Abstract
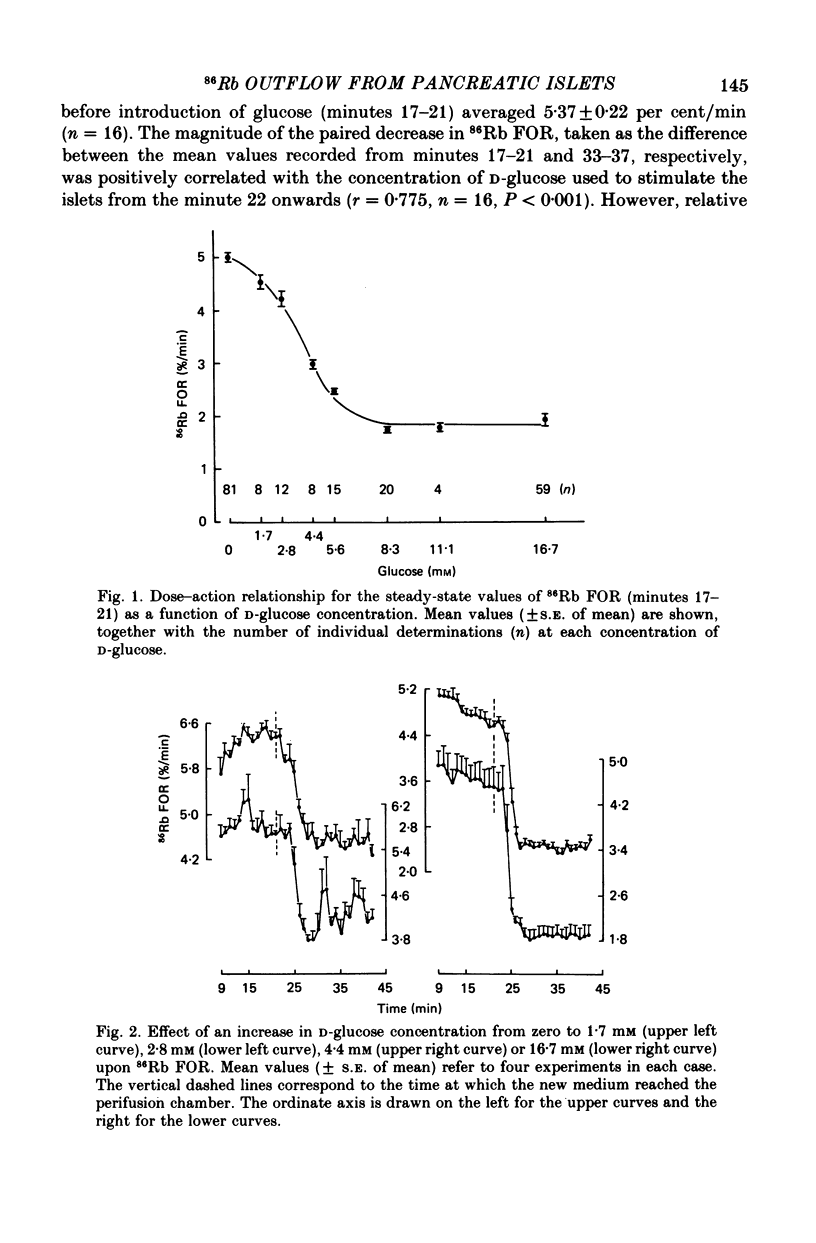
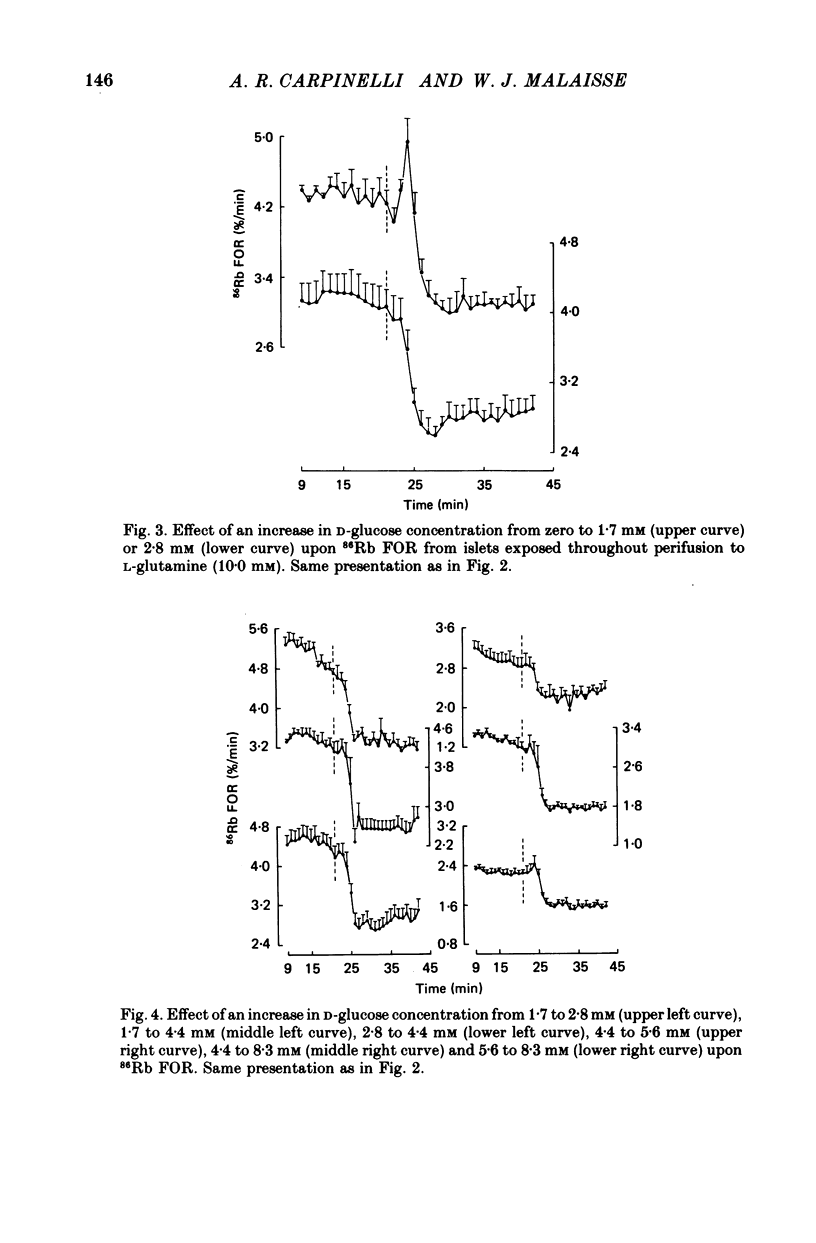
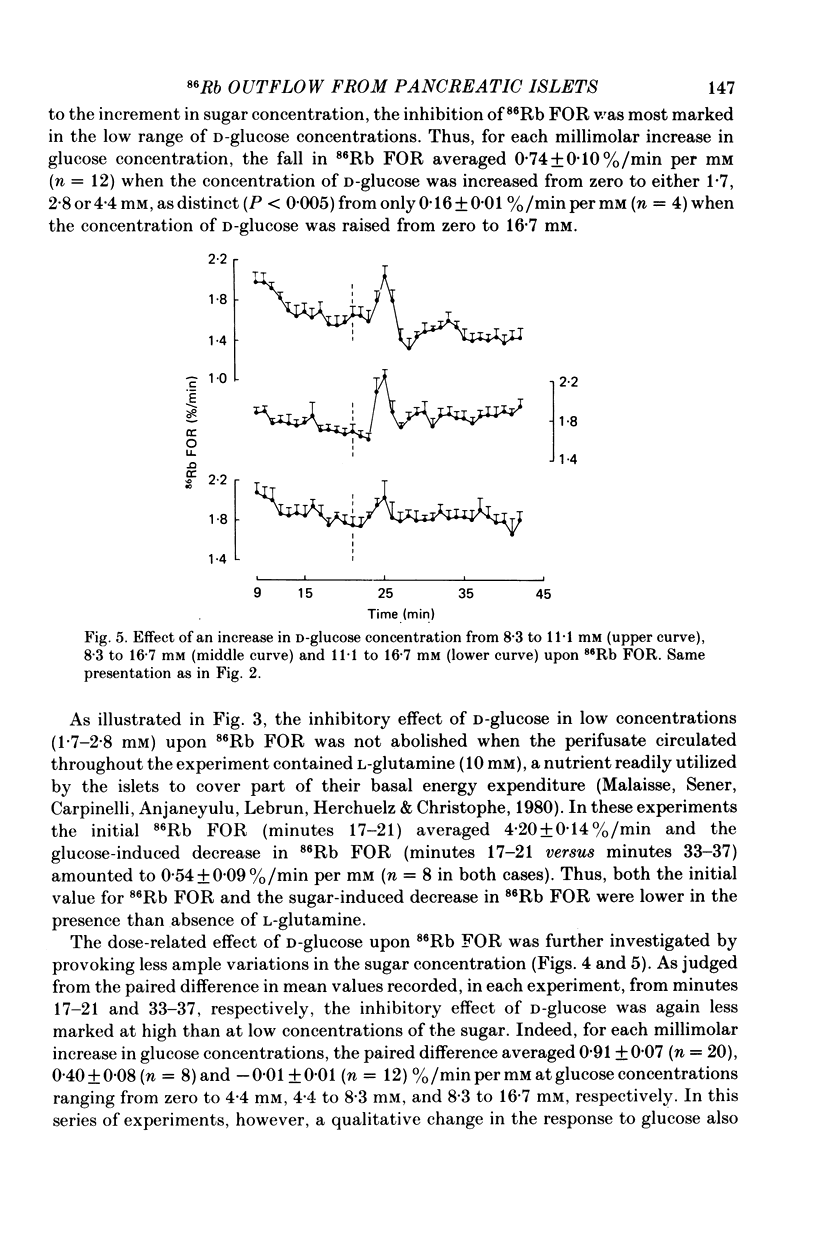
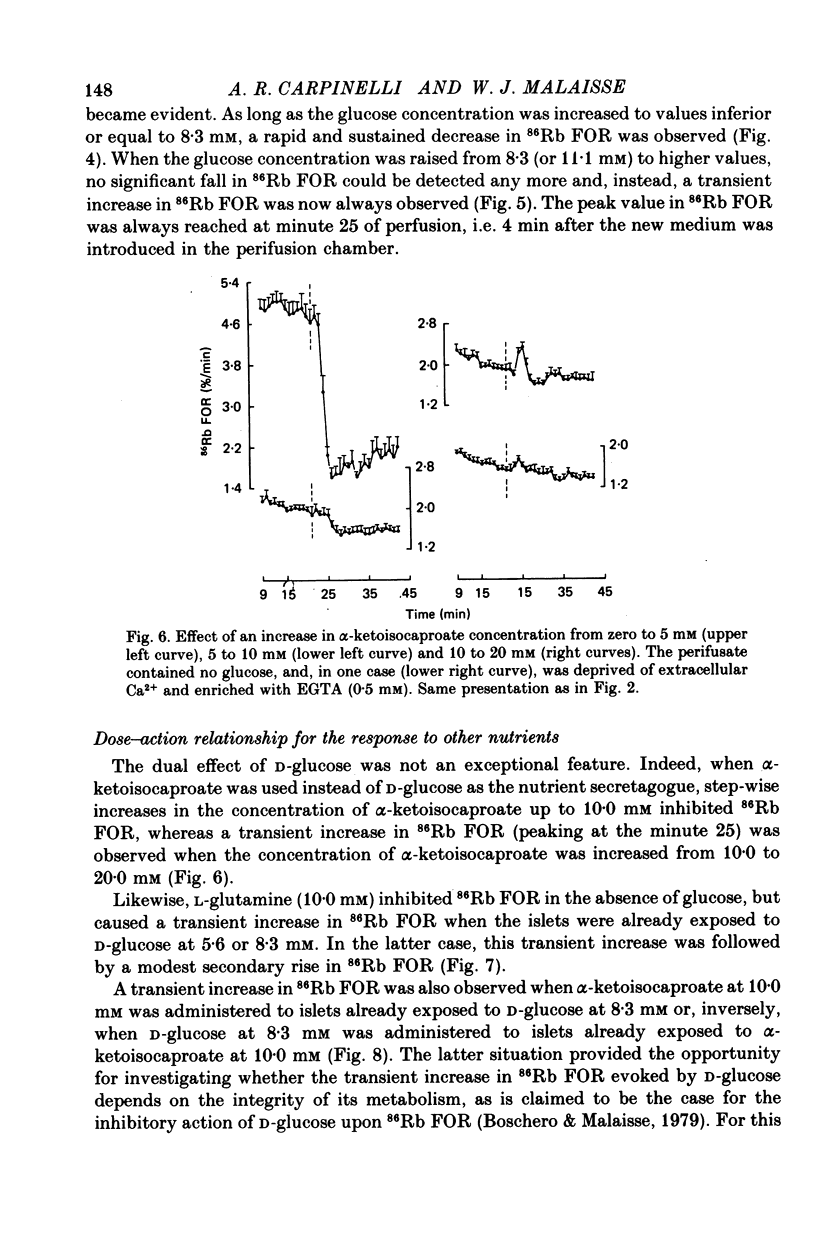
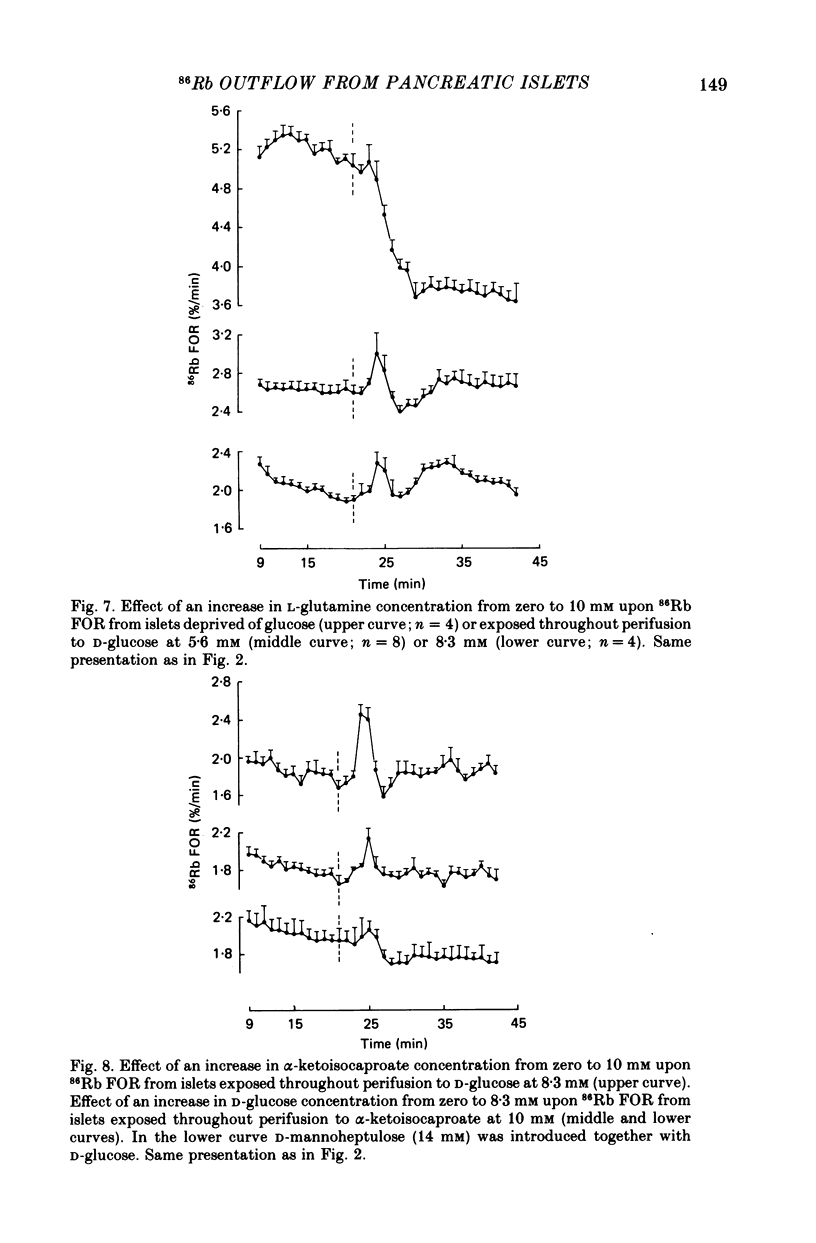
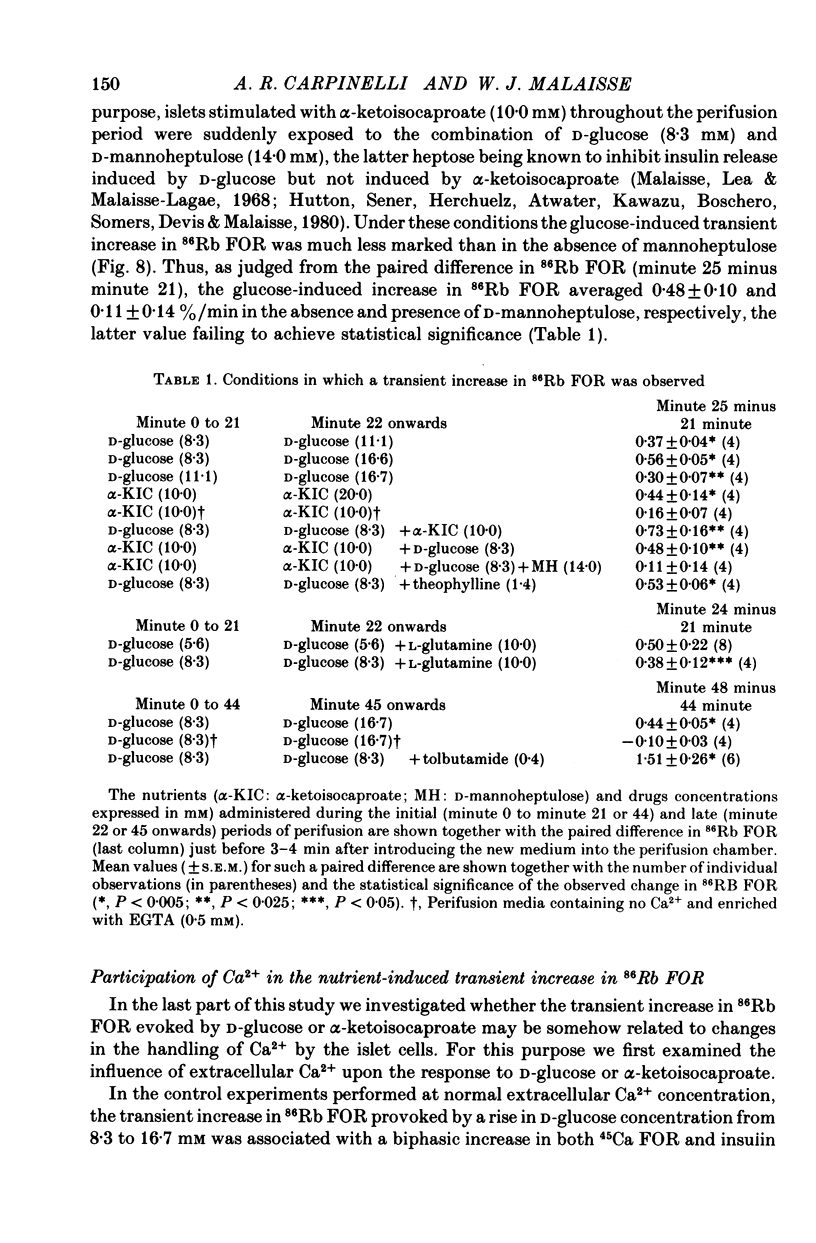
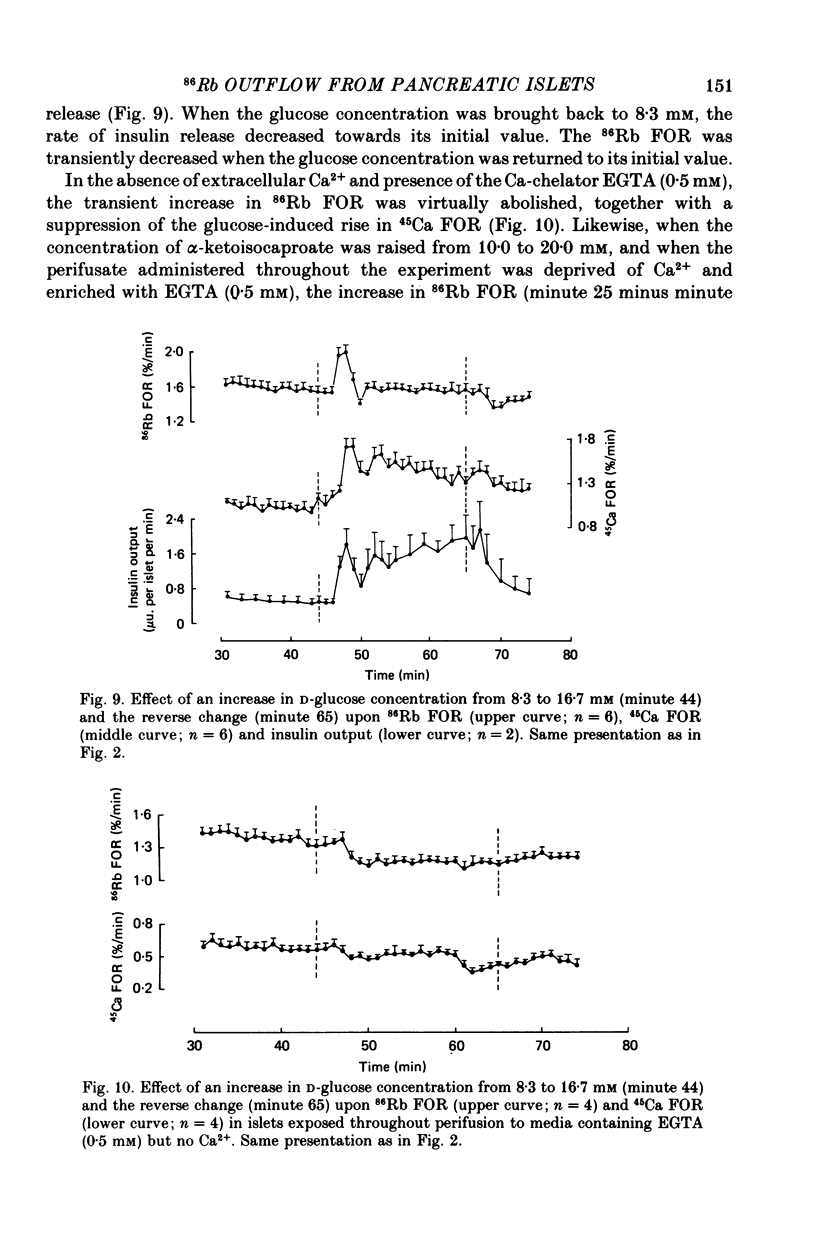
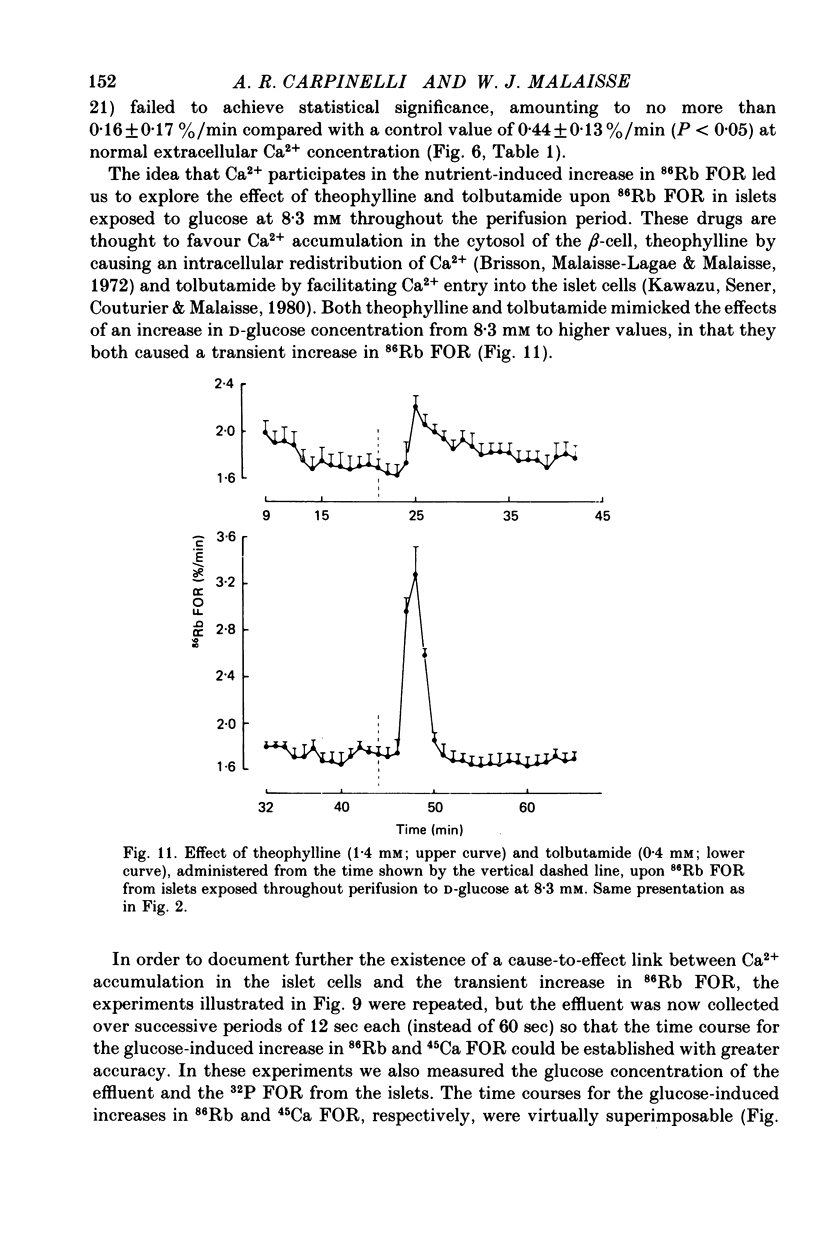
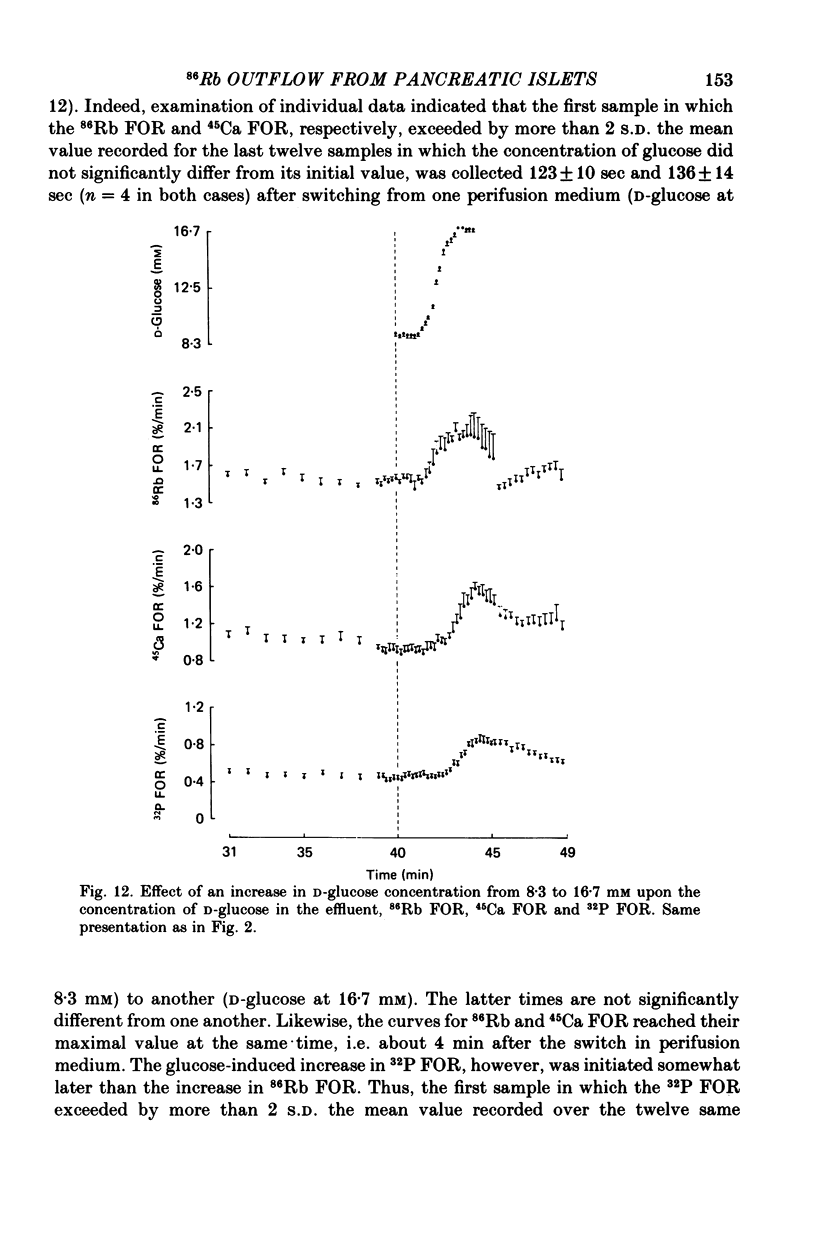
1. An increase in the concentration of extracellular D-glucose from zero to 1.7 mM or more (up to 16.7 mM) causes a rapid and sustained decrease in 86Rb fractional outflow rate (FOR) from prelabelled and perifused pancreatic rat islets. The 86Rb FOR also decreases when the concentration of D-glucose is raised from 1.7 mM or more (up to 5.6 mM) to higher values not exceeding 8.3 mM. 2. However, when the glucose concentration is raised from 8.3 mM (or 11.1 mM) to higher values, no decrease in 86Rb FOR is observed and, instead, a transient increase in 86Rb FOR now takes place. 