Abstract
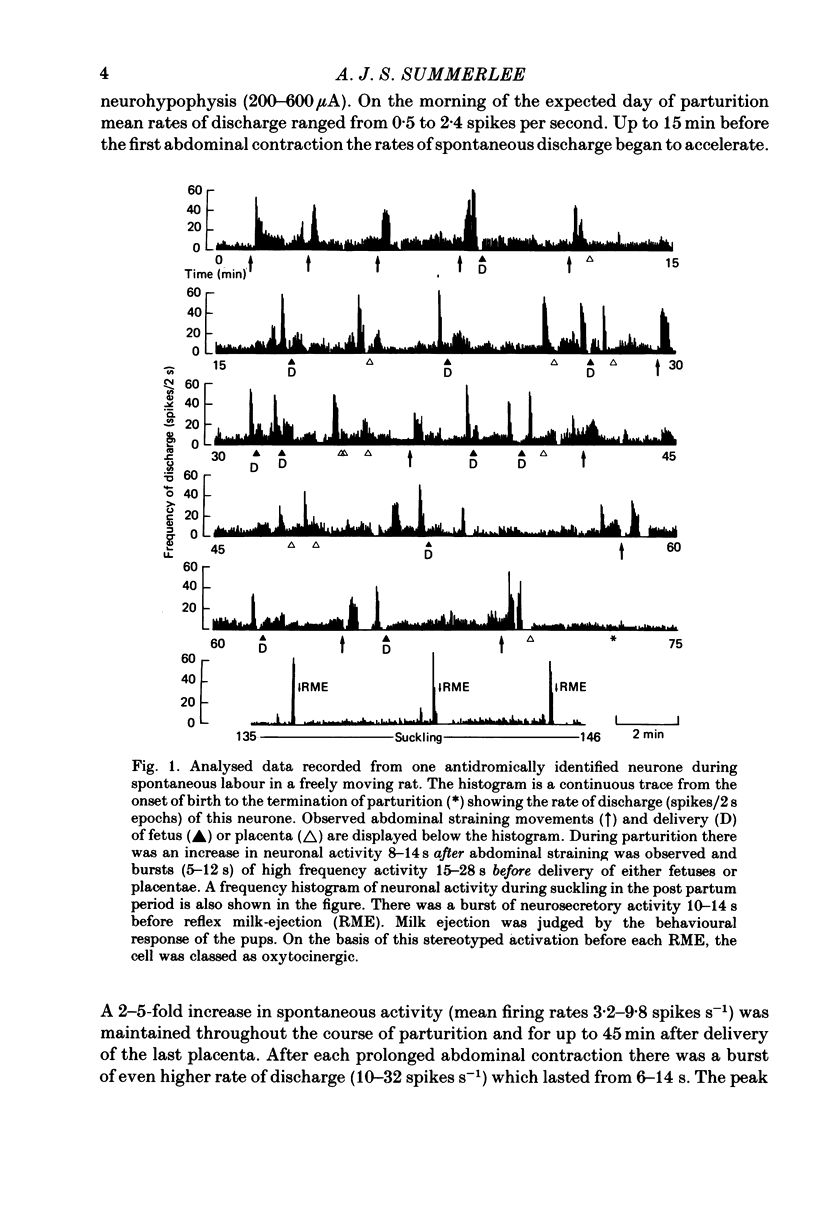
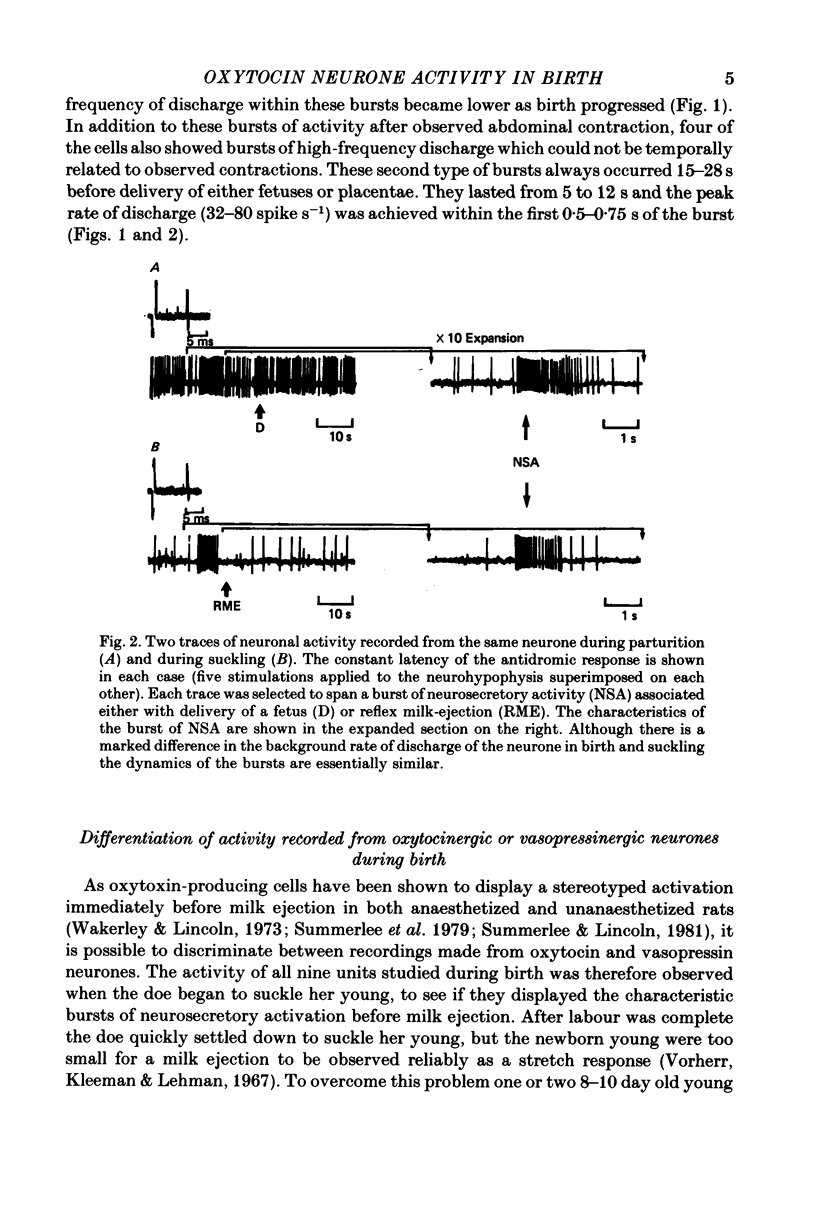
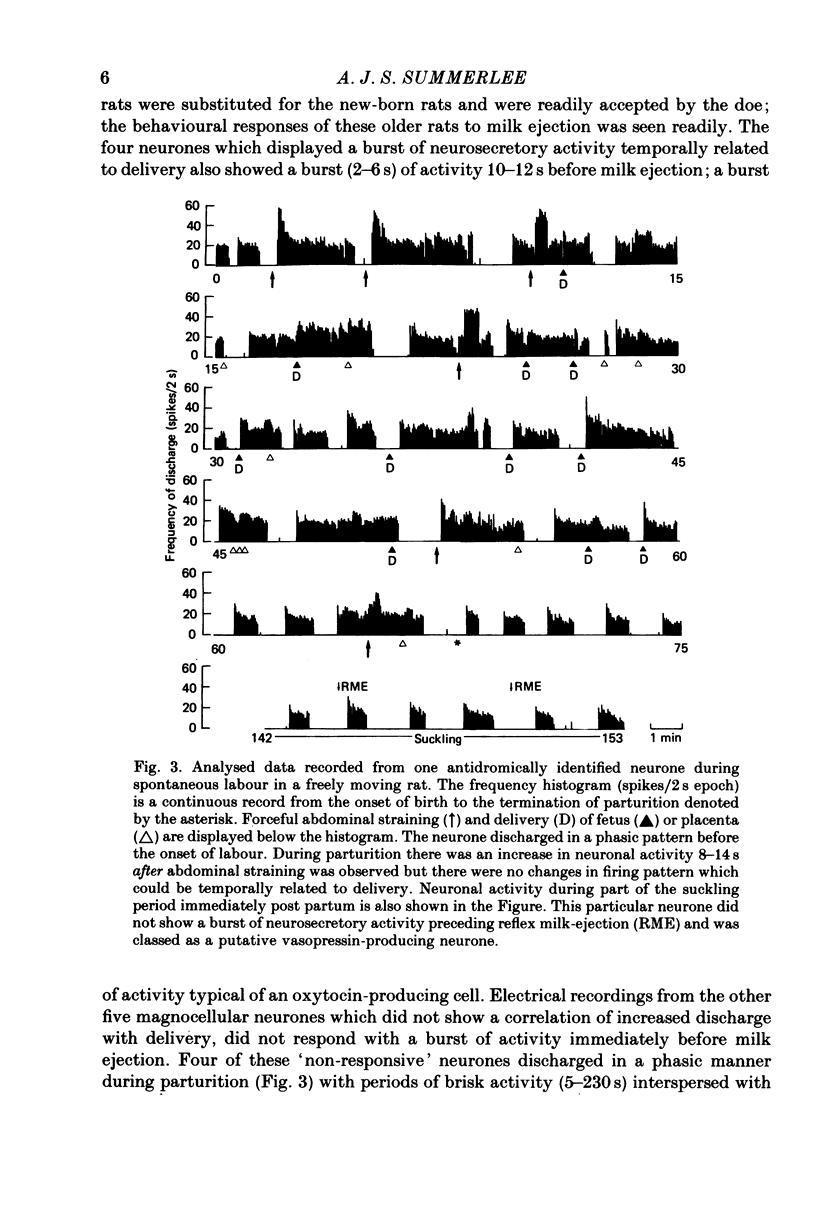
1. Extracellular electrical recordings were taken from nine antidromically identified paraventricular units in unanaesthetized, unrestrained rats. Neuronal activity was correlated with the observed events of parturition, i.e. abdominal contractions and delivery of young or placentae. 2. The level of spontaneous activity (0.15--3.2 spikes s-1) of all nine units began to increase 15 min before the first signs of abdominal contraction. This accelerated discharge (2--5 fold increase over the background activity) was maintained throughout parturition (58--93 min) and for up to 45 min after delivery of the last placenta. 3. All nine neurones displayed at 6--14 s periods of even higher rate of discharge (10--32 spikes s-1) after forceful abdominal contractions. The peak firing rates within these periods of accelerated discharge decreased as labour progressed. 4. Four cells also showed a burst (5--12 s) of high-frequency activity 15--28 s before delivery of either fetuses or placentae. These four units were later classified as oxytocinergic on the basis of their stereotyped activation 10--12 s before reflex milk-ejection. 5. The remaining five neurones which did not respond with a burst of high-frequency discharge before delivery were classed as potential vasopressin-producing cells. Four of these units displayed a phasic pattern of activity with periods of activity (5--230 s) alternating with periods of silence (4--31 s).
