Abstract
1. Experiments were performed on exteriorized fetal lambs of between 69 days' gestation and term (147 days) in order to observe changes in lung volume and lung liquid secretion rate, and to delineate any alterations in solute permeability, ion transport and tight junction morphology in the maturing lung epithelium. Whilst it was technically possible to measure solute permeability as early as 69 days it was not feasible to apply the Ussing flux ratio technique before 84 days.
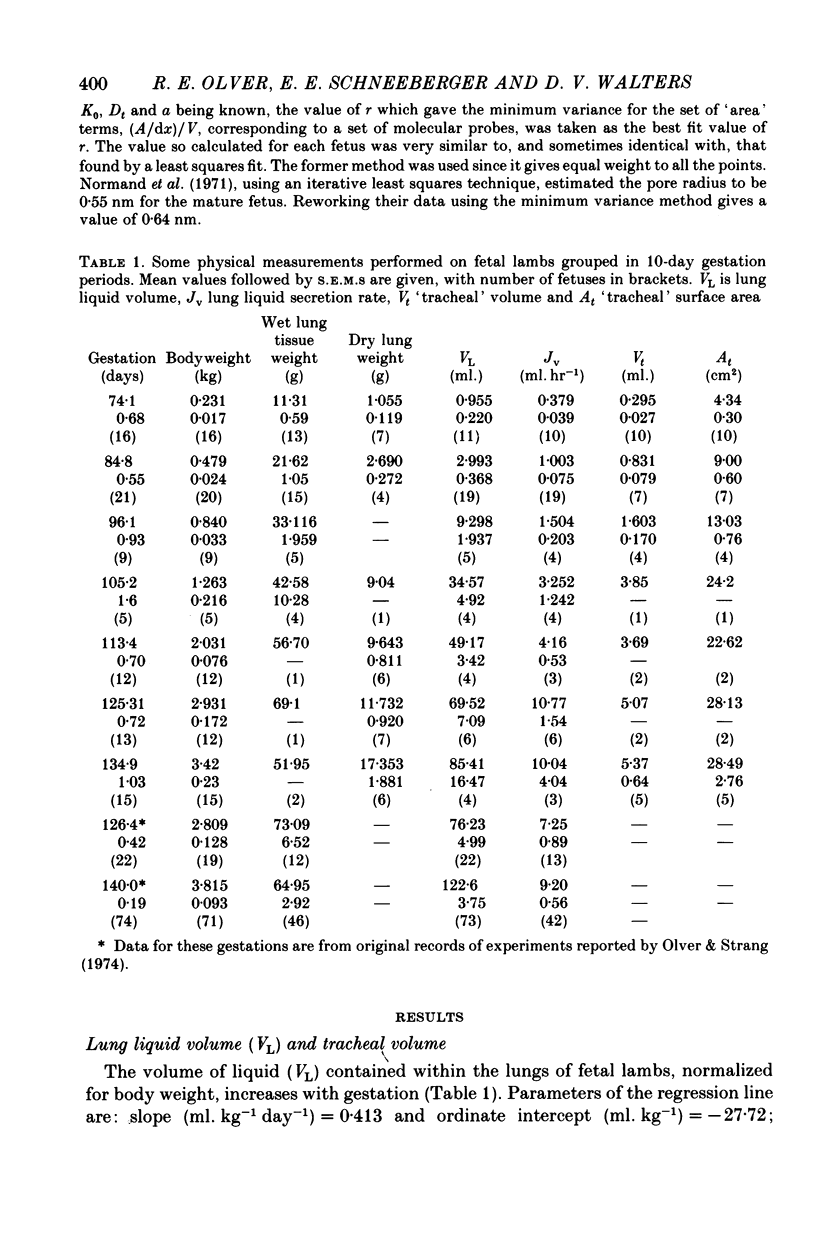
2. Fetal lung liquid volume and secretion rate, when normalized for body weight, increase linearly with gestation, whereas tracheal volume expressed in the same manner remains constant.
