Abstract
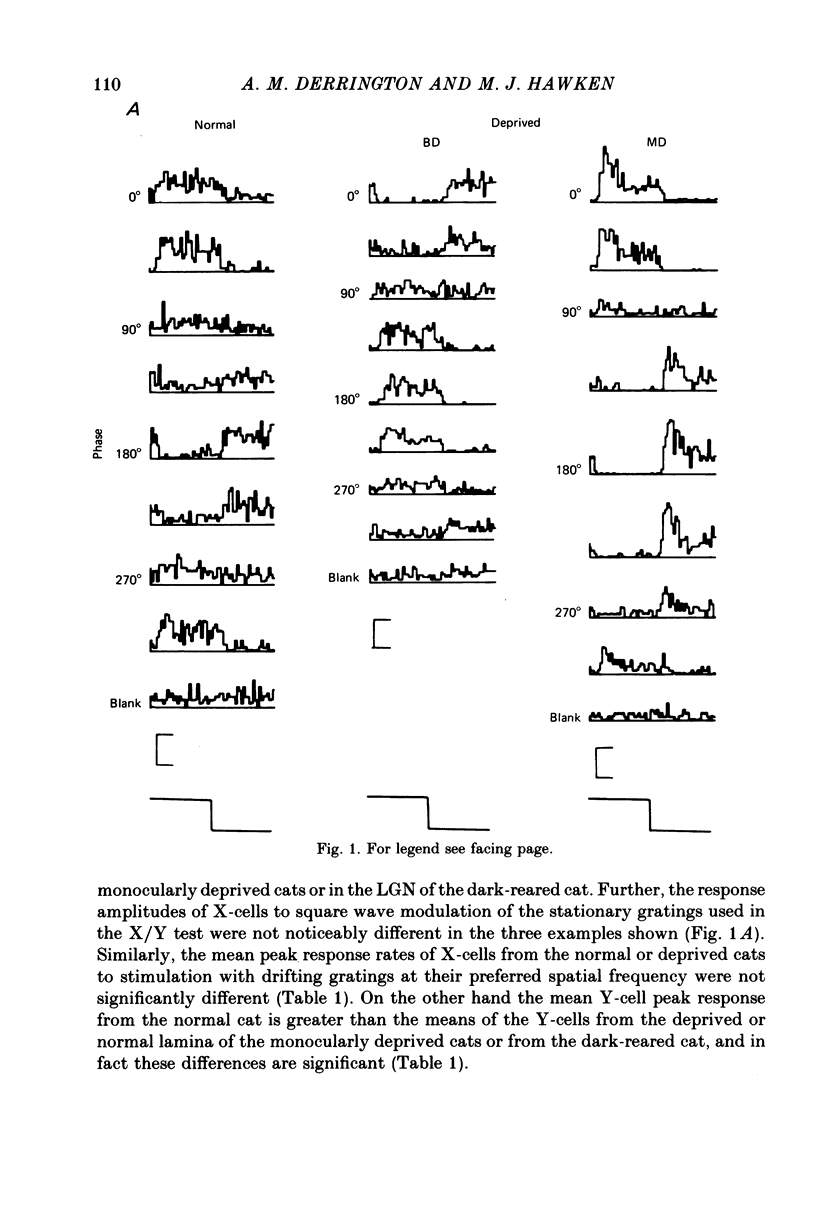
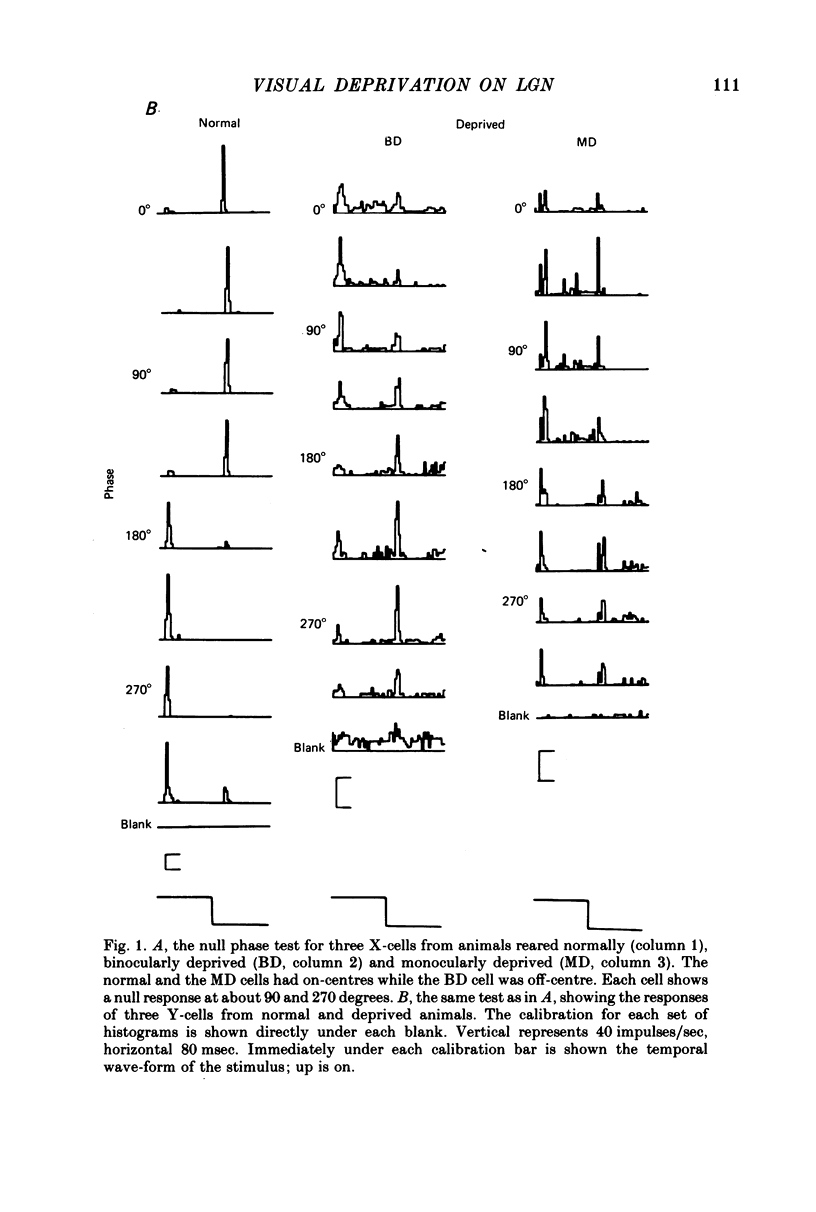
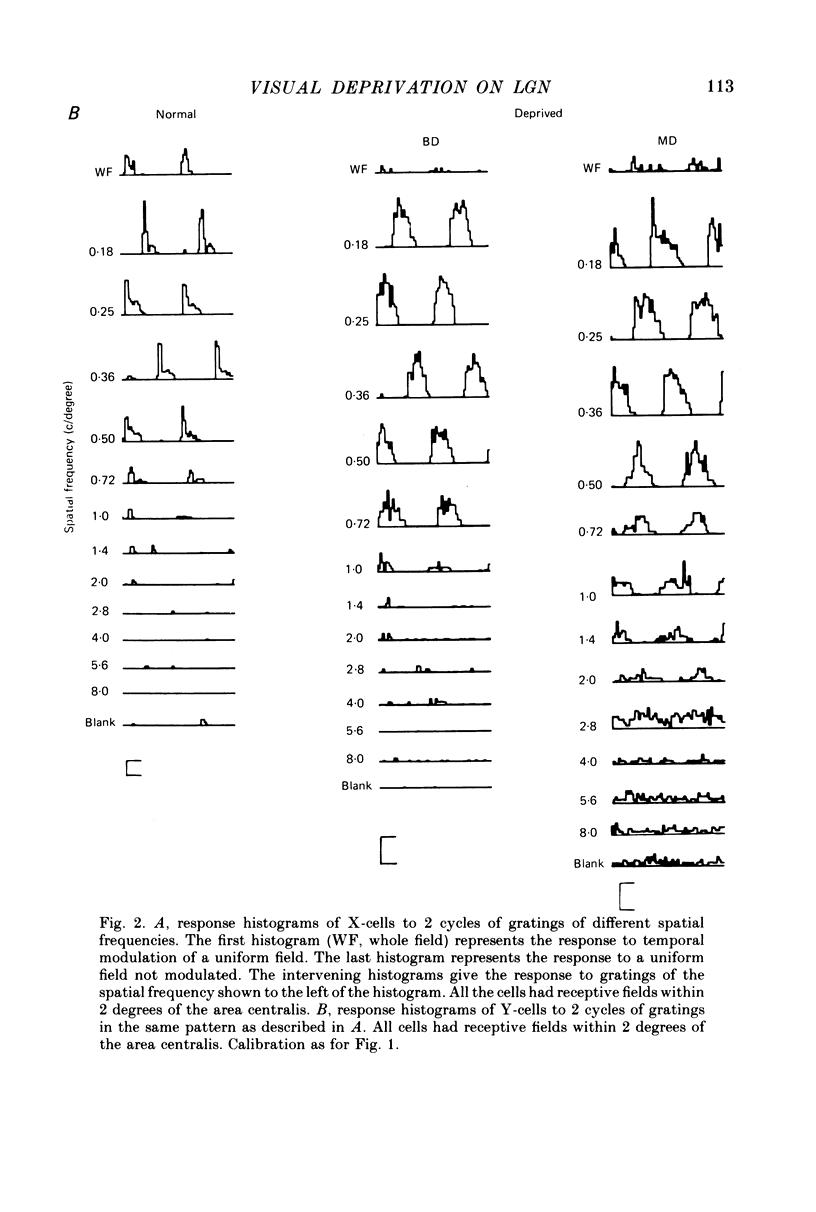
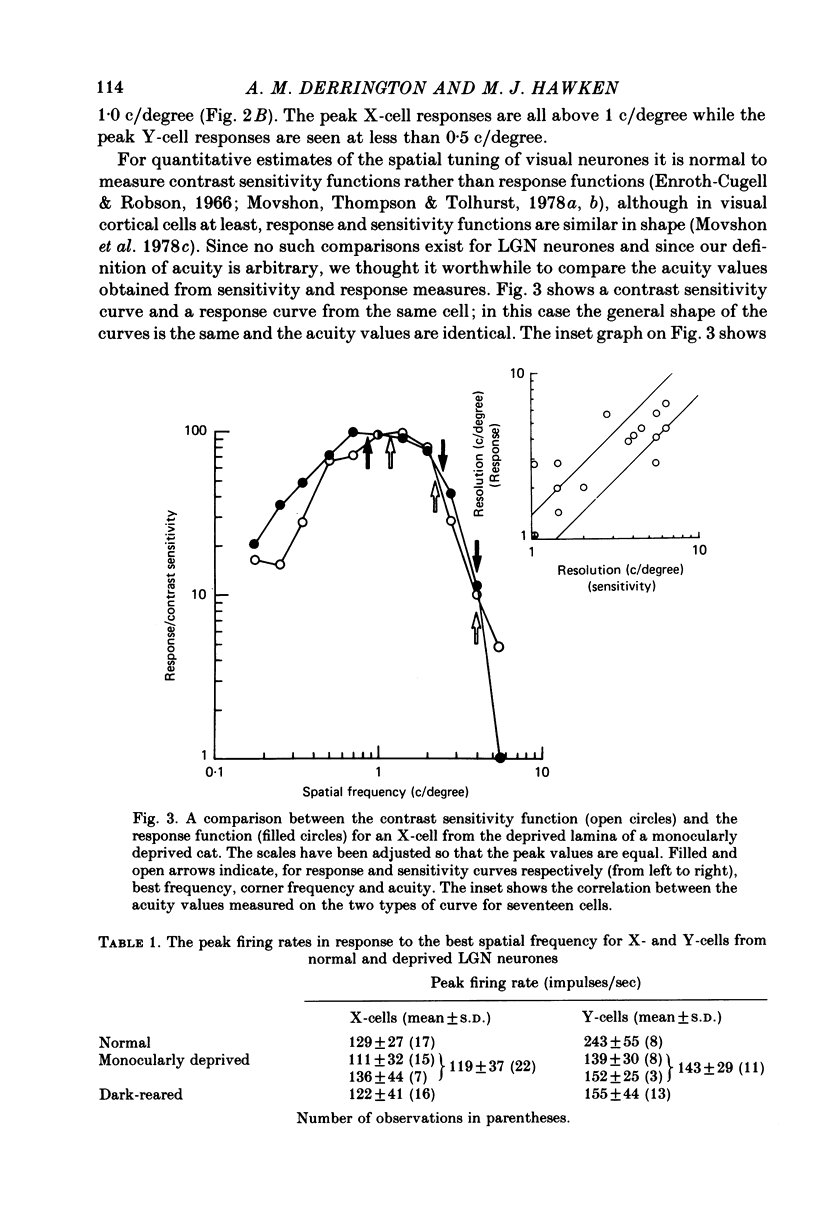
1. Extracellular recordings were made from the dorsal lateral geniculate nucleus of monocularly deprived, dark-reared and normal cats. The spatial and temporal properties of the neurones were studied. 2. The mean acuity of X-cells with receptive fields 3 degrees of the area centralis was 3.9 c/degree for deprived eye cells from monocularly deprived cats, compared was 3.8 c/degree for normal cells. 3. The mean activity of X-cells with receptive fields within 4 degrees of the area centralis was 4.3 c/degree for a dark-reared cat compared with 4.0 c/degree for a normal cat. 4. The peak response rates of X-cells to their best spatial frequency were determined. The mean values for the normal, monocularly deprived and dark-reared populations were all similar. 5 Measurement of the temporal frequency tuning of a number of cells was made. The mean peak temporal frequency for the dark-reared X-cells was lower than for monocularly deprived or normal X-cells. 6. The results are discussed with reference to the location of the primary neural deficit induced by visual deprivation.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blakemore C., Van Sluyters R. C. Innate and environmental factors in the development of the kitten's visual cortex. J Physiol. 1975 Jul;248(3):663–716. doi: 10.1113/jphysiol.1975.sp010995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C., Vital-Durand F. Development of the neural basis of visual acuity in monkeys: speculation on the origin of deprivation amblyopia. Trans Ophthalmol Soc U K. 1979;99(3):363–368. [PubMed] [Google Scholar]
