Abstract
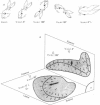
1. The gain and phase of the horizontal (H.v.o.r.) and vertical (V.v.o.r.) vestibulo-ocular reflexes were measured in rabbits. The V.v.o.r. was evoked by sinusoidal rolls about the longitudinal axis of the rabbit. This axis was maintained at different orientations with respect to the earth horizontal axis: V.v.o.r. 0°, prone; V.v.o.r. 90°, `nose-up'; V.v.o.r. 180°, supine; V.v.o.r. 0°L, left side down.
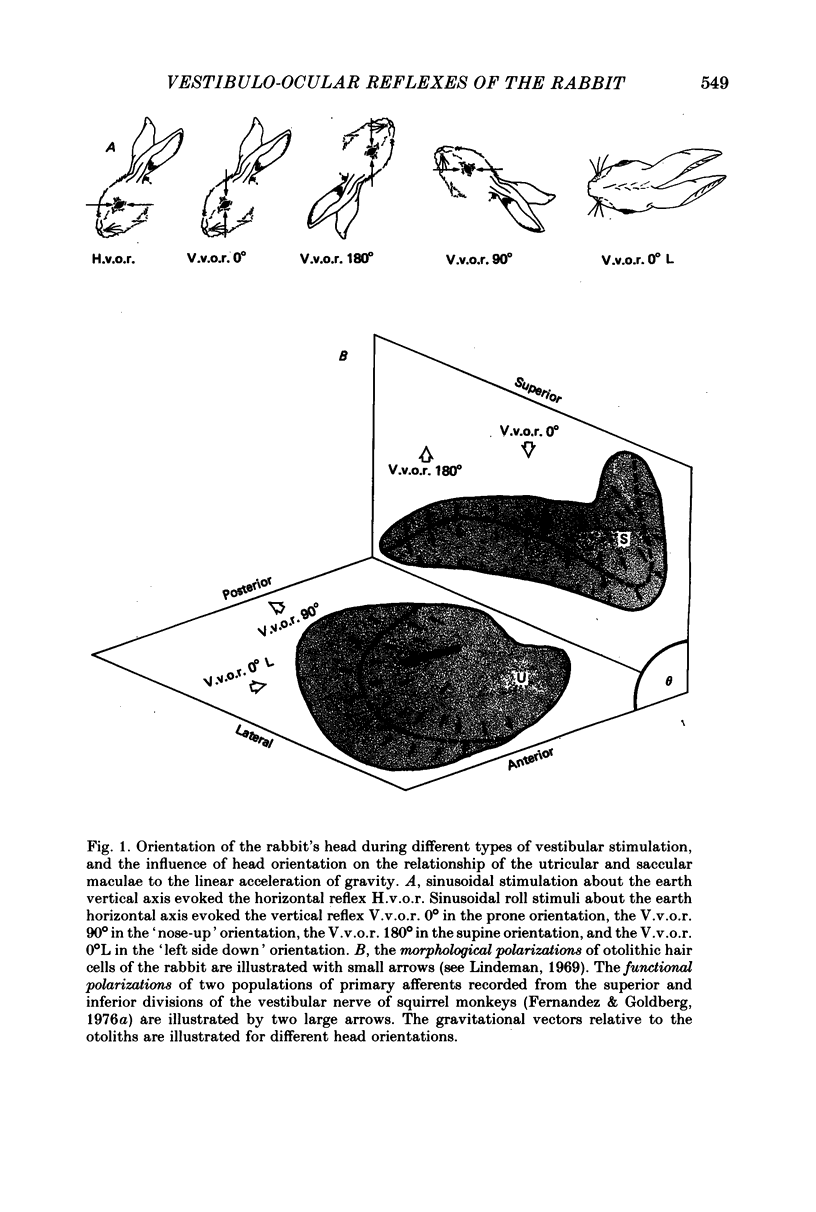
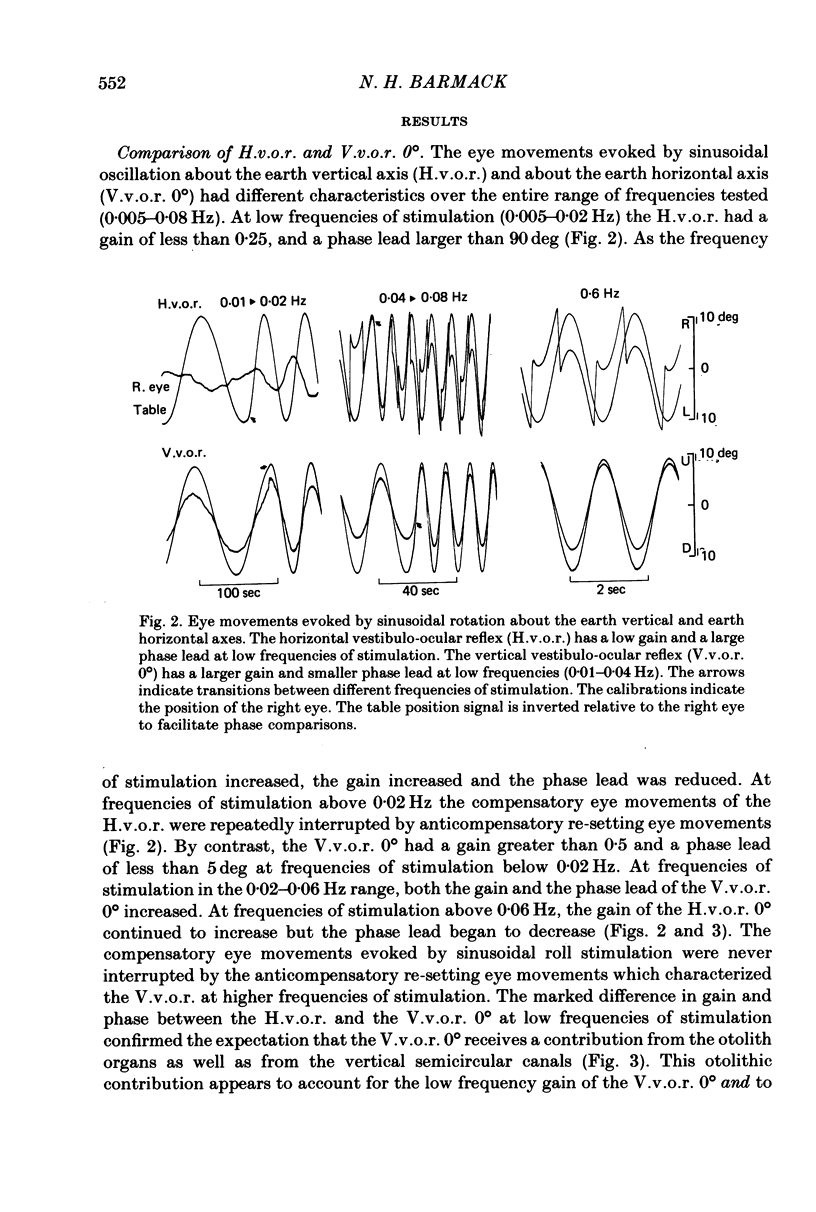
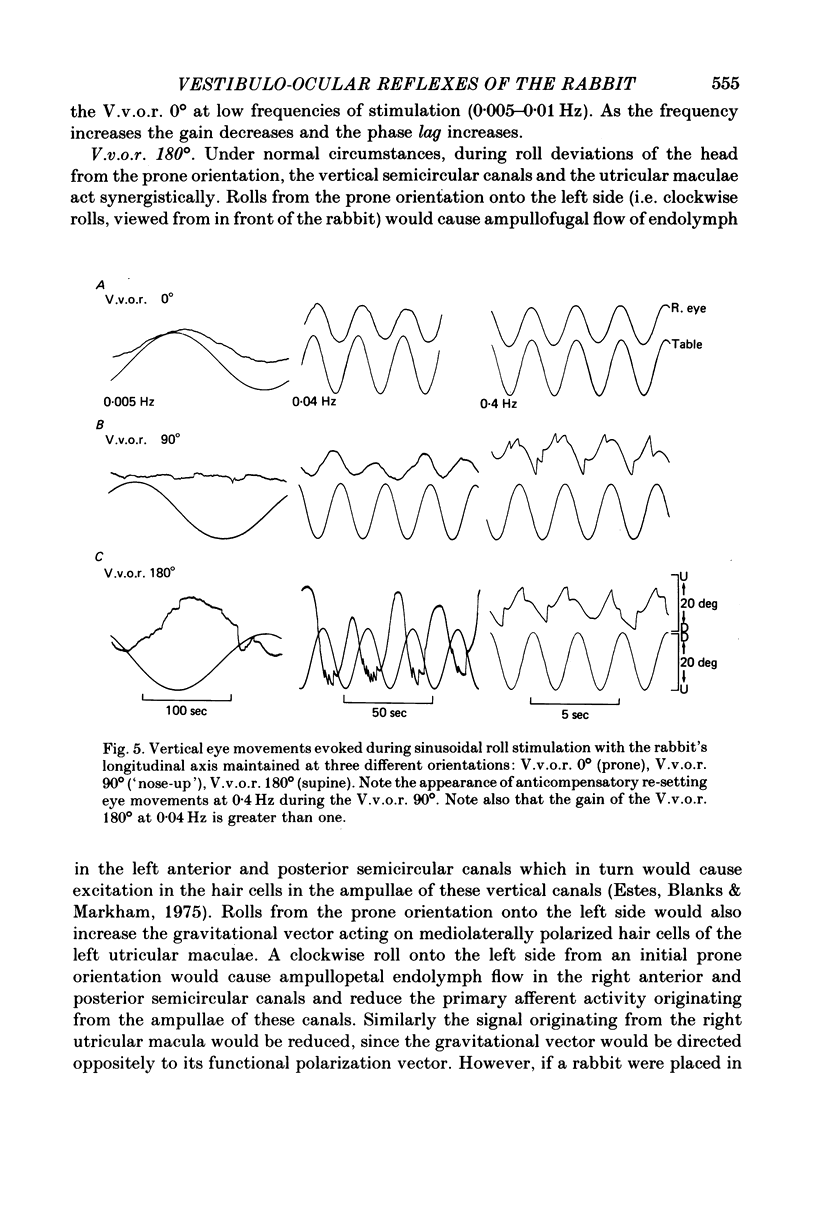
2. In contrast to the H.v.o.r., the V.v.o.r. 0° had a higher gain (eye velocity/head velocity) and a smaller phase lead (eye position + 180° with respect to head position) at low frequencies of sinusoidal