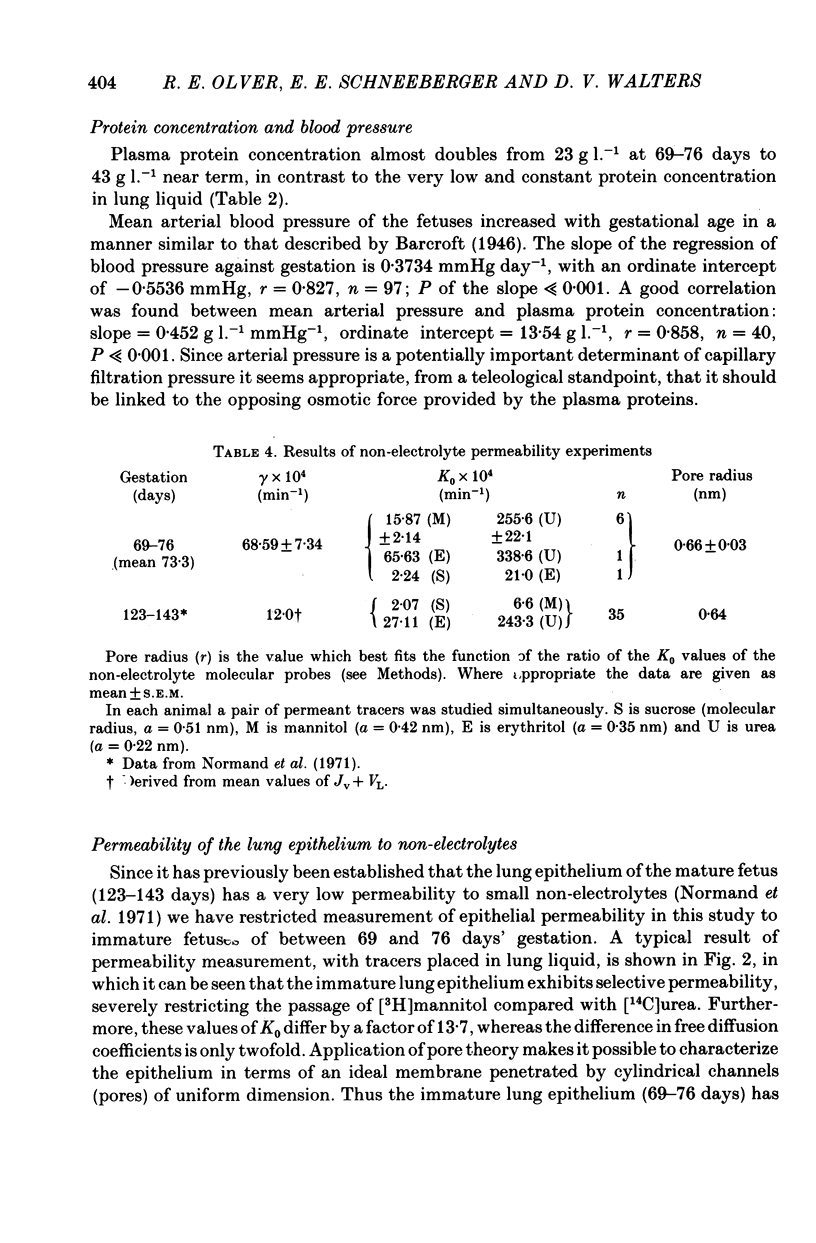
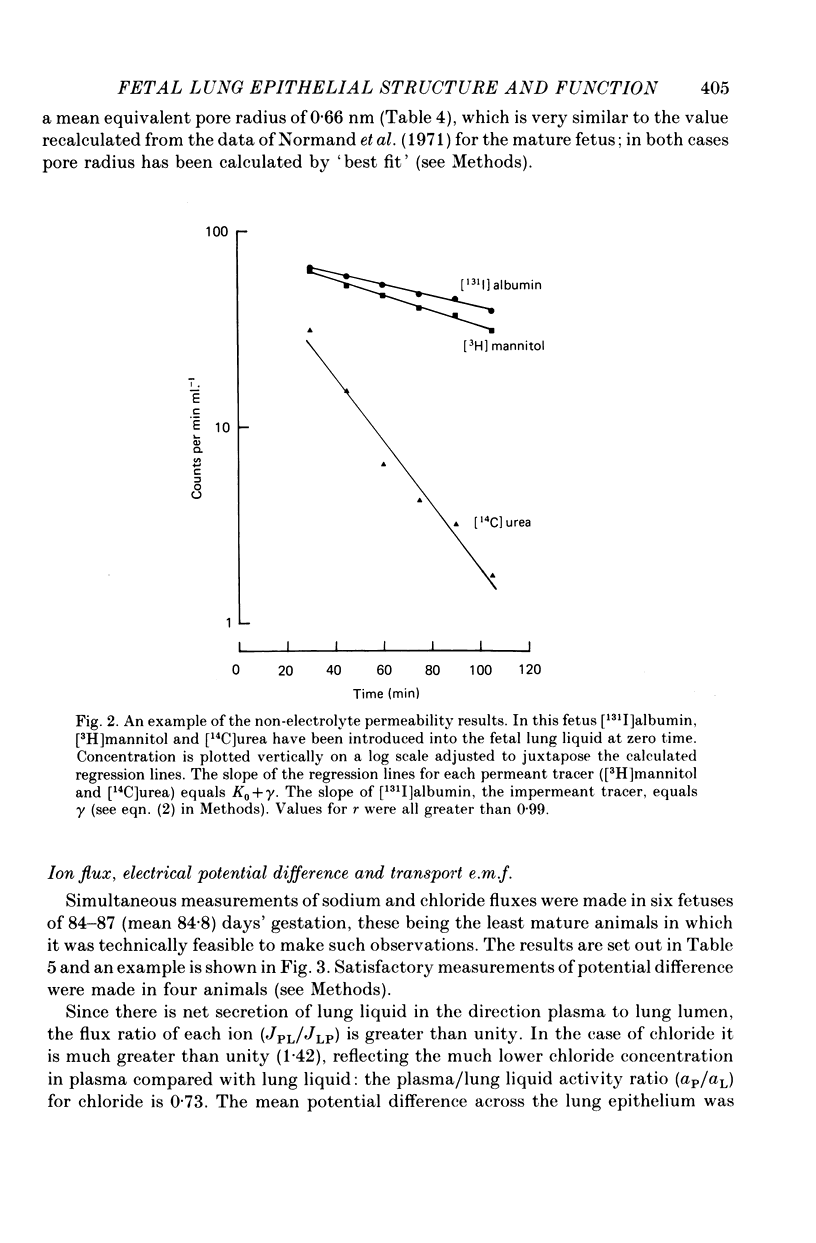
3. When expressed in terms of pore theory, epithelial permeability to small polar