Abstract
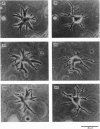
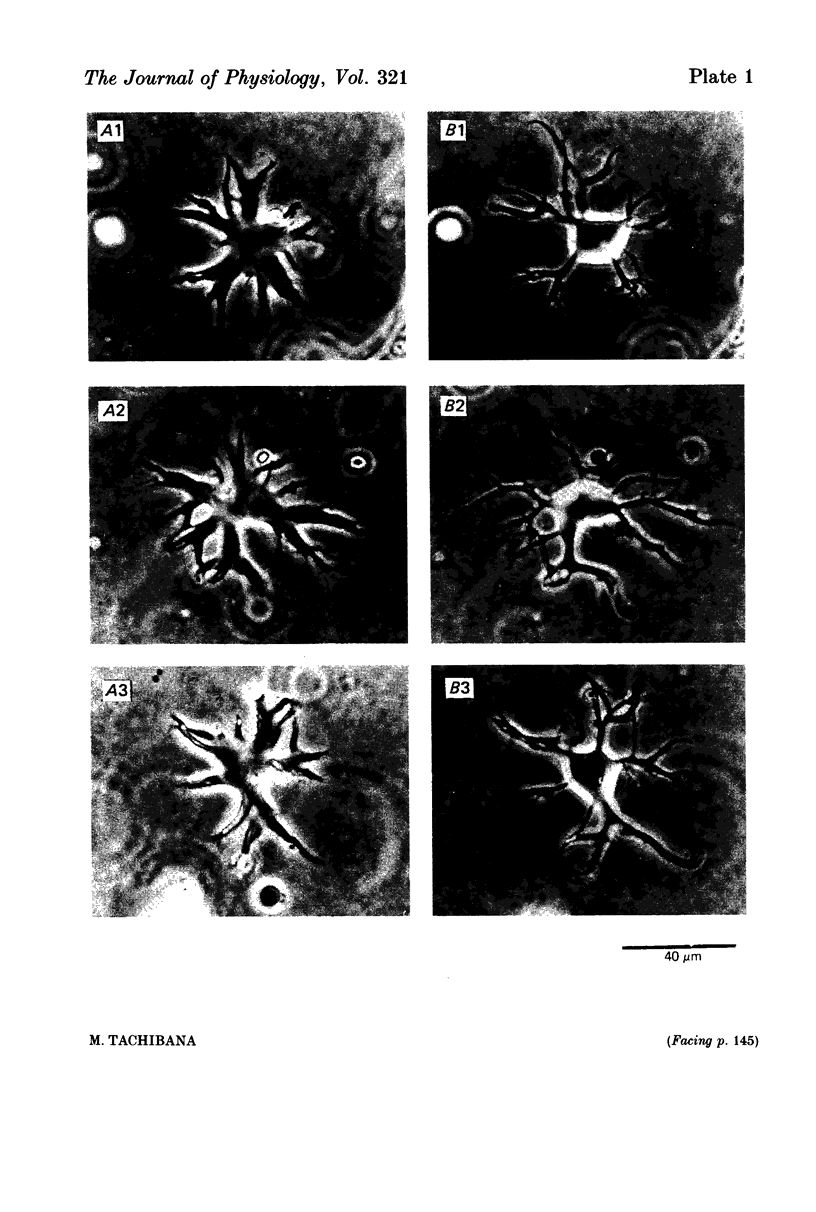
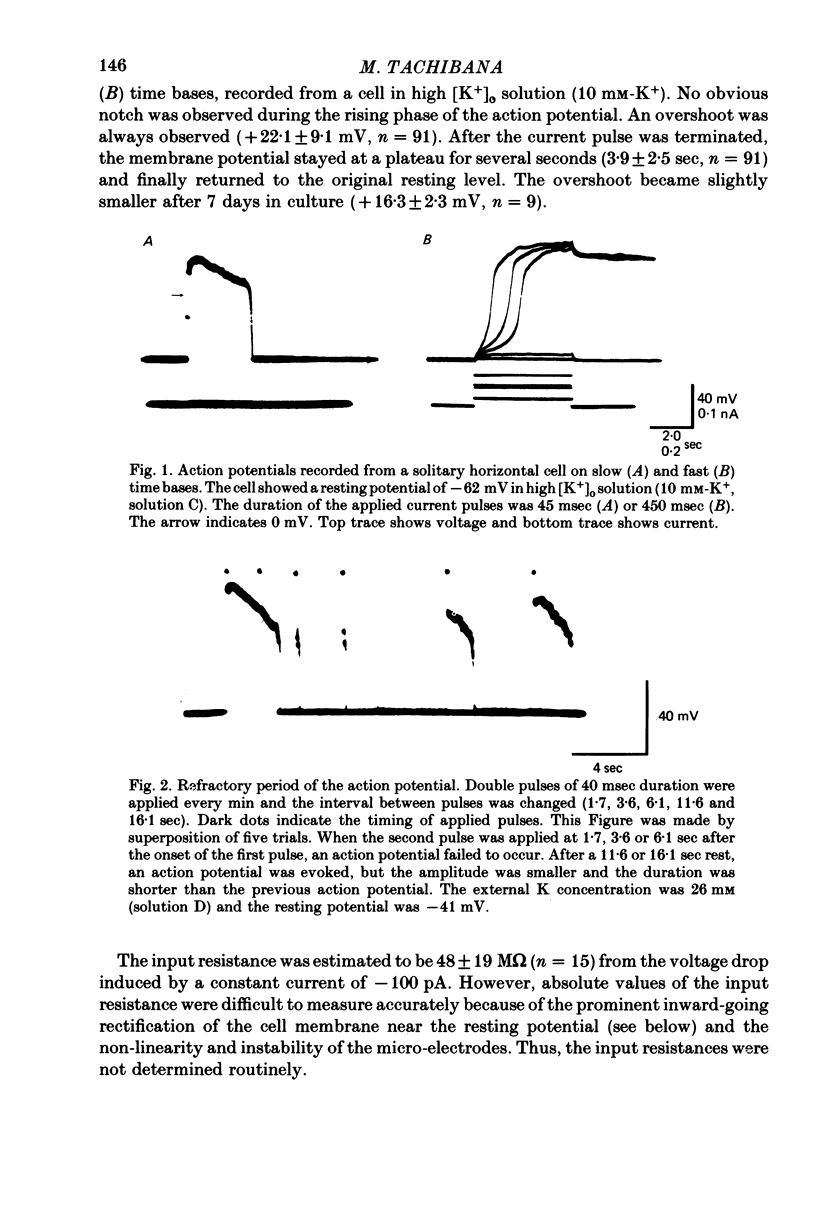
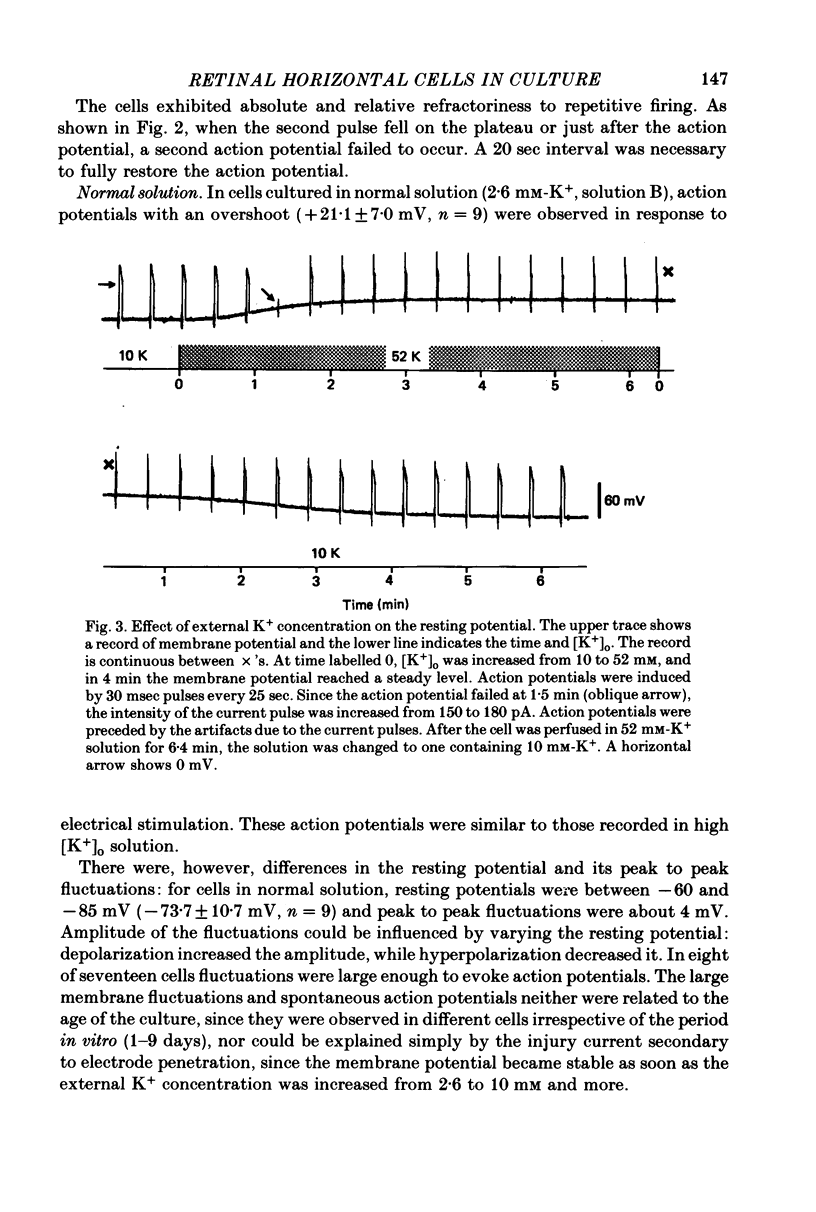
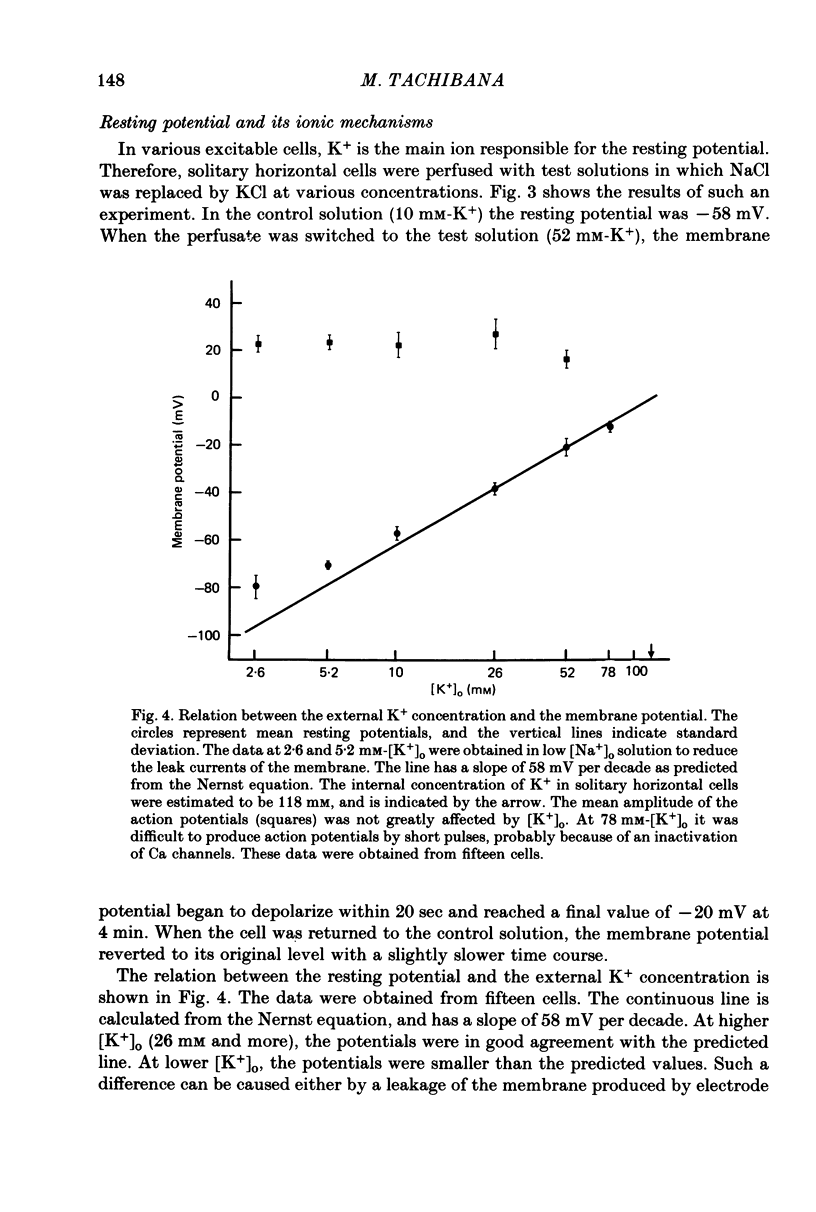
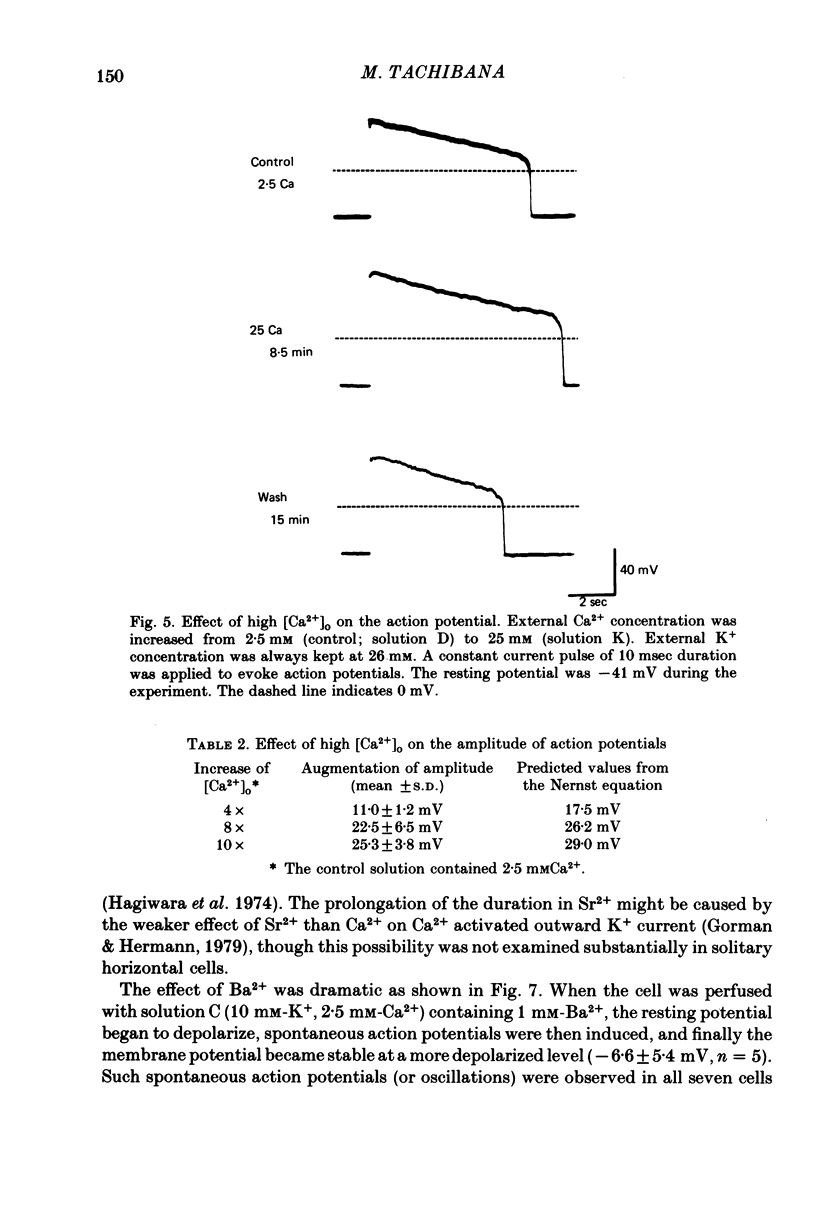
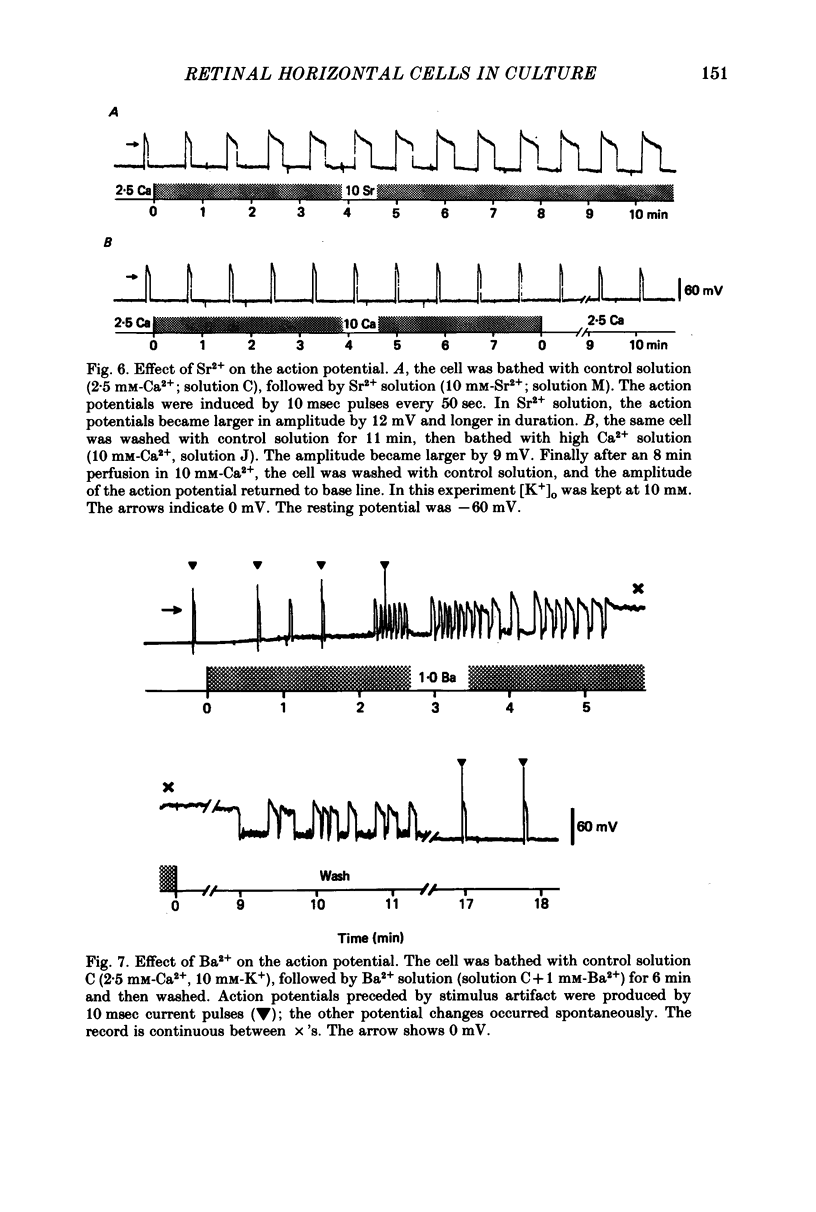
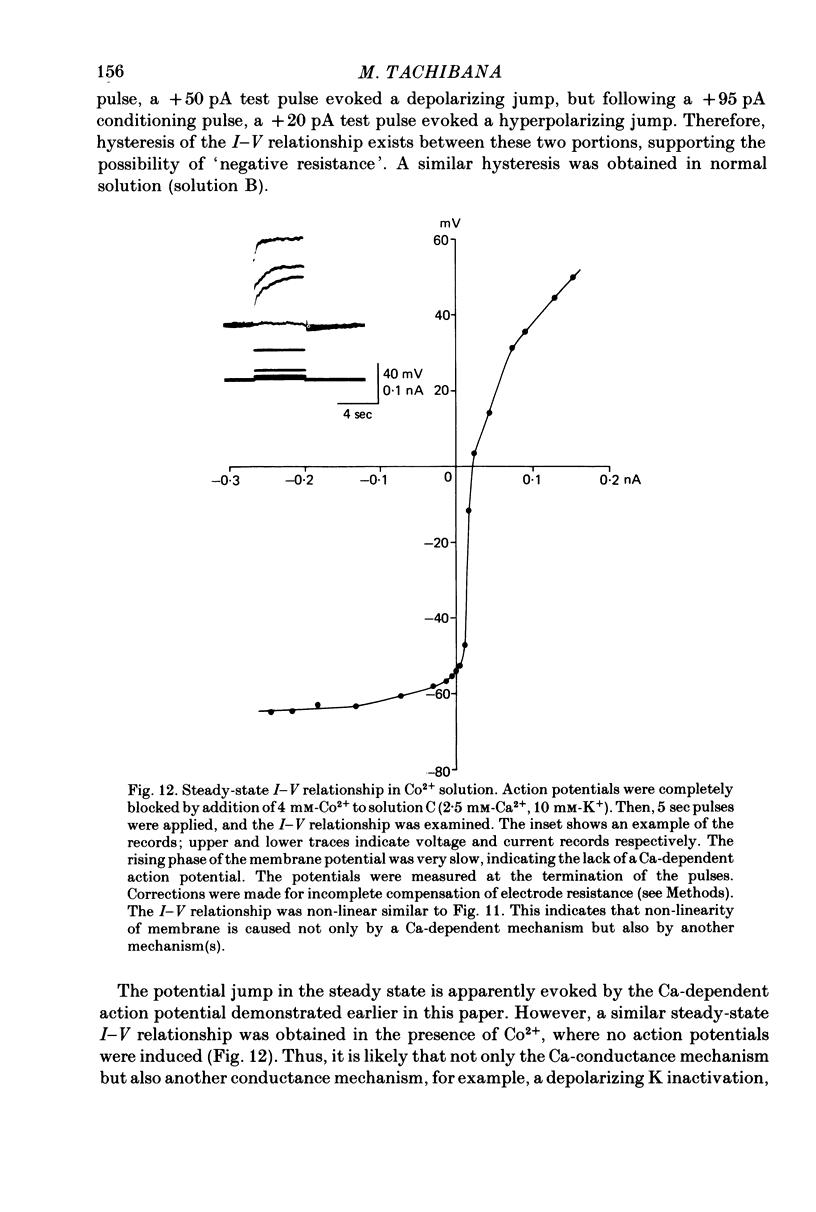
1. Solitary horizontal cells were obtained by dissociating the adult goldfish retina using the enzyme papain. The cells were identified on morphological grounds and could be kept in culture for over a week. 2. Solitary horizontal cells, penetrated with micro-electrodes, had resting potentials of about -75 mV in normal solution. When external K+ concentration was changed, the membrane potential varied from EK calculated from the Nernst equation. 3. All solitary horizontal cells tested showed an action potential in response to superthreshold depolarizing current pulses. The action potential had an overshoot of about +20 mV and a plateau potential lasting for several seconds. 