- Cleland B. G., Harding T. H., Tulunay-Keesey U. Visual resolution and receptive field size: examination of two kinds of cat retinal ganglion cell. Science. 1979 Sep 7;205(4410):1015–1017. doi: 10.1126/science.472720. [DOI] [PubMed] [Google Scholar]
- Cleland B. G., Mitchell D. E., Gillard-Crewther S., Crewther D. P. Visual resolution of retinal ganglion cells in monocularly-deprived cats. Brain Res. 1980 Jun 16;192(1):261–266. doi: 10.1016/0006-8993(80)91026-4. [DOI] [PubMed] [Google Scholar]
- Derrington A. M., Fuchs A. F. Spatial and temporal properties of X and Y cells in the cat lateral geniculate nucleus. J Physiol. 1979 Aug;293:347–364. doi: 10.1113/jphysiol.1979.sp012893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dews P. B., Wiesel T. N. Consequences of monocular deprivation on visual behaviour in kittens. J Physiol. 1970 Feb;206(2):437–455. doi: 10.1113/jphysiol.1970.sp009023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giffin F., Mitchell D. E. The rate of recovery of vision after early monocular deprivation in kittens. J Physiol. 1978 Jan;274:511–537. doi: 10.1113/jphysiol.1978.sp012164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Integrative action in the cat's lateral geniculate body. J Physiol. 1961 Feb;155:385–398. doi: 10.1113/jphysiol.1961.sp006635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstein S., Shapley R. M. Quantitative analysis of retinal ganglion cell classifications. J Physiol. 