non-electrolytes does not change between 69 days and term (equivalent pore radius 0.66 nm and 0.64 nm respectively).
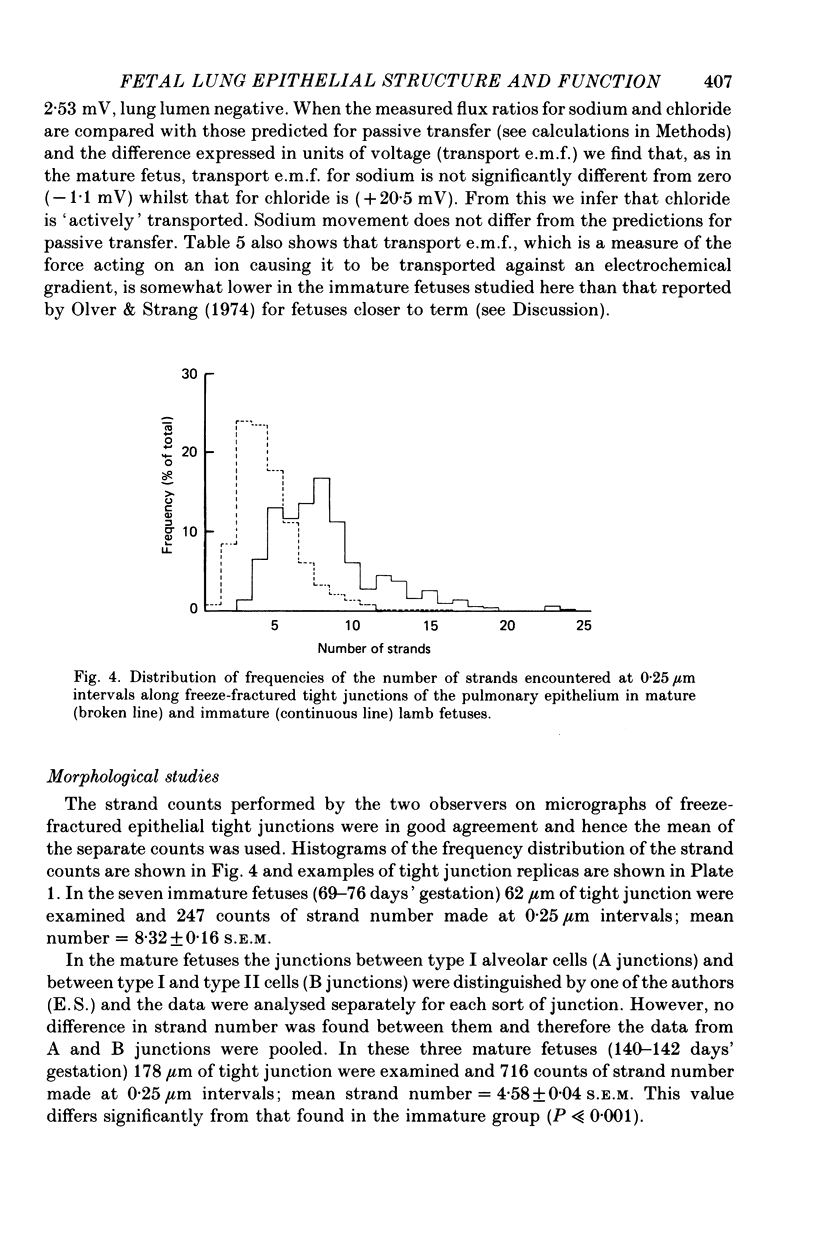