vestibular stimulation (± 10 deg, 0·005-0·05 Hz). At higher frequencies (0·05-0·8 Hz), the H.v.o.r. and V.v.o.r. 0° were equivalent in both gain and phase.
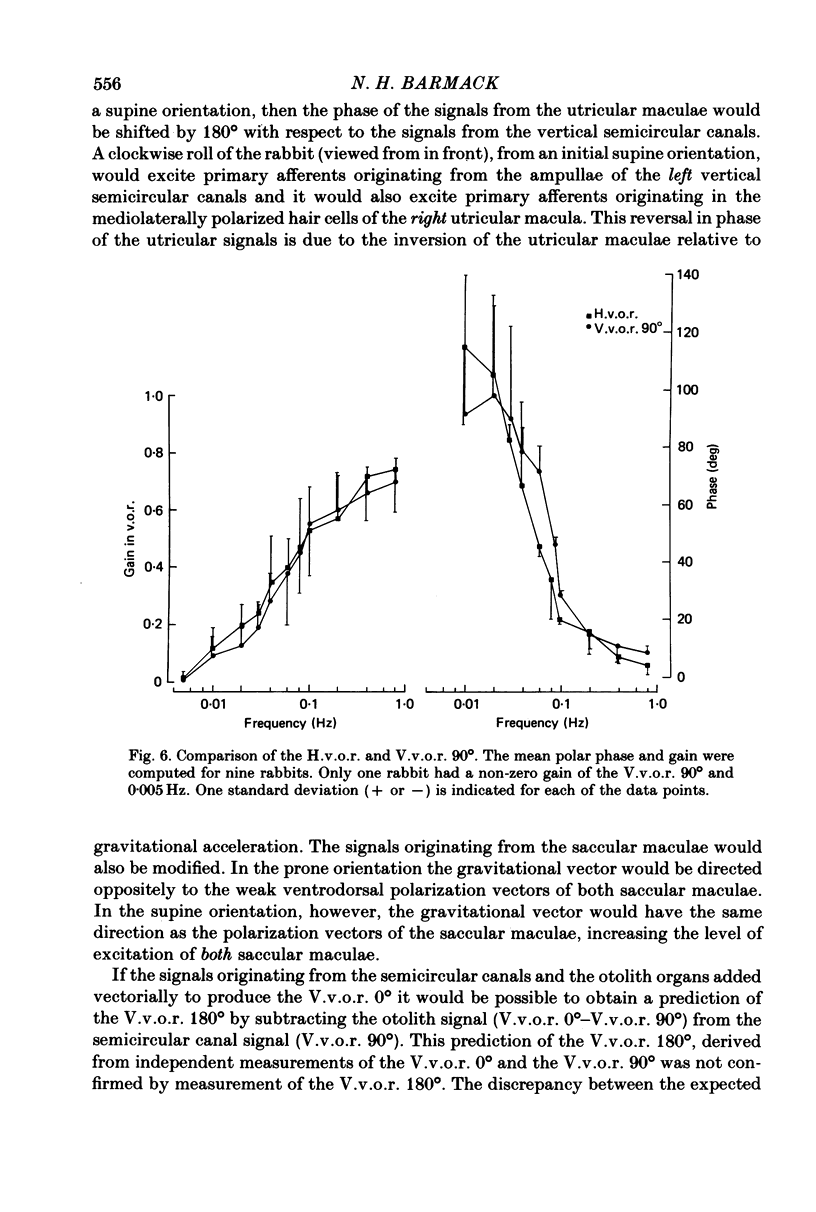
3. The low-frequency gain of the V.v.o.r. was smallest in the `nose-up' orientation. The V.v.o.r. 90° was equivalent in both gain and phase to the H.v.o.r. over the entire range of frequencies