4. The action potential appeared to be Ca-dependent for the following reasons: (a) TTX or low [Na+] did not affect the action potential, (b) Sr2+, Ba2+ or high [Ca2+] enhanced the action potential, while (c) Co2+ or high [Mg2+] blocked it. No regenerative activity has been observed in horizontal cells in the retina but it is possible that the regenerative mechanism is suppressed normally. 5. A role for K+ was indicated by an increase in the duration and amplitude of the action potential on the application of tetraethylammonium. 6. The steady-state current--voltage (I--V) curve, measured by applying constant current pulses, was S-shaped (current on the abscissa) and composed of inward- and outward-going rectifying regions and a transitional region between them. A similar non-linear I--V relationship has been reported in vivo. 7. The transitional region was characterized by a sudden potential jump and hysteresis, suggesting the presence of a 'negative resistance'. This potential jump appeared not to be produced by the Ca-conductance mechanism mentioned above, since similar jumps were observed in the presence of Co2+.
Full text
PDF




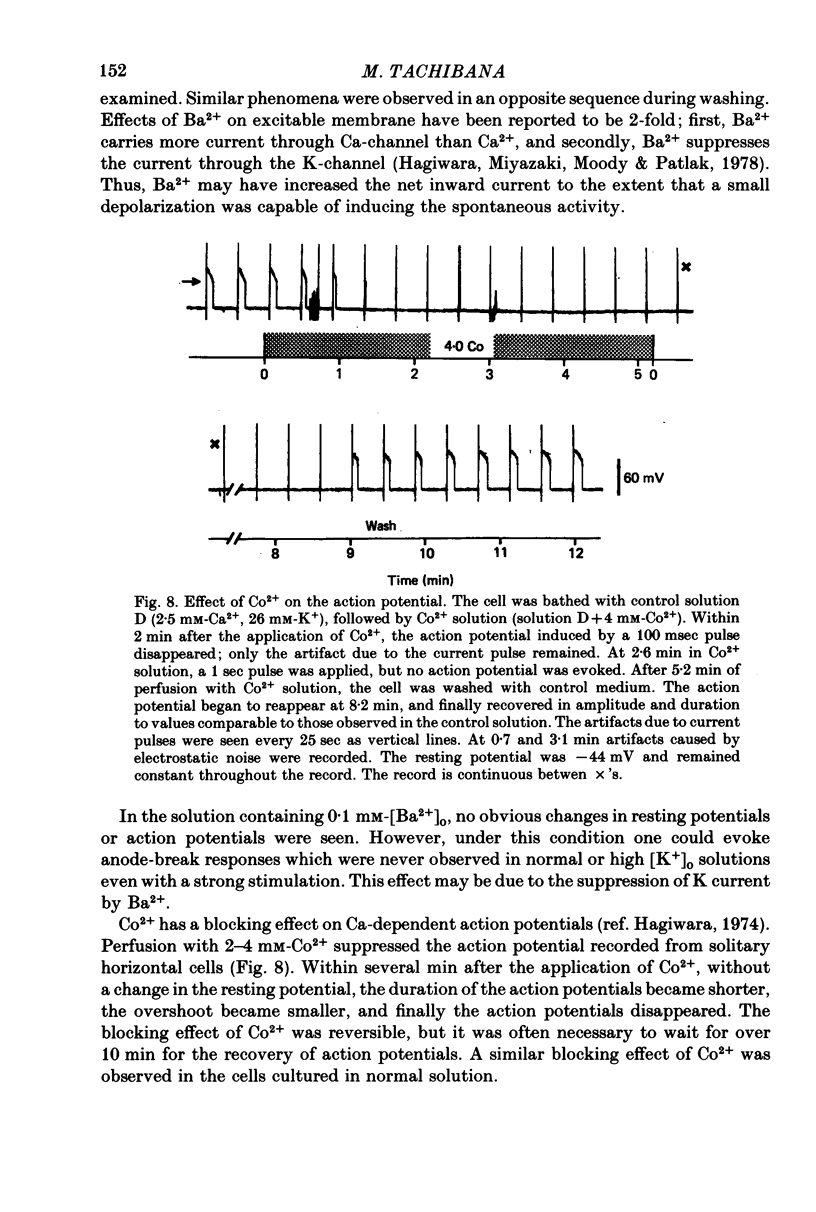





Images in this article
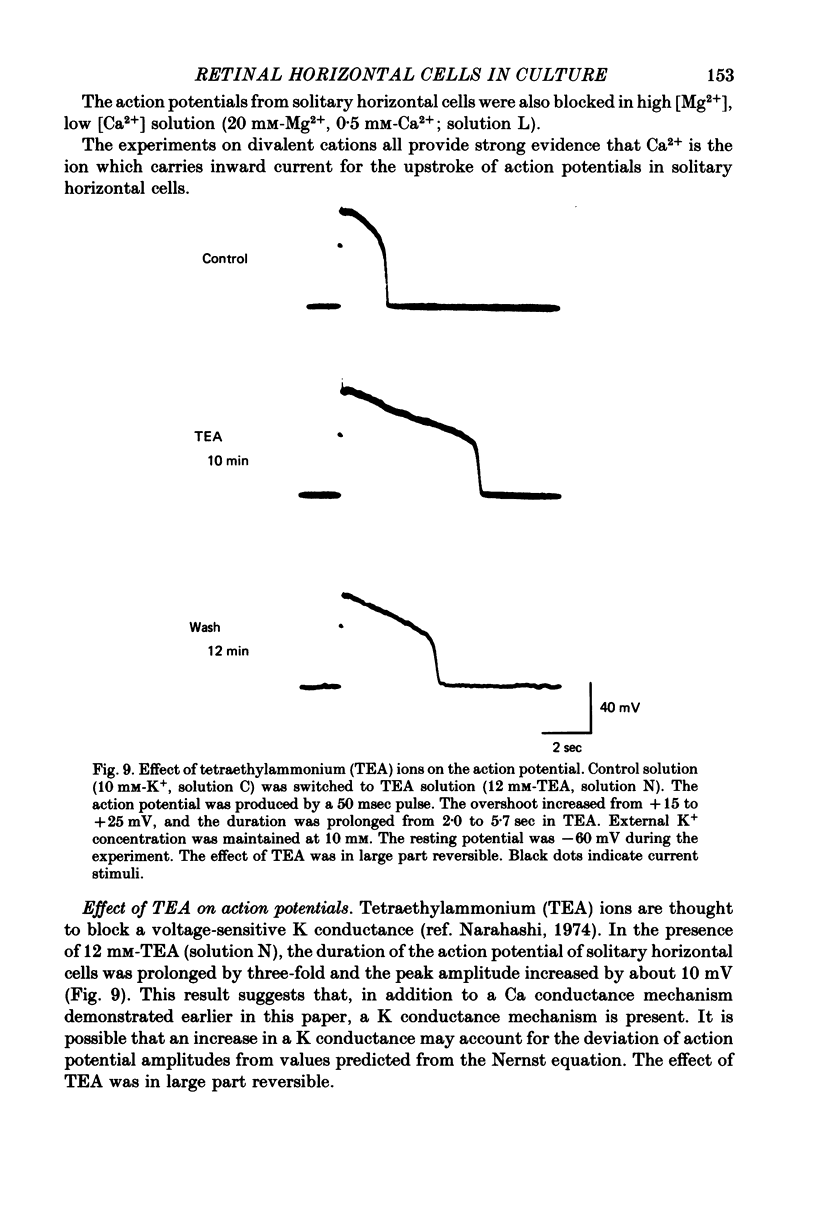
Selected References
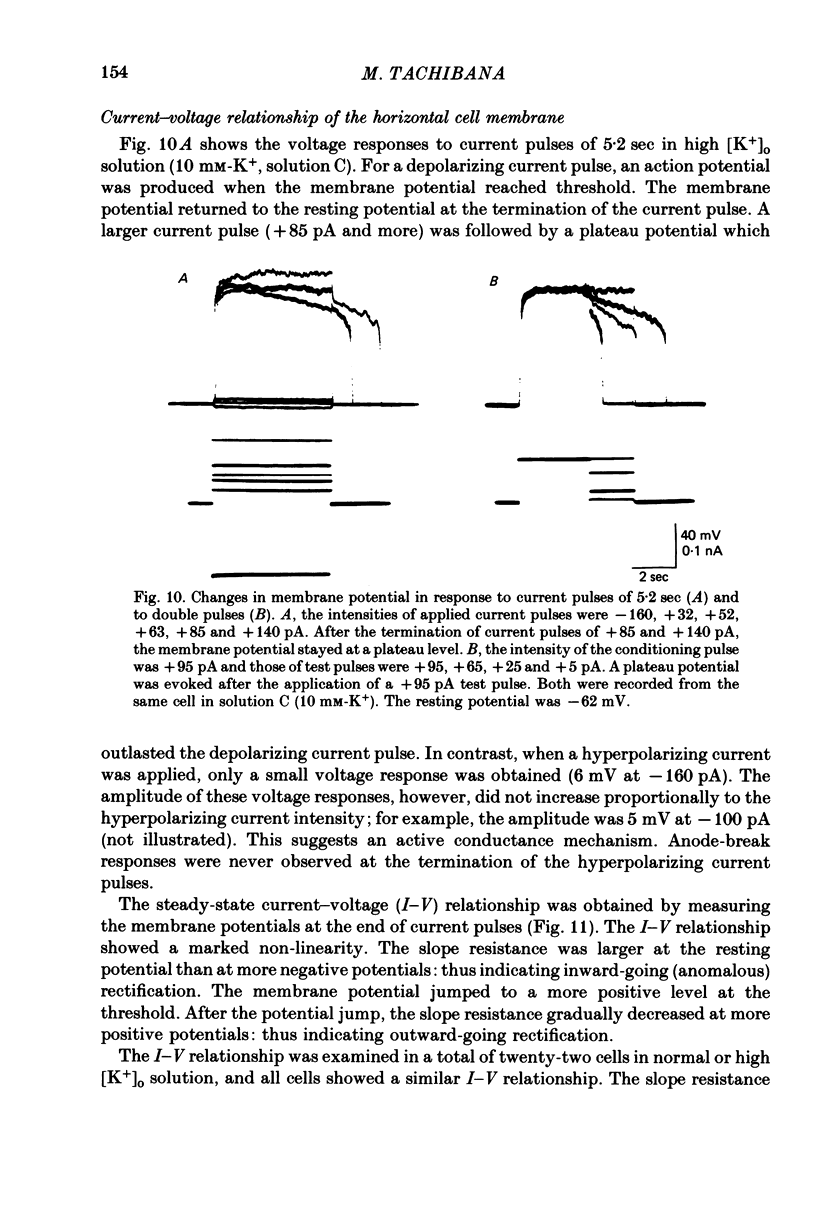
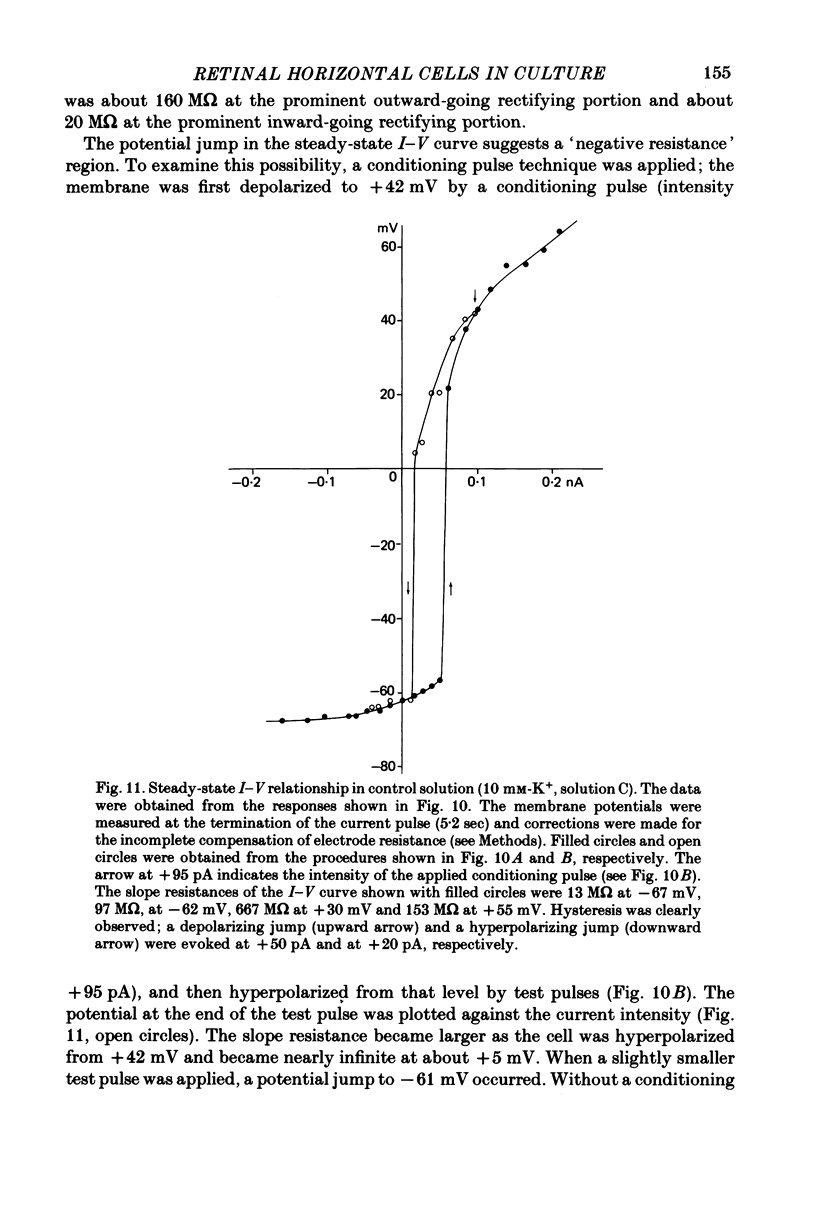