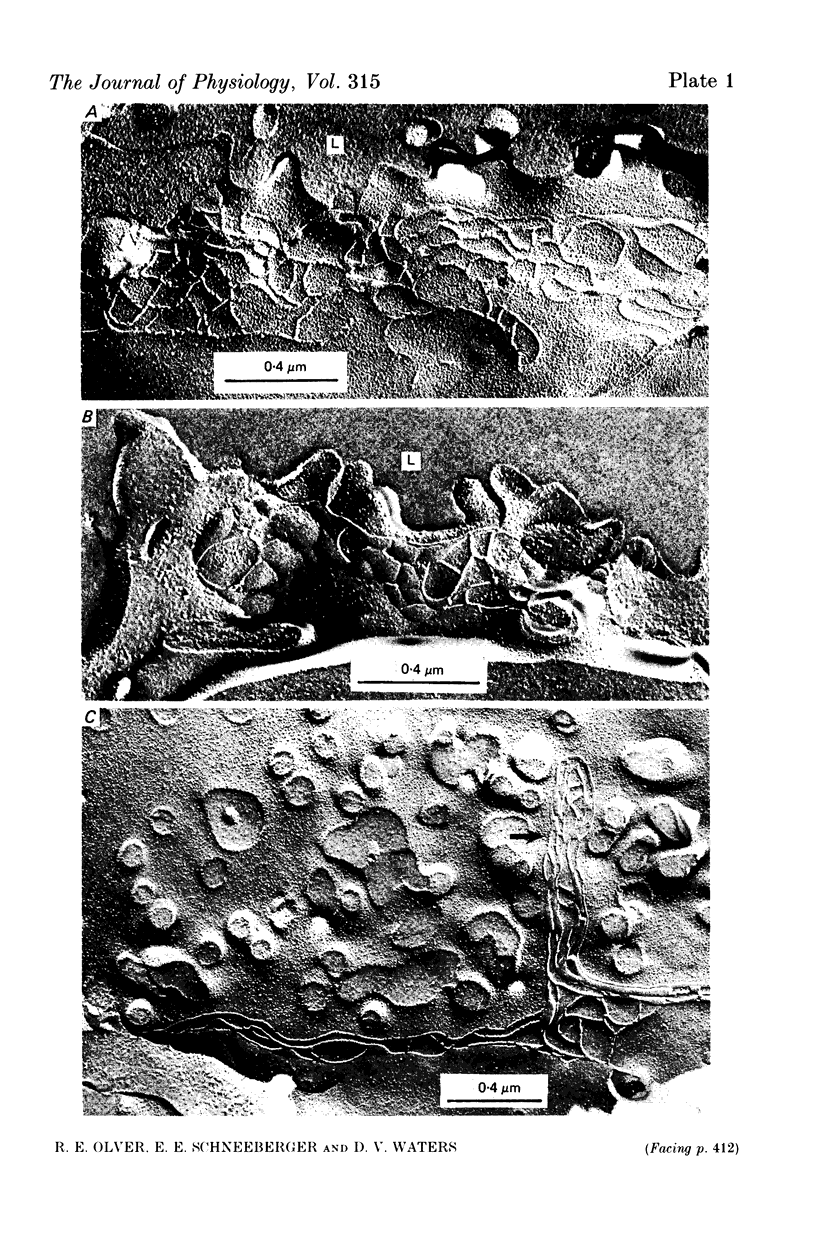
4. In the immature fetus of 69-76 days, mean epithelial tight junction strand number is 8.3, whereas by the end of gestation it has fallen to 4.6.