tested (0·005-0·8 Hz). Threshold angular accelerations for the H.v.o.r. and V.v.o.r. 90° were below 0·04 deg/sec2.
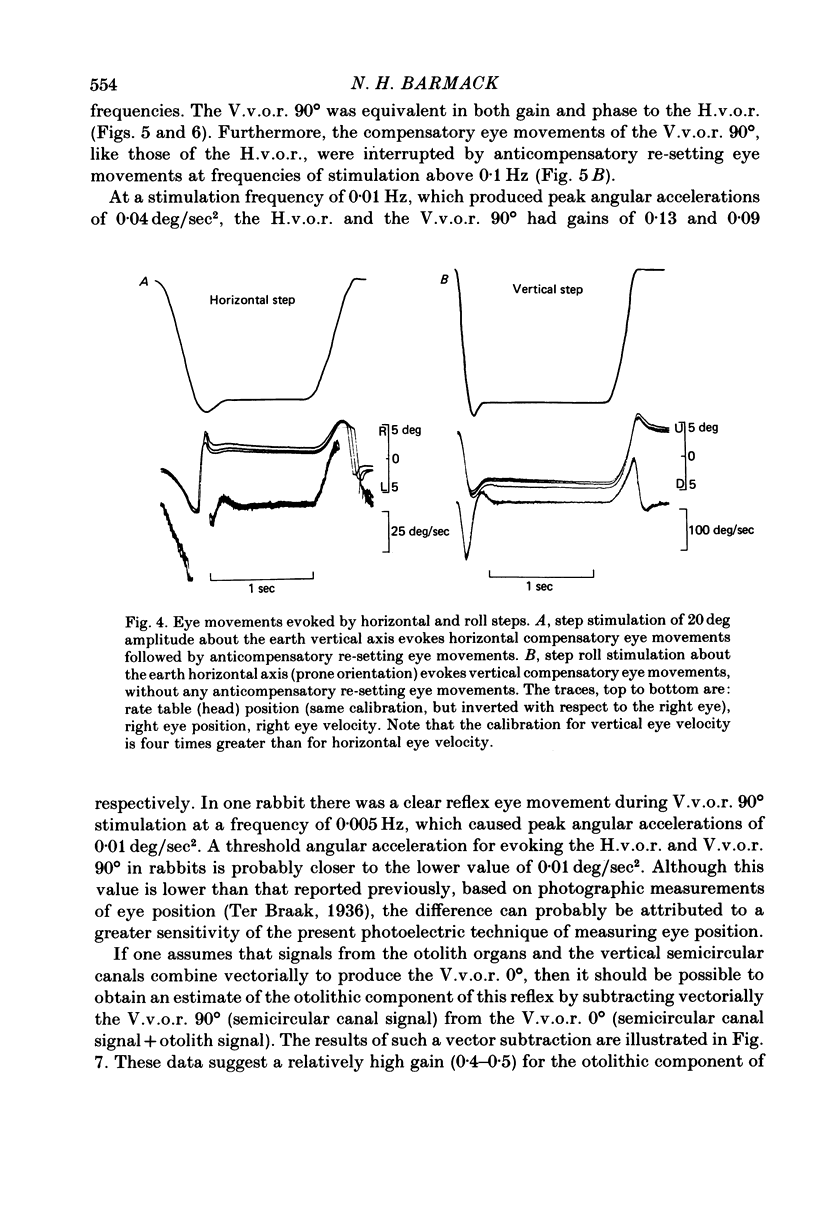
4. The compensatory eye movements of the H.v.o.r. were frequently interrupted by anticompensatory re-setting eye movements. These anticompensatory re-setting eye movements were present in the V.v.o.r. 90°, but not in the V.v.o.r. 0°.