These references are in PubMed. This may not be the complete list of references from this article.
- Anctil M., Ali M. A., Couillard P. Isolated retinal cells of some lower vertebrates. Rev Can Biol. 1973 Jun;32(2):107–119. [PubMed] [Google Scholar]
- Bader C. R., MacLeish P. R., Schwartz E. A. Responses to light of solitary rod photoreceptors isolated from tiger salamander retina. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3507–3511. doi: 10.1073/pnas.75.7.3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader C. R., Macleish P. R., Schwartz E. A. A voltage-clamp study of the light response in solitary rods of the tiger salamander. J Physiol. 1979 Nov;296:1–26. doi: 10.1113/jphysiol.1979.sp012988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Fuortes M. G. Electrical responses of single cones in the retina of the turtle. J Physiol. 1970 Mar;207(1):77–92. doi: 10.1113/jphysiol.1970.sp009049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Fuortes M. G., O'Bryan P. M. Receptive fields of cones in the retina of the turtle. J Physiol. 1971 Apr;214(2):265–294. doi: 10.1113/jphysiol.1971.sp009432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray D. Surface movements during the growth of single explanted neurons. Proc Natl Acad Sci U S A. 1970 Apr;65(4):905–910. doi: 10.1073/pnas.65.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt D. A. Responses and receptive-field organization of cones in perch retinas. J Neurophysiol. 1977 Jan;40(1):53–62. doi: 10.1152/jn.1977.40.1.53. [DOI] [PubMed] [Google Scholar]
- Byzov A. L., Trifonov J. A. The response to electric stimulation of horizontal cells in the carp retina. Vision Res. 1968 Jul;8(7):817–822. doi: 10.1016/0042-6989(68)90132-6. [DOI] [PubMed] [Google Scholar]
- Byzov A. L., Trifonov Y. A., Chailahian L. M., Golubtzov K. W. Amplification of graded potentials in horizontal cells of the retina. Vision Res. 1977 Feb;17(2):265–273. doi: 10.1016/0042-6989(77)90090-6. [DOI] [PubMed] [Google Scholar]
- Cervetto L., Piccolino M. Synaptic transmission between photoreceptors and horizontal cells in the turtle retina. Science. 1974 Feb 1;183(4123):417–419. doi: 10.1126/science.183.4123.417. [DOI] [PubMed] [Google Scholar]
- Colburn T. R., Schwartz E. A. Linear voltage control of current passed through a micropipette with variable resistance. Med Biol Eng. 1972 Jul;10(4):504–509. doi: 10.1007/BF02474198. [DOI] [PubMed] [Google Scholar]