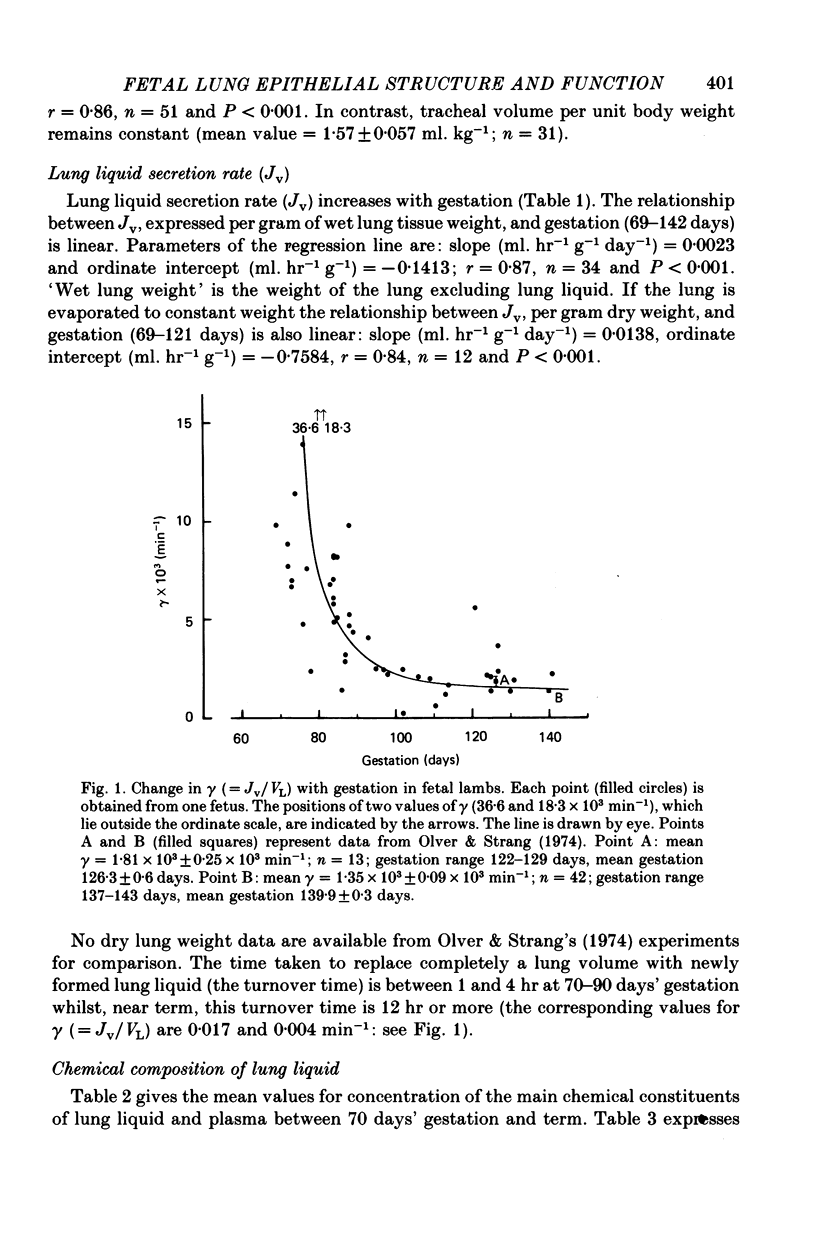
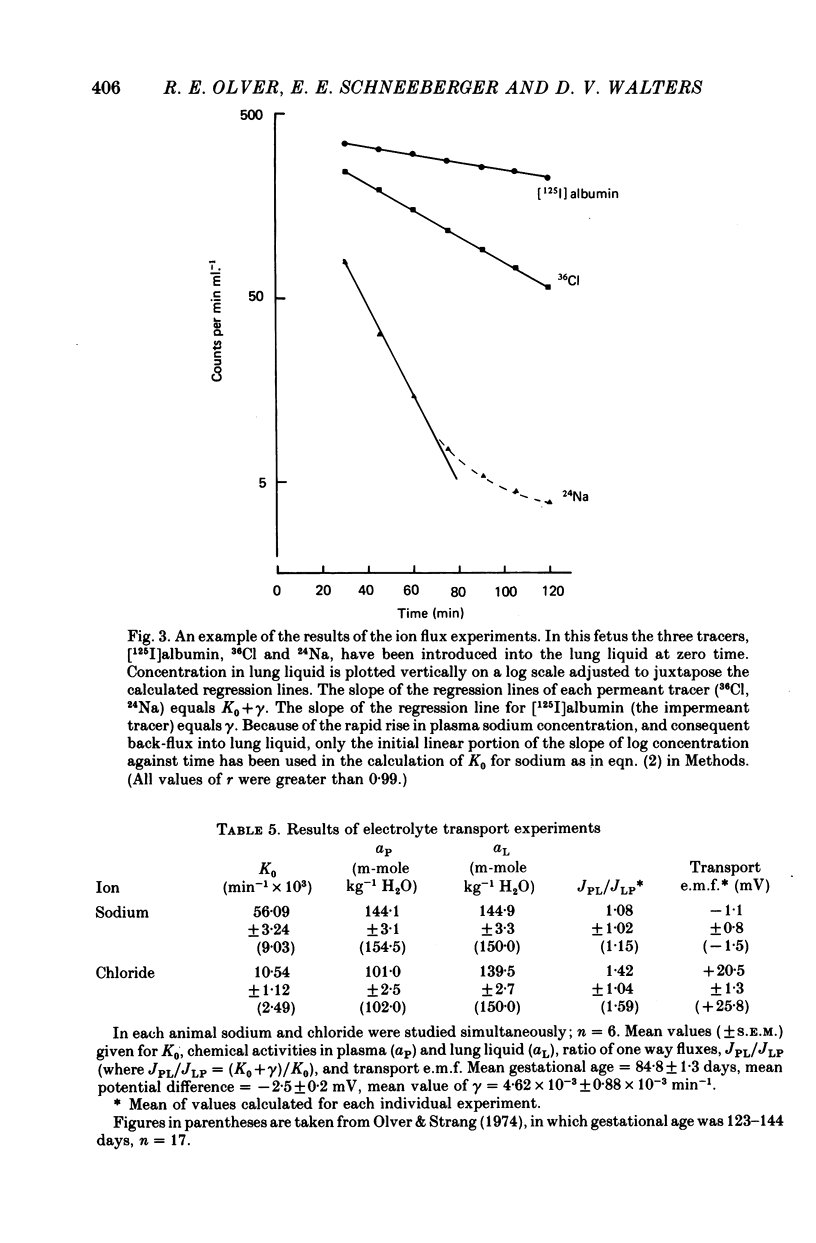
5. The transfer constants (min-1) for sodium and chloride movement in the direction lung liquid to plasma are, respectively, some 6 and 4 times greater at 84-87 days than at term.
6. As in the mature fetus, the lung epithelium at 84-87 days actively transports chloride from plasma to lung lumen, albeit