3. Such a dual effect on 86Rb FOR is also observed when alpha-ketoisocaproic acid is used as the nutrient secretagogue or when the latter keto acid is used in combination with D-glucose. 4. The transient increase in 86Rb FOR evoked by D-glucose in islets already exposed to alpha-ketoisocaproate is abolished by mannoheptulose, suggesting that it depends on the integrity of glucose metabolism. 5. The transient increase in 86Rb FOR evoked, under suitable experimental conditions, by D-glucose of alpha-ketoisocaproate is abolished in the absence of extracellular Ca2+ and mimicked by theophylline and tolbutamide, suggesting that it is attributable to an increase in cytosolic Ca2+ concentration. The latter view is supported by the fact that the increase in 86Rb FOR coincides with an increase in 45Ca FOR, provided that Ca2+ is not removed from the extracellular medium. 6. It is concluded that, in contrast to the situation found when the concentration of the nutrient secretagogue is increased from a non-insulinotropic to a higher value, the stimulation of Ca2+ entry into islet cells and the subsequent increase in insulin secretion evoked by D-glucose or alpha-ketoisocaproate when the concentration of these nutrients is increased from intermediate (8.3-10.0 mM) to higher values is not attributable to a decrease in K+ conductance.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atwater I., Dawson C. M., Ribalet B., Rojas E. Potassium permeability activated by intracellular calcium ion concentration in the pancreatic beta-cell. J Physiol. 1979 Mar;288:575–588. [PMC free article] [PubMed] [Google Scholar]
- Boschero A. C., Kawazu S., Duncan G., Malaisse W. J. Effect of glucose on K+ handling by pancreatic islets. FEBS Lett. 1977 Nov 1;83(1):151–154. doi: 10.1016/0014-5793(77)80662-5. [DOI] [PubMed] [Google Scholar]
