Abstract
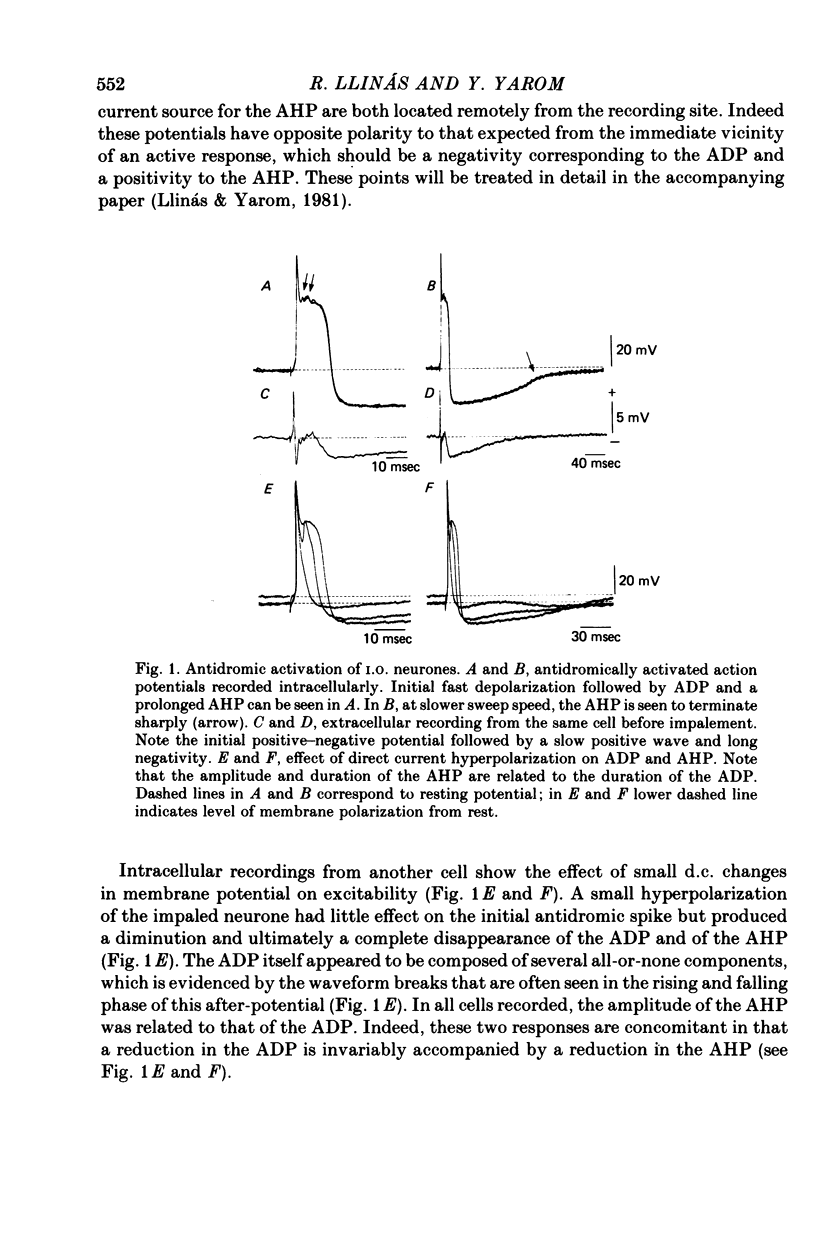
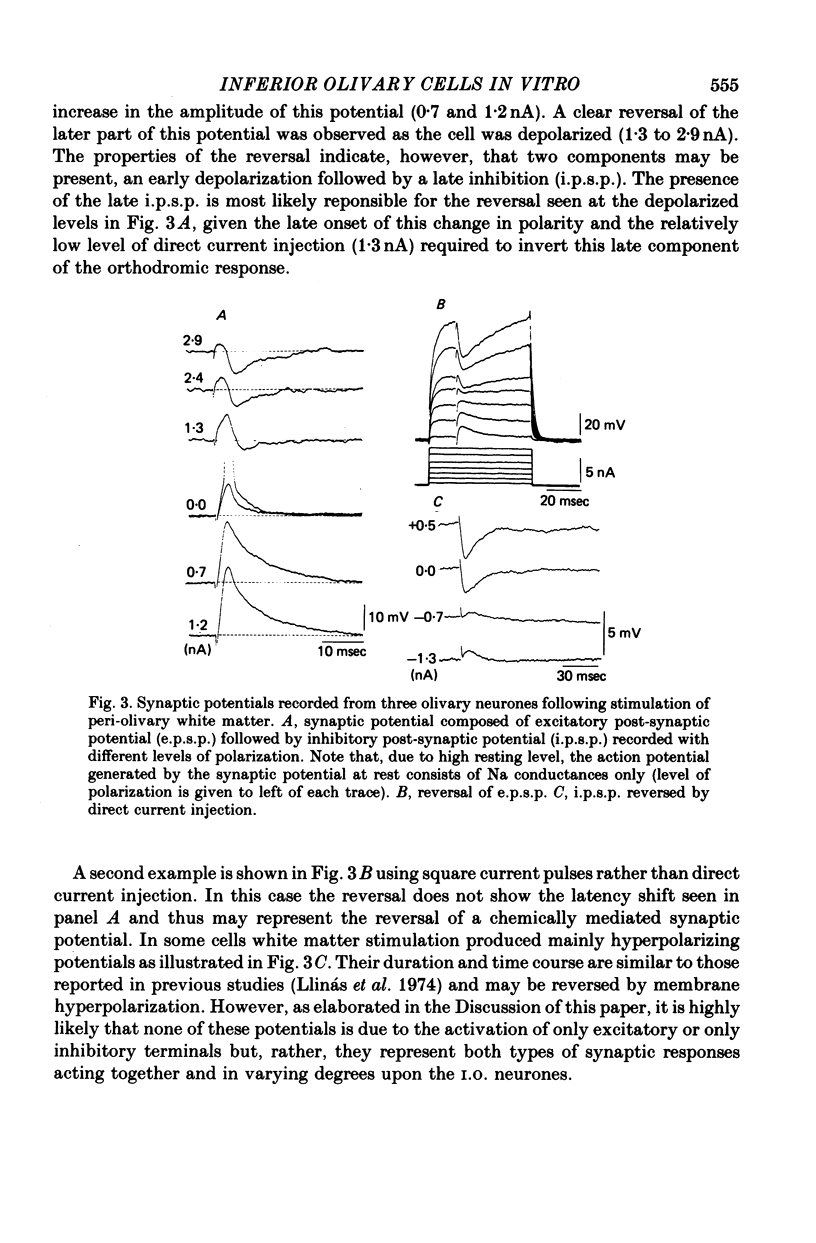
The electrophysiological properties of guinea-pig inferior olivary (I.O.) cells have been studied in an in vitro brain stem slice preparation. 1. Intracellular recordings from 185 neurones in this nucleus reveal that antidromic, orthodromic or direct stimulation generates action potentials consisting of a fast spike followed by an after-depolarizing potential (ADP). The ADP had an amplitude of 49 +/- 8 mV (mean +/- S.D.) and a duration which varied over a wide range with the level of depolarization. This ADP is followed by an after-hyperpolarizing potential (AHP) having an amplitude of 12 +/- 3 mV (mean +/- S.D.) from rest and lasting up to 250 msec. The AHP shows a rebound depolarization wave. 2. Synaptic activation may be obtained by peri-olivary stimulation with a bipolar electrode located in the immediate vicinity of the I.O. nucleus. These potentials are a mixture of depolarizing and hyperpolarizing synaptic events which can be reversed by direct membrane polarization. 3. Addition of tetrodotoxin (TTX) to the bath, or removal of extracellular Na, abolishes the fast initial action potential but does not modify the ADP or the AHP. Blockage of Ca conductance by Co, Mn, Cd or D600, or replacement of Ca by Mg, abolishes the ADP--AHP sequence. 4. Hyperpolarization of the neurone uncovers a low-threshold Ca conductance which is inactivated at rest and has similar pharmacological properties to the ADP. This low-threshold spike plays a central role in the rebound potential following the AHP. 5. Simultaneous impalement of I.O. neurone pairs demonstrated the presence of electrotonic coupling between neurones, which is especially prominent in the medial accessory olive.