with a slightly reduced transport e.m.f. Sodium movement does not, at any gestational age, differ from the predictions for passive transfer.
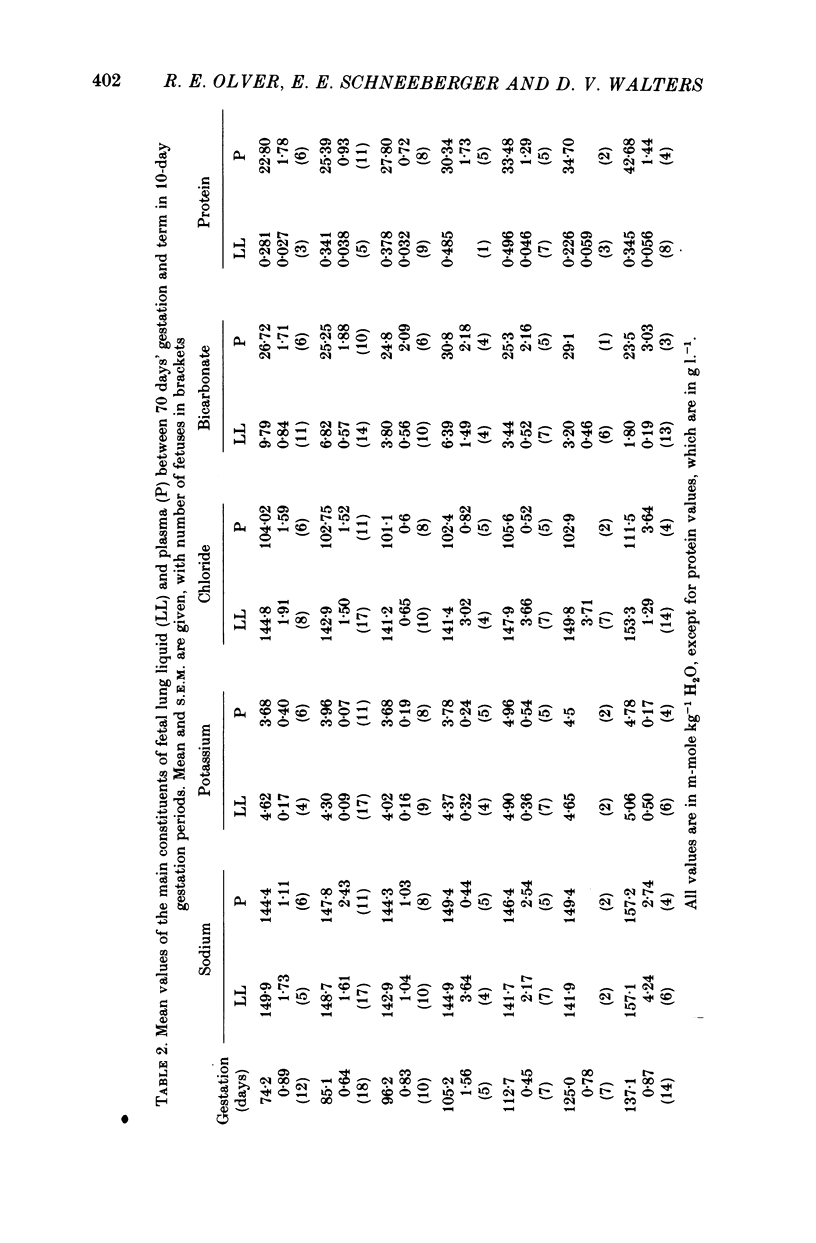
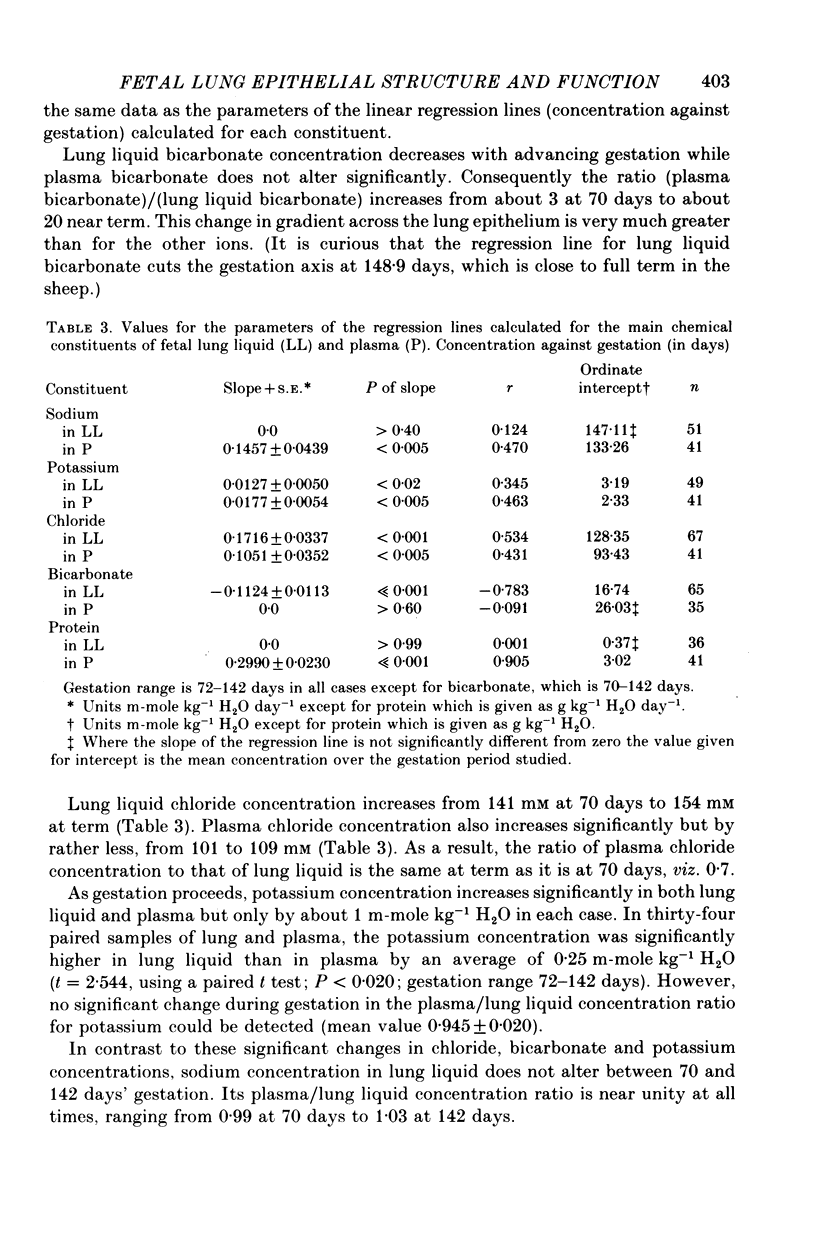
7. In lung liquid the concentrations of chloride and potassium increase and that of bicarbonate decreases during gestation, whilst that of sodium does not change. The rises in lung liquid chloride and potassium concentrations follow those in plasma, maintaining plasma/lung liquid ratios of 0.7 and 0.95 respectively. However, plasma bicarbonate remains constant and the plasma/lung liquid ratio for bicarbonate rises from 3 at 69-76 days to 20 near term as the lung liquid bicarbonate falls from 9.8 to under 2 m-mole kg-1 H2O.