- Boschero A. C., Malaisse W. J. Stimulus-secretion coupling of glucose-induced insulin release. XXIX. Regulation of 86Rb+ efflux from perfused islets. Am J Physiol. 1979 Feb;236(2):E139–E146. doi: 10.1152/ajpendo.1979.236.2.E139. [DOI] [PubMed] [Google Scholar]
- Brisson G. R., Malaisse-Lagae F., Malaisse W. J. The stimulus-secretion coupling of glucose-induced insulin release. VII. A proposed site of action for adenosine-3',5'-cyclic monophosphate. J Clin Invest. 1972 Feb;51(2):232–241. doi: 10.1172/JCI106808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpinelli A. R., Malaisse W. J. The stimulus-secretion coupling in glucose-induced insulin release xliv. A possible link between glucose metabolism and phosphate flush. Diabetologia. 1980 Nov;19(5):458–464. doi: 10.1007/BF00281826. [DOI] [PubMed] [Google Scholar]
- Carpinelli A., Malaisse W. J. Regulation of 86Rb+ outflow from pancreatic islets. I. Reciprocal changes in the response to glucose, tetraethylammonium and quinine. Mol Cell Endocrinol. 1980 Feb;17(2):103–110. doi: 10.1016/0303-7207(80)90122-7. [DOI] [PubMed] [Google Scholar]
- Henquin J. C. D-glucose inhibits potassium efflux from pancreatic islet cells. Nature. 1978 Jan 19;271(5642):271–273. doi: 10.1038/271271a0. [DOI] [PubMed] [Google Scholar]
- Henquin J. C. Metabolic control of potassium permeability in pancreatic islet cells. Biochem J. 1980 Feb 15;186(2):541–550. doi: 10.1042/bj1860541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herchuelz A., Couturier E., Malaisse W. J. Regulation of calcium fluxes in pancreatic islets: glucose-induced calcium-calcium exchange. Am J Physiol. 1980 Feb;238(2):E96–103. doi: 10.1152/ajpendo.1980.238.2.E96. [DOI] [PubMed] [Google Scholar]