1976 Nov;262(2):237–264. doi: 10.1113/jphysiol.1976.sp011594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H., Jacobson S. G. Nasal field loss in cats reared with convergent squint: behavioural studies. J Physiol. 1977 Sep;270(2):367–381. doi: 10.1113/jphysiol.1977.sp011957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H., Plant G. T., Tremain K. E. Nasal field loss in kittens reared with convergent squint: neurophysiological and morphological studies of the lateral geniculate nucleus. J Physiol. 1977 Sep;270(2):345–366. doi: 10.1113/jphysiol.1977.sp011956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H., Tremain K. E. Amblyopia occurs in retinal ganglion cells in cats reared with convergent squint without alternating fixation. Exp Brain Res. 1979 May 2;35(3):559–582. doi: 10.1007/BF00236772. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Tremain K. E., Einon G. Loss of spatial resolution of lateral geniculate nucleus neurones in kittens raised with convergent squint produced at different stages in development. Exp Brain Res. 1978 Feb 15;31(2):207–220. doi: 10.1007/BF00237600. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Tremain K. E. The development of spatial resolving power of LGN cells and its susceptibility to blur and strabismus. Arch Ital Biol. 1978 Sep;116(3-4):375–384. [PubMed] [Google Scholar]
- Ikeda H., Wright M. J. Properties of LGN cells in kittens reared with convergent squint: a neurophysiological demonstration of amblyopia. Exp Brain Res. 1976 May 10;25(1):63–77. doi: 10.1007/BF00237326. [DOI] [PubMed] [Google Scholar]