Full text
PDF
















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong B. D., Harvey R. J. Responses in the inferior olive to stimulation of the cerebellar and cerebral cortices in the cat. J Physiol. 1966 Dec;187(3):553–574. doi: 10.1113/jphysiol.1966.sp008108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D. M., Eccles J. C., Harvey R. J., Matthews P. B. Responses in the dorsal accessory olive of the cat to stimulation of hind limb afferents. J Physiol. 1968 Jan;194(1):125–145. doi: 10.1113/jphysiol.1968.sp008398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Hodgkin A. L., Ridgway E. B. Depolarization and calcium entry in squid giant axons. J Physiol. 1971 Nov;218(3):709–755. doi: 10.1113/jphysiol.1971.sp009641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker R., Llinás R. Electrotonic coupling between neurones in the rat mesencephalic nucleus. J Physiol. 1971 Jan;212(1):45–63. doi: 10.1113/jphysiol.1971.sp009309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E. F., Barret J. N. Separation of two voltage-sensitive potassium currents, and demonstration of a tetrodotoxin-resistant calcium current in frog motoneurones. J Physiol. 1976 Mar;255(3):737–774. doi: 10.1113/jphysiol.1976.sp011306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baux G., Simonneau M., Tauc L., Segundo J. P. Uncoupling of electrotonic synapses by calcium. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4577–4581. doi: 10.1073/pnas.75.9.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. V. Physiology of electrotonic junctions. Ann N Y Acad Sci. 1966 Jul 14;137(2):509–539. doi: 10.1111/j.1749-6632.1966.tb50178.x. [DOI] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol. 1971 Feb;213(1):21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crill W. E. Unitary multiple-spiked responses in cat inferior olive nucleus. J Neurophysiol. 1970 Mar;33(2):199–209. doi: 10.1152/jn.1970.33.2.199. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K. The excitatory synaptic action of climbing fibres on the Purkinje cells of the cerebellum. J Physiol. 1966 Jan;182(2):268–296. doi: 10.1113/jphysiol.1966.sp007824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGIWARA S., KUSANO K., SAITO N. Membrane changes of Onchidium nerve cell in potassium-rich media. J Physiol. 1961 Mar;155:470–489. doi: 10.1113/jphysiol.1961.sp006640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S. Ca spike. Adv Biophys. 1973;4:71–102. [PubMed] [Google Scholar]
- Headley P. M., Lodge D., Duggan A. W. Drug-induced rhythmical activity in the inferior olivary complex of the rat. Brain Res. 1976 Jan 23;101(3):461–478. doi: 10.1016/0006-8993(76)90471-6. [DOI] [PubMed] [Google Scholar]
- Headley P. M., Lodge D. Studies on field potentials and on single cells in the inferior olivary complex of the rat. Brain Res. 1976 Jan 23;101(3):445–459. doi: 10.1016/0006-8993(76)90470-4. [DOI] [PubMed] [Google Scholar]
- Heyer C. B., Lux H. D. Control of the delayed outward potassium currents in bursting pace-maker neurones of the snail, Helix pomatia. J Physiol. 1976 Nov;262(2):349–382. doi: 10.1113/jphysiol.1976.sp011599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer C. B., Lux H. D. Properties of a facilitating calcium current in pace-maker neurones of the snail, Helix pomatia. J Physiol. 1976 Nov;262(2):319–348. doi: 10.1113/jphysiol.1976.sp011598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotson J. R., Prince D. A. A calcium-activated hyperpolarization follows repetitive firing in hippocampal neurons. J Neurophysiol. 1980 Feb;43(2):409–419. doi: 10.1152/jn.1980.43.2.409. [DOI] [PubMed] [Google Scholar]
- King J. S. The synaptic cluster (glomerulus) in the inferior olivary nucleus. J Comp Neurol. 1976 Feb 1;165(3):387–400. doi: 10.1002/cne.901650307. [DOI] [PubMed] [Google Scholar]