- Herchuelz A., Malaisse W. J. Regulation of calcium fluxes in pancreatic islets: dissociation between calcium and insulin release. J Physiol. 1978 Oct;283:409–424. doi: 10.1113/jphysiol.1978.sp012509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herchuelz A., Sener A., Malaisse W. J. Regulation of calcium fluxes in rat pancreatic islets: calcium extrusion by sodium-calcium countertransport. J Membr Biol. 1980 Nov 15;57(1):1–12. doi: 10.1007/BF01868981. [DOI] [PubMed] [Google Scholar]
- Hutton J. C., Sener A., Herchuelz A., Atwater I., Kawazu S., Boschero A. C., Somers G., Devis G., Malaisse W. J. Similarities in the stimulus-secretion coupling mechanisms of glucose- and 2-keto acid-induced insulin release. Endocrinology. 1980 Jan;106(1):203–219. doi: 10.1210/endo-106-1-203. [DOI] [PubMed] [Google Scholar]
- Kawazu S., Sener A., Couturier E., Malaisse W. J. Metabolic, cationic and secretory effects of hypoglycemic sulfonylureas in pancreatic islets. Naunyn Schmiedebergs Arch Pharmacol. 1980 Jul;312(3):277–283. doi: 10.1007/BF00499158. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Hutton J. C., Kawazu S., Herchuelz A., Valverde I., Sener A. The stimulus-secretion coupling of glucose-induced insulin release. XXXV. The links between metabolic and cationic events. Diabetologia. 1979 May;16(5):331–341. doi: 10.1007/BF01223623. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Lea M. A., Malaisse-Lagae F. The effect of mannoheptulose on the phosphorylation of glucose and the secretion of insulin by islets of Langerhans. Metabolism. 1968 Feb;17(2):126–132. doi: 10.1016/0026-0495(68)90138-8. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Sener A., Carpinelli A. R., Anjaneyulu K., Lebrun P., Herchuelz A., Christophe J. The stimulus-secretion coupling of glucose-induced insulin release. XLVI. Physiological role of L-glutamine as a fuel for pancreatic islets. Mol Cell Endocrinol. 1980 Nov;20(2):171–189. doi: 10.1016/0303-7207(80)90080-5. [DOI] [PubMed] [Google Scholar]
- Meissner H. P., Preissler M. Glucose-induced changes of the membrane potential of pancreatic B-cells: their significance for the regulation of insulin release. Adv Exp Med Biol. 1979;119:97–107. doi: 10.1007/978-1-4615-9110-8_15. [DOI] [PubMed] [Google Scholar]
- Meissner H. P., Preissler M. Ionic mechanisms of the glucose-induced membrane potential changes in B-cells. Horm Metab Res Suppl. 1980;Suppl 10:91–99. [PubMed] [Google Scholar]
- Sehlin J., Täljedal I. B. Transport of rubidium and sodium in pancreatic islets. J Physiol. 1974 Oct;242(2):505–515. doi: 10.1113/jphysiol.1974.sp010720. [DOI] [PMC free article] [PubMed] [Google Scholar]