8. Whereas lung liquid protein concentration remains constant and low at about 0.35 g l.-1, plasma protein concentration rises from 23 g l.-1 at 69-76 days to 43 g l.-1 near term. During the same period arterial blood pressure doubles.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADAMS F. H., MOSS A. J., FAGAN L. THE TRACHEAL FLUID IN THE FETAL LAMB. Biol Neonat. 1963;5:151–158. doi: 10.1159/000239867. [DOI] [PubMed] [Google Scholar]
- Adamson T. M., Boyd R. D., Platt H. S., Strang L. B. Composition of alveolar liquid in the foetal lamb. J Physiol. 1969 Sep;204(1):159–168. doi: 10.1113/jphysiol.1969.sp008905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcorn D., Adamson T. M., Lambert T. F., Maloney J. E., Ritchie B. C., Robinson P. M. Morphological effects of chronic tracheal ligation and drainage in the fetal lamb lung. J Anat. 1977 Jul;123(Pt 3):649–660. [PMC free article] [PubMed] [Google Scholar]
- Claude P., Goodenough D. A. Fracture faces of zonulae occludentes from "tight" and "leaky" epithelia. J Cell Biol. 1973 Aug;58(2):390–400. doi: 10.1083/jcb.58.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J. M., Wright E. M. Biological membranes: the physical basis of ion and nonelectrolyte selectivity. Annu Rev Physiol. 1969;31:581–646. doi: 10.1146/annurev.ph.31.030169.003053. [DOI] [PubMed] [Google Scholar]
- Dziegielewska K. M., Evans C. A., Malinowska D. H., Møllgård K., Reynolds J. M., Reynolds M. L., Saunders N. R. Studies of the development of brain barrier systems to lipid insoluble molecules in fetal sheep. J Physiol. 1979 Jul;292:207–231. doi: 10.1113/jphysiol.1979.sp012847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOEFOED-JOHNSEN V., USSING H. H. The contributions of diffusion and flow to the passage of D2O through living membranes; effect of neurohypophyseal hormone on isolated anuran skin. Acta Physiol Scand. 1953 Mar 31;28(1):60–76. doi: 10.1111/j.1748-1716.1953.tb00959.x. [DOI] [PubMed] [Google Scholar]
- Mescher E. J., Platzker A. C., Ballard P. L., Kitterman J. A., Clements J. A., Tooley W. H. Ontogeny of tracheal fluid, pulmonary surfactant, and plasma corticoids in the fetal lamb. J Appl Physiol. 1975 Dec;39(6):1017–1021. doi: 10.1152/jappl.1975.39.6.1017. [DOI] [PubMed] [Google Scholar]
- Normand I. C., Olver R. E., Reynolds E. O., Strang L. B. Permeability of lung capillaries and alveoli to non-electrolytes in the foetal lamb. J Physiol. 1971 Dec;219(2):303–330. doi: 10.1113/jphysiol.1971.sp009663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olver R. E., Strang L. B. Ion fluxes across the pulmonary epithelium and the secretion of lung liquid in the foetal lamb. J Physiol. 1974 Sep;241(2):327–357. doi: 10.1113/jphysiol.1974.sp010659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeberger E. E., Walters D. V., Olver R. E. Development of intercellular junctions in the pulmonary epithelium of the foetal lamb. J Cell Sci. 1978 Aug;32:307–324. doi: 10.1242/jcs.32.1.307. [DOI] [PubMed] [Google Scholar]
- USSING H. H., ZERAHN K. Active transport of sodium as the source of electric current in the short-circuited isolated frog skin. Acta Physiol Scand. 1951 Aug 25;23(2-3):110–127. doi: 10.1111/j.1748-1716.1951.tb00800.x. [DOI] [PubMed] [Google Scholar]
- Walters D. V., Olver R. E. The role of catecholamines in lung liquid absorption at birth. Pediatr Res. 1978 Mar;12(3):239–242. doi: 10.1203/00006450-197803000-00017. [DOI] [PubMed] [Google Scholar]