- Jacobson S. G., Ikeda H. Behavioural studies of spatial vision in cats reared with convergent squint: is amblyopia due to arrest of development? Exp Brain Res. 1979 Jan 2;34(1):11–26. doi: 10.1007/BF00238338. [DOI] [PubMed] [Google Scholar]
- Kratz K. E., Mangel S. C., Lehmkuhle S., Sherman M. Retinal X- and Y-cells in monocularly lid-sutured cats: normality of spatial and temporal properties. Brain Res. 1979 Aug 31;172(3):545–551. doi: 10.1016/0006-8993(79)90586-9. [DOI] [PubMed] [Google Scholar]
- Kratz K. E., Sherman S. M., Kalil R. Lateral geniculate nucleus in dark-reared cats: loss of Y cells without changes in cell size. Science. 1979 Mar 30;203(4387):1353–1355. doi: 10.1126/science.424758. [DOI] [PubMed] [Google Scholar]
- Kratz K. E., Spear P. D. Postcritical-period reversal of effects of monocular deprivation on striate cortex cells in the cat. J Neurophysiol. 1976 May;39(3):501–511. doi: 10.1152/jn.1976.39.3.501. [DOI] [PubMed] [Google Scholar]
- Lehmkuhle S., Kratz K. E., Mangel S. C., Sherman S. M. Effects of early monocular lid suture on spatial and temporal sensitivity of neurons in dorsal lateral geniculate nucleus of the cat. J Neurophysiol. 1980 Feb;43(2):542–556. doi: 10.1152/jn.1980.43.2.542. [DOI] [PubMed] [Google Scholar]
- Leventhal A. G., Hirsch H. V. Receptive-field properties of different classes of neurons in visual cortex of normal and dark-reared cats. J Neurophysiol. 1980 Apr;43(4):1111–1132. doi: 10.1152/jn.1980.43.4.1111. [DOI] [PubMed] [Google Scholar]
- Maffei L., Fiorentini A. Monocular deprivation in kittens impairs the spatial resolution of geniculate neurones. Nature. 1976 Dec 23;264(5588):754–755. doi: 10.1038/264754a0. [DOI] [PubMed] [Google Scholar]
