Abstract
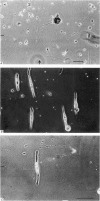
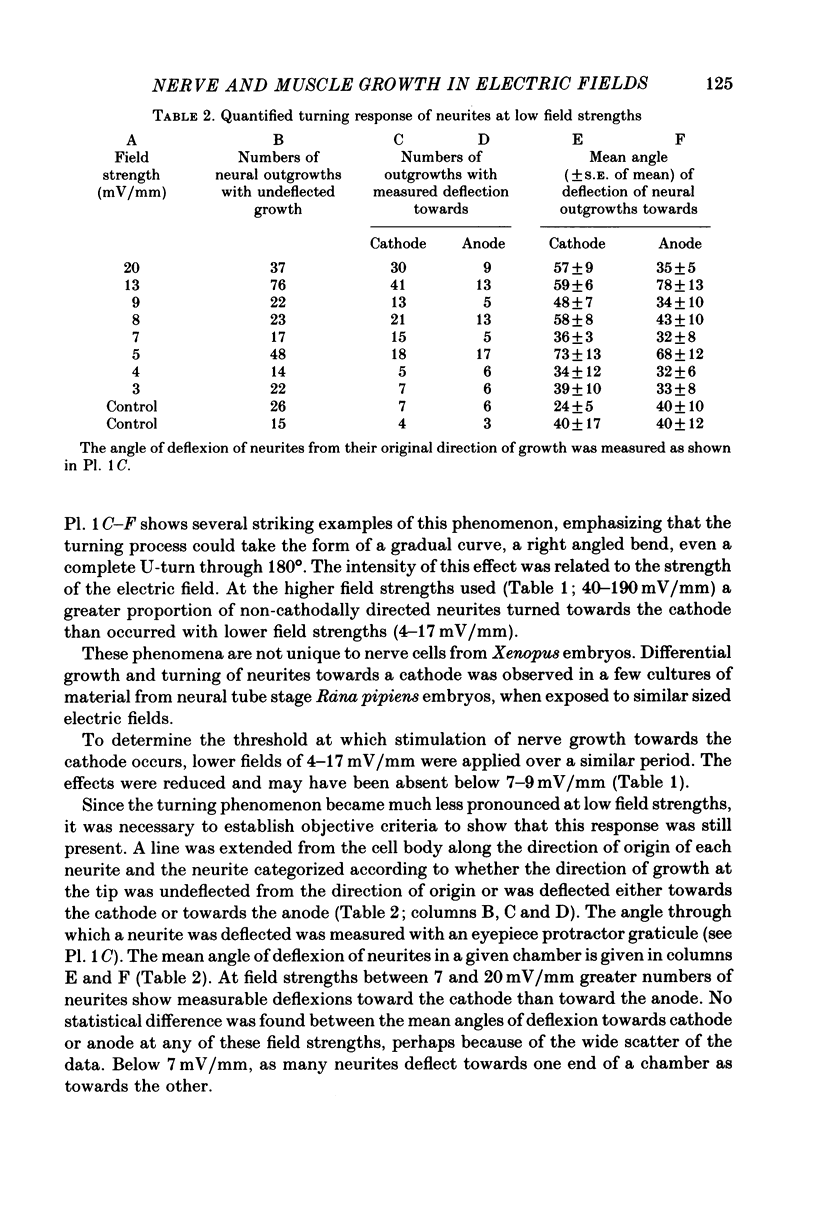
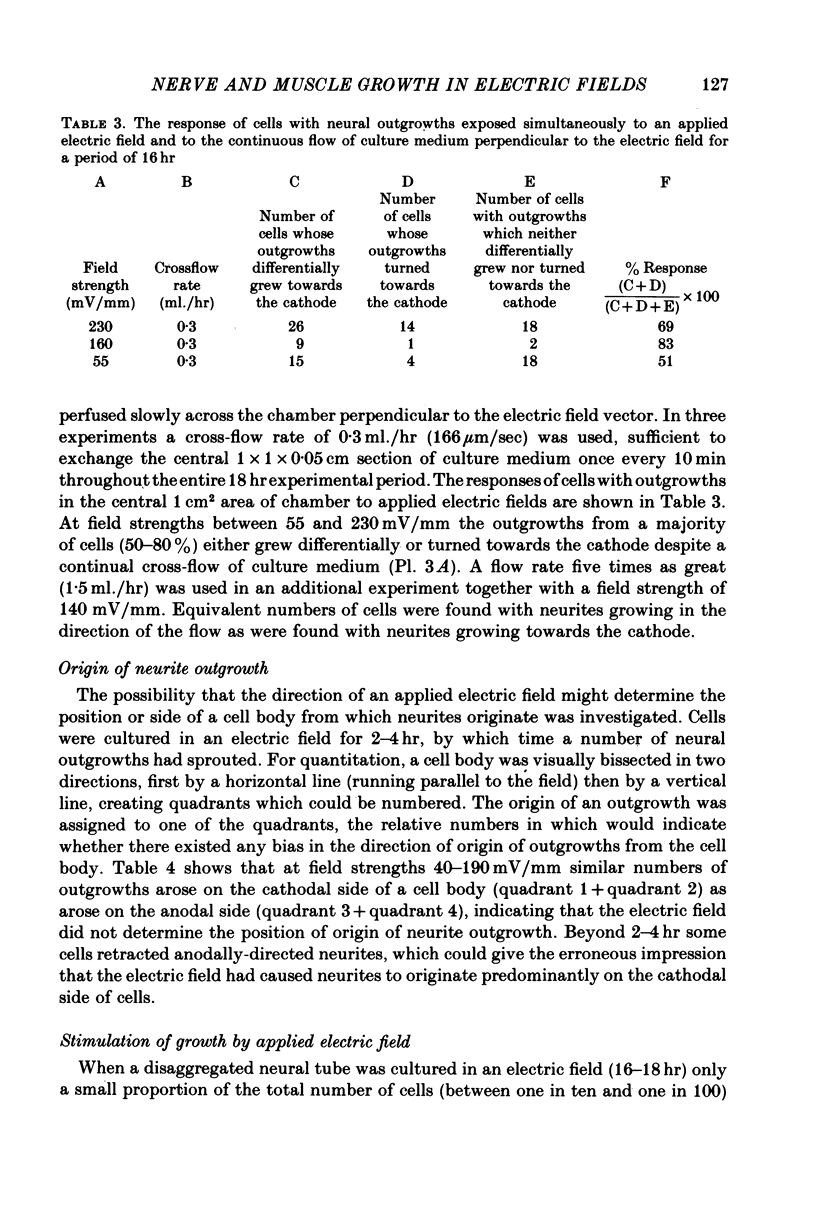
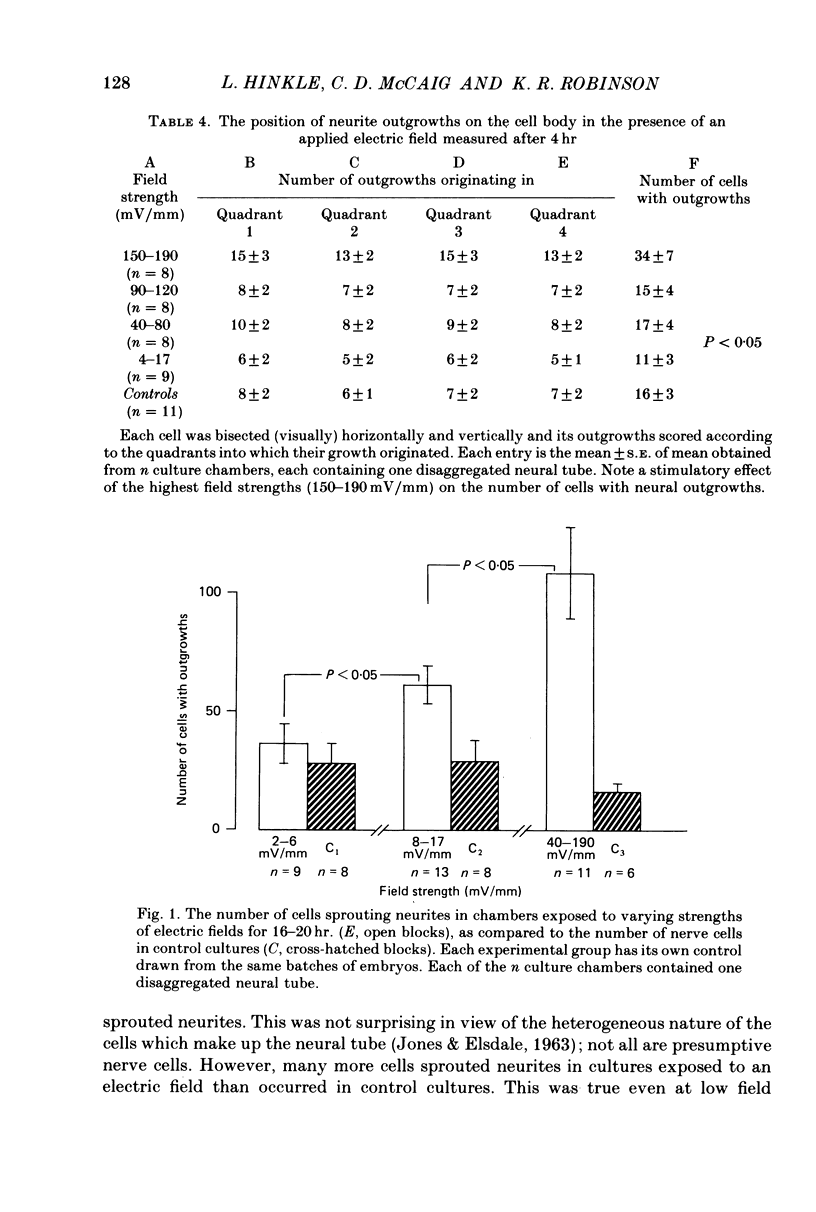
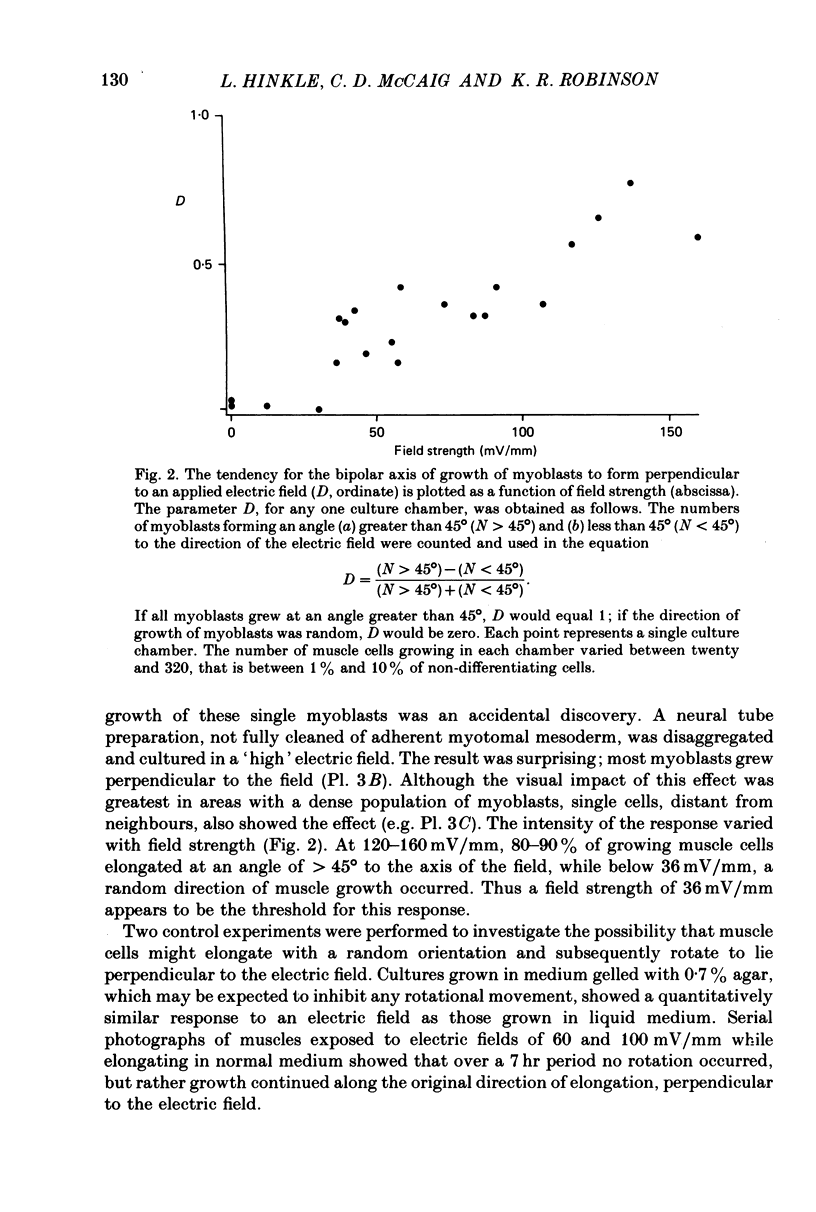
1. Disaggregated single neurones and myoblasts obtained from the neural tube and somites of Xenopus laevis embryos (stages 17-21) were cultured in the presence of steady small electric fields. 2. Neurites grew preferentially towards the negative pole, or cathode, in field strengths of 7-190 mV/mm. Many turned through considerable angles to do so. This effect disappeared below a threshold level of about 7 mV/mm. 3. Greater numbers of neurones sprouted neurites in cultures exposed to an electric field compared to control cultures. The difference could be as much as tenfold. The threshold level of this phenomenon was about 6-8 mV/mm. Other cell types such as pigment cells and fibroblasts were also stimulated to differentiate in culture by an electric field, although to a lesser extent than neurones. 4. Applied electric fields had no effect on the location of the origin of neurites on the cell body. 5. Spherical myoblasts cultured in applied electric fields (36-170 mV/mm) elongated with a bipolar axis of growth