- Dowling J. E., Ehinger B. The interplexiform cell system. I. Synapses of the dopaminergic neurons of the goldfish retina. Proc R Soc Lond B Biol Sci. 1978 Apr 13;201(1142):7–26. doi: 10.1098/rspb.1978.0030. [DOI] [PubMed] [Google Scholar]
- Dowling J. E., Ripps H. Effect of magnesium on horizontal cell activity in the skate retina. Nature. 1973 Mar 9;242(5393):101–103. doi: 10.1038/242101a0. [DOI] [PubMed] [Google Scholar]
- Drujan B. D., Svaetichin G. Characterization of different classes of isolated retinal cells. Vision Res. 1972 Nov;12(11):1777–1784. doi: 10.1016/0042-6989(72)90068-5. [DOI] [PubMed] [Google Scholar]
- Ehinger B., Falck B., Laties A. M. Adrenergic neurons in teleost retina. Z Zellforsch Mikrosk Anat. 1969 May 23;97(2):285–297. doi: 10.1007/BF00344763. [DOI] [PubMed] [Google Scholar]
- Fain G. L., Gerschenfeld H. M., Quandt F. N. Calcium spikes in toad rods. J Physiol. 1980 Jun;303:495–513. doi: 10.1113/jphysiol.1980.sp013300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain G. L., Quandt F. N., Gerschenfeld H. M. Calcium-dependent regenerative responses in rods. Nature. 1977 Oct 20;269(5630):707–710. doi: 10.1038/269707a0. [DOI] [PubMed] [Google Scholar]