- Merrill E. G., Ainsworth A. Glass-coated platinum-plated tungsten microelectrodes. Med Biol Eng. 1972 Sep;10(5):662–672. doi: 10.1007/BF02476084. [DOI] [PubMed] [Google Scholar]
- Movshon J. A., Thompson I. D., Tolhurst D. J. Receptive field organization of complex cells in the cat's striate cortex. J Physiol. 1978 Oct;283:79–99. doi: 10.1113/jphysiol.1978.sp012489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movshon J. A., Thompson I. D., Tolhurst D. J. Spatial and temporal contrast sensitivity of neurones in areas 17 and 18 of the cat's visual cortex. J Physiol. 1978 Oct;283:101–120. doi: 10.1113/jphysiol.1978.sp012490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movshon J. A., Thompson I. D., Tolhurst D. J. Spatial summation in the receptive fields of simple cells in the cat's striate cortex. J Physiol. 1978 Oct;283:53–77. doi: 10.1113/jphysiol.1978.sp012488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHADE O. H., Sr Optical and photoelectric analog of the eye. J Opt Soc Am. 1956 Sep;46(9):721–739. doi: 10.1364/josa.46.000721. [DOI] [PubMed] [Google Scholar]
- Shapley R., So Y. T. Is there an effect of monocular deprivation on the proportions of X and Y cells in the cat lateral geniculate nucleus? Exp Brain Res. 1980;39(1):41–48. doi: 10.1007/BF00237068. [DOI] [PubMed] [Google Scholar]