which was perpendicular to the electric field. The response was graded and disappeared at field strengths below 36 mV/mm. 6. It is suggested that in vivo, the direction of neural outgrowth from the neural tube and the strict spatial organization of somites might be under the control, in part, of endogenous electric fields. Possible sources of these are discussed.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarado R. H., Moody A. Sodium and chloride transport in tadpoles of the bullfrog Rana catesbeiana. Am J Physiol. 1970 May;218(5):1510–1516. doi: 10.1152/ajplegacy.1970.218.5.1510. [DOI] [PubMed] [Google Scholar]
- Baker P. C., Schroeder T. E. Cytoplasmic filaments and morphogenetic movement in the amphibian neural tube. Dev Biol. 1967 May;15(5):432–450. doi: 10.1016/0012-1606(67)90036-x. [DOI] [PubMed] [Google Scholar]
- Blackshaw S. E., Warner A. E. Alterations in resting membrane properties during neural plate stages of development of the nervous system. J Physiol. 1976 Feb;255(1):231–247. doi: 10.1113/jphysiol.1976.sp011277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshaw S. E., Warner A. E. Low resistance junctions between mesoderm cells during development of trunk muscles. J Physiol. 1976 Feb;255(1):209–230. doi: 10.1113/jphysiol.1976.sp011276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray D. Model for membrane movements in the neural growth cone. Nature. 1973 Jul 13;244(5411):93–96. doi: 10.1038/244093a0. [DOI] [PubMed] [Google Scholar]
- Bray D. Surface movements during the growth of single explanted neurons. Proc Natl Acad Sci U S A. 1970 Apr;65(4):905–910. doi: 10.1073/pnas.65.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge M. B. Fine structure of nerve fibers and growth cones of isolated sympathetic neurons in culture. J Cell Biol. 1973 Mar;56(3):713–735. doi: 10.1083/jcb.56.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebendal T. The relative roles of contact inhibition and contact guidance in orientation of axons extending on aligned collagen fibrils in vitro. Exp Cell Res. 1976 Mar 1;98(1):159–169. doi: 10.1016/0014-4827(76)90475-4. [DOI] [PubMed] [Google Scholar]
- Gundersen R. W., Barrett J. N. Neuronal chemotaxis: chick dorsal-root axons turn toward high concentrations of nerve growth factor. Science. 1979 Nov 30;206(4422):1079–1080. doi: 10.1126/science.493992. [DOI] [PubMed] [Google Scholar]
- Hamilton L. The formation of somites in Xenopus. J Embryol Exp Morphol. 1969 Sep;22(2):253–264. [PubMed] [Google Scholar]