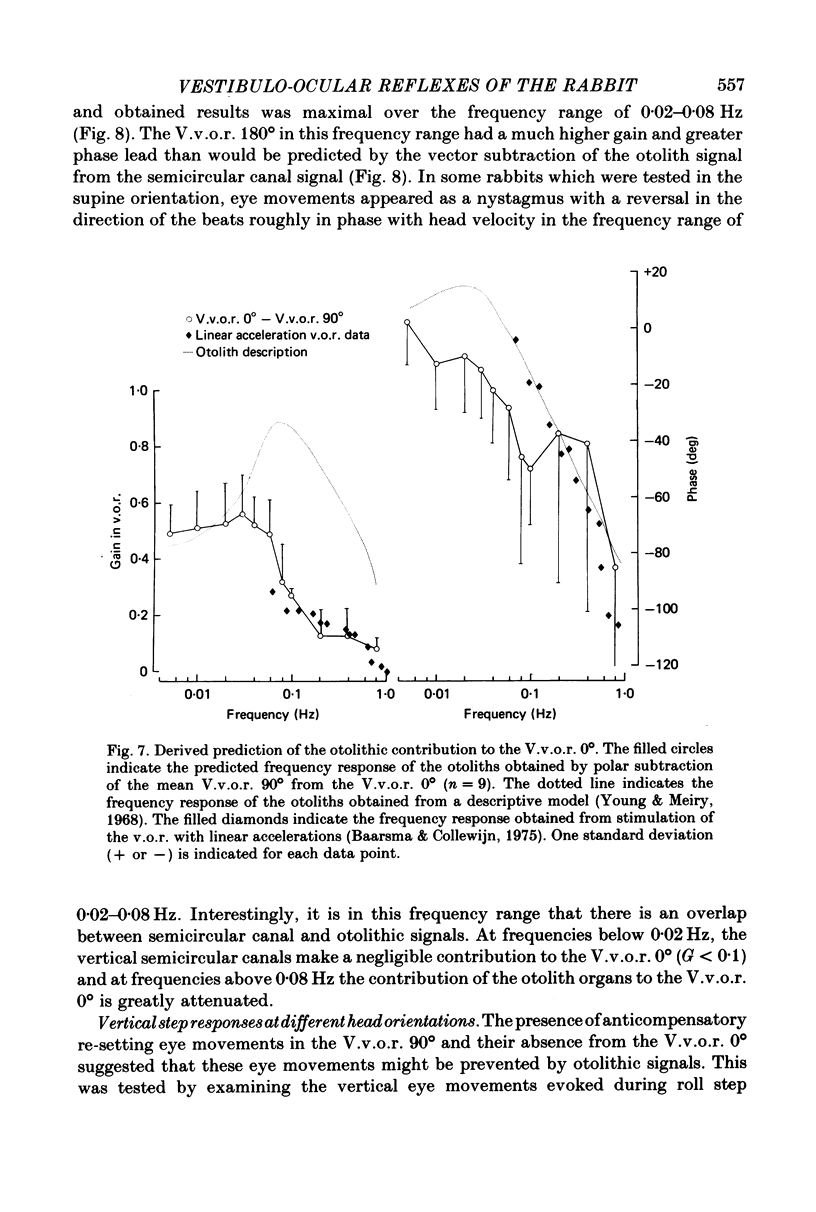
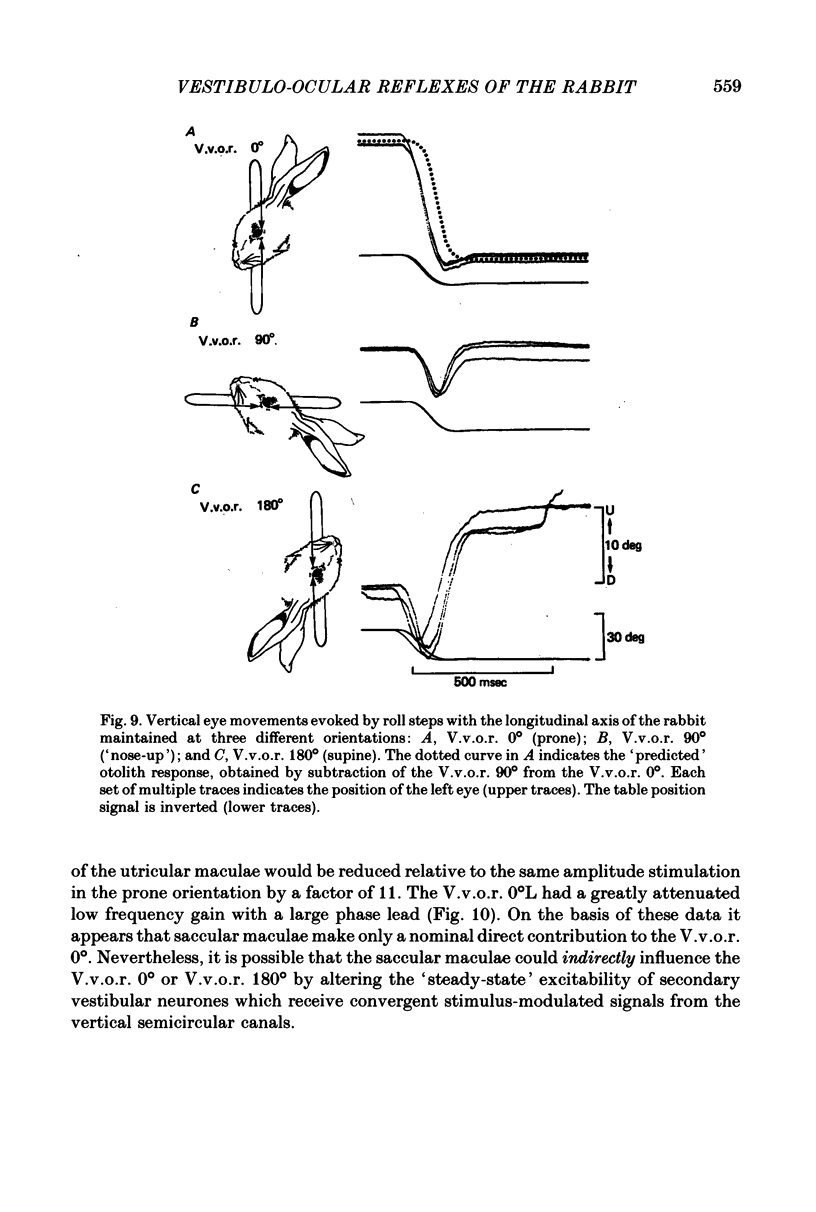
5. An estimate gain and phase of the otolithic component of the V.v.o.r. 0° was derived by subtraction of the V.v.o.r. 90° (semicircular canal signal) from the V.v.o.r. 0° (semicircular canal signal+otolith signal). This procedure was based on the assumption that the signals from the otolith organs and vertical semicircular canals combine linearly.
