [PubMed] [Google Scholar]
- 44.Shirozu M., Nakano, T., Inazawa, J., Tashiro, K., Tada, H., Shinohara, T. & Honjo, T. (1995) Genomics 28, 495-500. [DOI] [PubMed] [Google Scholar]
- 45.Bajetto A., Bonavia, R., Barbero, S., Florio, T. & Schettini, G. (2001) Front. Neuroendocrinol. 22, 147-184. [DOI] [PubMed] [Google Scholar]
- 46.Tashiro K., Tada, H., Heilker, R., Shirozu, M., Nakano, T. & Honjo, T. (1993) Science 261, 600-603. [DOI] [PubMed] [Google Scholar]
- 47.Robinson S., Tani, M., Strieter, R. M., Ransohoff, R. M. & Miller, R. H. (1998) J. Neurosci. 18, 10457-10463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bell M. D., Taub, D. D. & Perry, V. H. (1996) Neuroscience 74, 283. [DOI] [PubMed] [Google Scholar]
- 49.Sprenger H., Rosler, A., Tonn, P., Braune, H. J., Huffmann, G. & Gemsa, D. (1996) Clin. Immunol. Immunopathol. 80, 155-161. [DOI] [PubMed] [Google Scholar]
- 50.Asensio V. & Campbell, I. L. (1997) J. Virol. 71, 7832-7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Charles P. C., Chen, X., Horwitz, M. S. & Brosnan, C. F. (1999) J. Neurovirol. 5, 55-64. [DOI] [PubMed] [Google Scholar]
- 52.Carr D. J., Noisakran, S., Halford, W. P., Lukacs, N., Asensio, V. & Campbell, I. L. (1998) J. Neuroimmunol. 85, 111-121. [DOI] [PubMed] [Google Scholar]
- 53.Rempel S. A., Dudas, S., Ge, S. & Gutirrez, J. A. (2000) Clin. Cancer Res. 6, 102-111. [PubMed] [Google Scholar]
- 54.Schmidtmayerova H., Nottet, H. S., Nuovo, G., Raabe, T., Flanagan, C. R., Dubrovsky, L., Gendelman, H. E., Cerami, A., Bukrinsky, M. & Sherry, B. (1996) Proc. Natl. Acad. Sci. USA 93, 700-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wetzel M. A., Steele, A. D., Eisenstein, T. K., Adler, M. W., Henderson, E. E. & Rogers, T. J. (2000) J. Immunol. 165, 6519-6524. [DOI] [PubMed] [Google Scholar]
- 56.Wetzel M. A., Steele, A. D., Henderson, E. E. & Rogers, T. J. (2002) Virology 292, 6-15. [DOI] [PubMed] [Google Scholar]
- 57.Giovannelli A., Limatola, C., Ragozzino, D., Mileo, A. M., Ruggieri, A., Ciotti, M. T., Mercanti, D., Santoni, A. & Eusebi, F. (1998) J. Neuroimmunol. 92, 122. [DOI] [PubMed] [Google Scholar]
- 58.Zubieta J. K., Smith, Y. R., Bueller, J. A., Xu, Y., Kilbourn, M. R., Jewett, D. M., Meyer, C. R., Koeppe, R. A. & Stohler, C. S. (2001) Science 293, 311-315. [DOI] [PubMed] [Google Scholar]
- 59.Watkins L. R., Maier, S. F. & Goehler, L. E. (1995) Pain 63, 289-302. [DOI] [PubMed] [Google Scholar]
- 60.Junger H. & Sorkin, L. S. (2000) Pain 85, 145-151. [DOI] [PubMed] [Google Scholar]