- Jaffe L. F., Nuccitelli R. An ultrasensitive vibrating probe for measuring steady extracellular currents. J Cell Biol. 1974 Nov;63(2 Pt 1):614–628. doi: 10.1083/jcb.63.2.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe L. F., Nuccitelli R. Electrical controls of development. Annu Rev Biophys Bioeng. 1977;6:445–476. doi: 10.1146/annurev.bb.06.060177.002305. [DOI] [PubMed] [Google Scholar]
- Jaffe L. F., Poo M. M. Neurites grow faster towards the cathode than the anode in a steady field. J Exp Zool. 1979 Jul;209(1):115–128. doi: 10.1002/jez.1402090114. [DOI] [PubMed] [Google Scholar]
- Ludueña M. A., Wessells N. K. Cell locomotion, nerve elongation, and microfilaments. Dev Biol. 1973 Feb;30(2):427–440. doi: 10.1016/0012-1606(73)90100-0. [DOI] [PubMed] [Google Scholar]
- Messenger E. A., Warner A. E. The function of the sodium pump during differentiation of amphibian embryonic neurones. J Physiol. 1979 Jul;292:85–105. doi: 10.1113/jphysiol.1979.sp012840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrill G. A., Kostellow A. B., Watson D. E. The electropotential difference between the blastocoel and the external medium in the amphibian embryo: its similarity to the adult frog trans-skin potential. Life Sci. 1966 Apr;5(8):705–709. doi: 10.1016/0024-3205(66)90209-8. [DOI] [PubMed] [Google Scholar]
- Poo M. M., Poo W. J., Lam J. W. Lateral electrophoresis and diffusion of Concanavalin A receptors in the membrane of embryonic muscle cell. J Cell Biol. 1978 Feb;76(2):483–501. doi: 10.1083/jcb.76.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo M., Robinson K. R. Electrophoresis of concanavalin A receptors along embryonic muscle cell membrane. Nature. 1977 Feb 17;265(5595):602–605. doi: 10.1038/265602a0. [DOI] [PubMed] [Google Scholar]
- Slack C., Warner A. E. Intracellular and intercellular potentials in the early amphibian embryo. J Physiol. 1973 Jul;232(2):313–330. doi: 10.1113/jphysiol.1973.sp010272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR R. E., Jr, BARKER S. B. TRANSEPIDERMAL POTENTIAL DIFFERENCE: DEVELOPMENT IN ANURAN LARVAE. Science. 1965 Jun 18;148(3677):1612–1613. doi: 10.1126/science.148.3677.1612. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Spooner B. S., Wessells N. K. Ultrastructure and function of growth cones and axons of cultured nerve cells. J Cell Biol. 1971 Jun;49(3):614–635. doi: 10.1083/jcb.49.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]