6. The V.v.o.r. 180° provided an interesting test of the assumption of linear combination of otolithic and semicircular canal signals by reversing the phase of the modulated otolithic signal. The data indicated that the V.v.o.r. 180° is non-linear.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abend W. K. Functional organization of the superior vestibular nucleus of the squirrel monkey. Brain Res. 1977 Aug 19;132(1):65–84. doi: 10.1016/0006-8993(77)90706-5. [DOI] [PubMed] [Google Scholar]
- Abend W. K. Response to constant angular accelerations of neurons in the monkey superior vestibular nucleus. Exp Brain Res. 1978 Apr 14;31(4):459–473. doi: 10.1007/BF00239805. [DOI] [PubMed] [Google Scholar]
- Baarsma E. A., Collewijn H. Eye movements due to linear accelerations in the rabbit. J Physiol. 1975 Feb;245(1):227–247. doi: 10.1113/jphysiol.1975.sp010842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baarsma E., Collewijn H. Vestibulo-ocular and optokinetic reactions to rotation and their interaction in the rabbit. J Physiol. 1974 May;238(3):603–625. doi: 10.1113/jphysiol.1974.sp010546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barmack N. H. Measurements of stiffness of extraocular muscles of the rabbit. J Neurophysiol. 1976 Sep;39(5):1009–1019. doi: 10.1152/jn.1976.39.5.1009. [DOI] [PubMed] [Google Scholar]
- Benson A. J., Bodin M. A. Interaction of linear and angular accelerations on vestibular receptors in man. Aerosp Med. 1966 Feb;37(2):144–154. [PubMed] [Google Scholar]
- Estes M. S., Blanks R. H., Markham C. H. Physiologic characteristics of vestibular first-order canal neurons in the cat. I. Response plane determination and resting discharge characteristics. J Neurophysiol. 1975 Sep;38(5):1232–1249. doi: 10.1152/jn.1975.38.5.1232. [DOI] [PubMed] [Google Scholar]
- FLOCK A. STRUCTURE OF THE MACULA UTRICULI WITH SPECIAL REFERENCE TO DIRECTIONAL INTERPLAY OF SENSORY RESPONSES AS REVEALED BY MORPHOLOGICAL POLARIZATION. J Cell Biol. 1964 Aug;22:413–431. doi: 10.1083/jcb.22.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez C., Goldberg J. M. Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey. II. Response to sinusoidal stimulation and dynamics of peripheral vestibular system. J Neurophysiol. 1971 Jul;34(4):661–675. doi: 10.1152/jn.1971.34.4.661. [DOI] [PubMed] [Google Scholar]
- Fernández C., Goldberg J. M. Physiology of peripheral neurons innervating otolith organs of the squirrel monkey. I. Response to static tilts and to long-duration centrifugal force. J Neurophysiol. 1976 Sep;39(5):970–984. doi: 10.1152/jn.1976.39.5.970. [DOI] [PubMed] [Google Scholar]
- Fernández C., Goldberg J. M. Physiology of peripheral neurons innervating otolith organs of the squirrel monkey. II. Directional selectivity and force-response relations. J Neurophysiol. 1976 Sep;39(5):985–995. doi: 10.1152/jn.1976.39.5.985. [DOI] [PubMed] [Google Scholar]
- Fernández C., Goldberg J. M. Physiology of peripheral neurons innervating otolith organs of the squirrel monkey. III. Response dynamics. J Neurophysiol. 1976 Sep;39(5):996–1008. doi: 10.1152/jn.1976.39.5.996. [DOI] [PubMed] [Google Scholar]
- Goldberg J. M., Fernandez C. Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey. I. Resting discharge and response to constant angular accelerations. J Neurophysiol. 1971 Jul;34(4):635–660. doi: 10.1152/jn.1971.34.4.635. [DOI] [PubMed] [Google Scholar]
- Jones G. M., Milsum J. H. Frequency-response analysis of central vestibular unit activity resulting from rotational stimulation of the semicircular canals. J Physiol. 1971 Dec;219(1):191–215. doi: 10.1113/jphysiol.1971.sp009657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindeman H. H. Studies on the morphology of the sensory regions of the vestibular apparatus with 45 figures. Ergeb Anat Entwicklungsgesch. 1969;42(1):1–113. [PubMed] [Google Scholar]
- Loe P. R., Tomko D. L., Werner G. The neural signal of angular head position in primary afferent vestibular nerve axons. J Physiol. 1973 Apr;230(1):29–50. doi: 10.1113/jphysiol.1973.sp010173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schor R. H. Responses of cat vestibular neurons to sinusoidal roll tilt. Exp Brain Res. 1974;20(4):347–362. doi: 10.1007/BF00237380. [DOI] [PubMed] [Google Scholar]
- Vidal J., Jeannerod M., Lifschitz W., Levitan H., Rosenberg J., Segundo J. P. Static and dynamic properties of gravity-sensitive receptors in the cat vestibular system. Kybernetik. 1971 Dec;9(6):205–215. doi: 10.1007/BF00289582. [DOI] [PubMed] [Google Scholar]
- Young L. R., Meiry J. L. A revised dynamic otolith model. Aerosp Med. 1968 Jun;39(6):606–608. [PubMed] [Google Scholar]