- Korn H., Sotelo C., Crepel F. Electronic coupling between neurons in the rat lateral vestibular nucleus. Exp Brain Res. 1973 Jan 29;16(3):255–275. [PubMed] [Google Scholar]
- Kostyuk P. G., Krishtal O. A., Shakhovalov Y. A. Separation of sodium and calcium currents in the somatic membrane of mollusc neurones. J Physiol. 1977 Sep;270(3):545–568. doi: 10.1113/jphysiol.1977.sp011968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamarre Y., Puil E. Induction of rhythmic activity by harmaline. Can J Physiol Pharmacol. 1974 Aug;52(4):905–908. doi: 10.1139/y74-118. [DOI] [PubMed] [Google Scholar]
- Llinas R., Baker R., Sotelo C. Electrotonic coupling between neurons in cat inferior olive. J Neurophysiol. 1974 May;37(3):560–571. doi: 10.1152/jn.1974.37.3.560. [DOI] [PubMed] [Google Scholar]
- Llinas R., Nicholson C. Electrophysiological properties of dendrites and somata in alligator Purkinje cells. J Neurophysiol. 1971 Jul;34(4):532–551. doi: 10.1152/jn.1971.34.4.532. [DOI] [PubMed] [Google Scholar]
- Llinás R. Eighteenth Bowditch lecture. Motor aspects of cerebellar control. Physiologist. 1974 Feb;17(1):19–46. [PubMed] [Google Scholar]
- Llinás R., Hess R. Tetrodotoxin-resistant dendritic spikes in avian Purkinje cells. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2520–2523. doi: 10.1073/pnas.73.7.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell dendrites in mammalian cerebellar slices. J Physiol. 1980 Aug;305:197–213. doi: 10.1113/jphysiol.1980.sp013358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J Physiol. 1980 Aug;305:171–195. doi: 10.1113/jphysiol.1980.sp013357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Volkind R. A. The olivo-cerebellar system: functional properties as revealed by harmaline-induced tremor. Exp Brain Res. 1973 Aug 31;18(1):69–87. doi: 10.1007/BF00236557. [DOI] [PubMed] [Google Scholar]
- Llinás R., Yarom Y. Properties and distribution of ionic conductances generating electroresponsiveness of mammalian inferior olivary neurones in vitro. J Physiol. 1981 Jun;315:569–584. doi: 10.1113/jphysiol.1981.sp013764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W. Calcium-dependent potassium activation in nervous tissues. Annu Rev Biophys Bioeng. 1978;7:1–18. doi: 10.1146/annurev.bb.07.060178.000245. [DOI] [PubMed] [Google Scholar]
- Meech R. W., Standen N. B. Potassium activation in Helix aspersa neurones under voltage clamp: a component mediated by calcium influx. J Physiol. 1975 Jul;249(2):211–239. doi: 10.1113/jphysiol.1975.sp011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose B., Loewenstein W. R. Permeability of cell junction depends on local cytoplasmic calcium activity. Nature. 1975 Mar 20;254(5497):250–252. doi: 10.1038/254250a0. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin P. A., Slawsky M. Probable calcium spikes in hippocampal neurons. Brain Res. 1977 Oct 21;135(1):157–161. doi: 10.1016/0006-8993(77)91060-5. [DOI] [PubMed] [Google Scholar]
- Sotelo C., Llinas R., Baker R. Structural study of inferior olivary nucleus of the cat: morphological correlates of electrotonic coupling. J Neurophysiol. 1974 May;37(3):541–559. doi: 10.1152/jn.1974.37.3.541. [DOI] [PubMed] [Google Scholar]
- Spira M. E., Bennett M. V. Synaptic control of electrotonic coupling between neurons. Brain Res. 1972 Feb 25;37(2):294–300. doi: 10.1016/0006-8993(72)90674-9. [DOI] [PubMed] [Google Scholar]
- Wong R. K., Prince D. A., Basbaum A. I. Intradendritic recordings from hippocampal neurons. Proc Natl Acad Sci U S A. 1979 Feb;76(2):986–990. doi: 10.1073/pnas.76.2.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Montigny C., Lamarre Y. Rhythmic activity induced by harmaline in the olivo-cerebello-bulbar system of the cat. Brain Res. 1973 Apr 13;53(1):81–95. doi: 10.1016/0006-8993(73)90768-3. [DOI] [PubMed] [Google Scholar]