- Shatz C. J., Stryker M. P. Ocular dominance in layer IV of the cat's visual cortex and the effects of monocular deprivation. J Physiol. 1978 Aug;281:267–283. doi: 10.1113/jphysiol.1978.sp012421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sireteanu R., Hoffmann K. P. Relative frequency and visual resolution of X- and Y-cells in the LGN of normal and monocularly deprived cats: interlaminar differences. Exp Brain Res. 1979 Feb 15;34(3):591–603. doi: 10.1007/BF00239151. [DOI] [PubMed] [Google Scholar]
- So Y. T., Shapley R. Spatial properties of X and Y cells in the lateral geniculate nucleus of the cat and conduction veolcities of their inputs. Exp Brain Res. 1979 Aug 1;36(3):533–550. doi: 10.1007/BF00238521. [DOI] [PubMed] [Google Scholar]
- Timney B., Mitchell D. E., Giffin F. The development of vision in cats after extended periods of dark-rearing. Exp Brain Res. 1978 Apr 14;31(4):547–560. doi: 10.1007/BF00239811. [DOI] [PubMed] [Google Scholar]
- WIESEL T. N., HUBEL D. H. EFFECTS OF VISUAL DEPRIVATION ON MORPHOLOGY AND PHYSIOLOGY OF CELLS IN THE CATS LATERAL GENICULATE BODY. J Neurophysiol. 1963 Nov;26:978–993. doi: 10.1152/jn.1963.26.6.978. [DOI] [PubMed] [Google Scholar]
- WIESEL T. N., HUBEL D. H. SINGLE-CELL RESPONSES IN STRIATE CORTEX OF KITTENS DEPRIVED OF VISION IN ONE EYE. J Neurophysiol. 1963 Nov;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- Wiesel T. N., Hubel D. H. Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens. J Neurophysiol. 1965 Nov;28(6):1029–1040. doi: 10.1152/jn.1965.28.6.1029. [DOI] [PubMed] [Google Scholar]
- Wiesel T. N., Hubel D. H. Extent of recovery from the effects of visual deprivation in kittens. J Neurophysiol. 1965 Nov;28(6):1060–1072. doi: 10.1152/jn.1965.28.6.1060. [DOI] [PubMed] [Google Scholar]