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen W. E., Chard T., Forsling M. L. Peripheral plasma levels of oxytocin and vasopressin in the mare during parturition. J Endocrinol. 1973 Apr;57(1):175–176. doi: 10.1677/joe.0.0570175. [DOI] [PubMed] [Google Scholar]
- Boer K., Nolten J. W. Hypothalamic paraventricular unit activity during labour in the rat. J Endocrinol. 1978 Jan;76(1):155–163. doi: 10.1677/joe.0.0760155. [DOI] [PubMed] [Google Scholar]
- Burns B. D., Webb A. C. The spontaneous activity of neurones in the cat's cerebral cortex. Proc R Soc Lond B Biol Sci. 1976 Oct 15;194(1115):211–223. doi: 10.1098/rspb.1976.0074. [DOI] [PubMed] [Google Scholar]
- Chard T., Boyd N. R., Forsling M. L., McNeilly A. S., Landon J. The development of a radioimmunoassay for oxytocin: the extraction of oxytocin from plasma, and its measurement during parturition in human and goat blood. J Endocrinol. 1970 Oct;48(2):223–234. doi: 10.1677/joe.0.0480223. [DOI] [PubMed] [Google Scholar]
- Dale H. H. On some physiological actions of ergot. J Physiol. 1906 May 31;34(3):163–206. doi: 10.1113/jphysiol.1906.sp001148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreifuss J. J., Tribollet E., Baertschi A. J. Excitation of supraoptic neurones by vaginal distention in lactating rats; correlation with neurohypophysial hormone release. Brain Res. 1976 Sep 3;113(3):600–605. doi: 10.1016/0006-8993(76)90062-7. [DOI] [PubMed] [Google Scholar]
- Fuchs A. R., Saito S. Pituitary oxytocin and vasopressin content of pregnant rats before, during and after parturition. Endocrinology. 1971 Mar;88(3):574–578. doi: 10.1210/endo-88-3-574. [DOI] [PubMed] [Google Scholar]
- Gibbens G. L., Chard T. Observations on maternal oxytocin release during human labor and the effect of intravenous alcohol administration. Am J Obstet Gynecol. 1976 Sep 15;126(2):243–246. doi: 10.1016/0002-9378(76)90283-0. [DOI] [PubMed] [Google Scholar]
- Haldar J. Independent release of oxytocin and vasopressin during parturition in the rabbit. J Physiol. 1970 Mar;206(3):723–730. doi: 10.1113/jphysiol.1970.sp009040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain M. K., Manger W. M., Rock T. W., Weiss R. J., Frantz A. G. Vasopressin release due to manual restraint in the rat: role of body compression and comparison with other stressful stimuli. Endocrinology. 1979 Mar;104(3):641–644. doi: 10.1210/endo-104-3-641. [DOI] [PubMed] [Google Scholar]
- Lincoln D. W. Correlation of unit activity in the hypothalamus with EEG patterns associated with the sleep cycle. Exp Neurol. 1969 May;24(1):1–18. doi: 10.1016/0014-4886(69)90002-8. [DOI] [PubMed] [Google Scholar]
- Lincoln D. W., Hentzen K., Hin T., van der Schoot P., Clarke G., Summerlee A. J. Sleep: a prerequisite for reflex milk ejection in the rat. Exp Brain Res. 1980 Jan;38(2):151–162. doi: 10.1007/BF00236736. [DOI] [PubMed] [Google Scholar]
- Summerlee A. J., Lincoln D. W. Electrophysiological recordings from oxytocinergic neurones during suckling in the unanaesthetized lactating rat. J Endocrinol. 1981 Aug;90(2):255–265. doi: 10.1677/joe.0.0900255. [DOI] [PubMed] [Google Scholar]
- Summerlee A. J., Lincoln D. W., Webb A. C. Long-term electrical recordings from single neurosecretory cells in the conscious lactating rat [proceedings]. J Endocrinol. 1979 Oct;83(1):41P–42P. [PubMed] [Google Scholar]
- Vorherr H., Kleeman C. R., Lehman E. Oxytocin-induced stretch reaction in suckling mice and rats: a semiquantitative bio-assay for oxytocin. Endocrinology. 1967 Oct;81(4):711–715. doi: 10.1210/endo-81-4-711. [DOI] [PubMed] [Google Scholar]
- Wakerley J. B., Lincoln D. W. The milk-ejection reflex of the rat: a 20- to 40-fold acceleration in the firing of paraventricular neurones during oxytocin release. J Endocrinol. 1973 Jun;57(3):477–493. doi: 10.1677/joe.0.0570477. [DOI] [PubMed] [Google Scholar]