- Gerschenfeld H. M., Piccolino M. Sustained feedback effects of L-horizontal cells on turtle cones. Proc R Soc Lond B Biol Sci. 1980 Jan 17;206(1165):465–480. doi: 10.1098/rspb.1980.0008. [DOI] [PubMed] [Google Scholar]
- Gorman A. L., Hermann A. Internal effects of divalent cations on potassium permeability in molluscan neurones. J Physiol. 1979 Nov;296:393–410. doi: 10.1113/jphysiol.1979.sp013012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S. Ca-dependent action potential. Membranes. 1975;3:359–381. [PubMed] [Google Scholar]
- Hagiwara S., Fukuda J., Eaton D. C. Membrane currents carried by Ca, Sr, and Ba in barnacle muscle fiber during voltage clamp. J Gen Physiol. 1974 May;63(5):564–578. doi: 10.1085/jgp.63.5.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Moody W., Patlak J. Blocking effects of barium and hydrogen ions on the potassium current during anomalous rectification in the starfish egg. J Physiol. 1978 Jun;279:167–185. doi: 10.1113/jphysiol.1978.sp012338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Nakajima S. Differences in Na and Ca spikes as examined by application of tetrodotoxin, procaine, and manganese ions. J Gen Physiol. 1966 Mar;49(4):793–806. doi: 10.1085/jgp.49.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y., Kato A., Inokuchi M., Watanabe K. Re-examination of horizontal cells in the carp retina with procion yellow electrode. Vision Res. 1976 Jan;16(1):25–29. doi: 10.1016/0042-6989(76)90072-9. [DOI] [PubMed] [Google Scholar]
- Hedden W. L., Jr, Dowling J. E. The interplexiform cell system. II. Effects of dopamine on goldfish retinal neurones. Proc R Soc Lond B Biol Sci. 1978 Apr 13;201(1142):27–55. doi: 10.1098/rspb.1978.0031. [DOI] [PubMed] [Google Scholar]
- Kaneko A. Electrical connexions between horizontal cells in the dogfish retina. J Physiol. 1971 Feb;213(1):95–105. doi: 10.1113/jphysiol.1971.sp009370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A., Lam D. M., Wiesel T. N. Isolated horizontal cells of elasmobranch retinae. Brain Res. 1976 Apr 9;105(3):567–572. doi: 10.1016/0006-8993(76)90605-3. [DOI] [PubMed] [Google Scholar]
- Kaneko A. Physiological and morphological identification of horizontal, bipolar and amacrine cells in goldfish retina. J Physiol. 1970 May;207(3):623–633. doi: 10.1113/jphysiol.1970.sp009084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A., Shimazaki H. Effects of external ions on the synaptic transmission from photorecptors to horizontal cells in the carp retina. J Physiol. 1975 Nov;252(2):509–522. doi: 10.1113/jphysiol.1975.sp011155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Tetrodotoxin-resistant electric activity in presynaptic terminals. J Physiol. 1969 Aug;203(2):459–487. doi: 10.1113/jphysiol.1969.sp008875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam D. M. Biosynthesis of acetylcholine in turtle photoreceptors. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1987–1991. doi: 10.1073/pnas.69.7.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam D. M. Biosynthesis of gamma-aminobutyric acid by isolated axons of cone horizontal cells in the goldfish retina. Nature. 1975 Mar 27;254(5498):345–347. doi: 10.1038/254345a0. [DOI] [PubMed] [Google Scholar]
- Lam D. M., Lasater E. M., Naka K. I. gamma-Aminobutyric acid: a neurotransmitter candidate for cone horizontal cells of the catfish retina. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6310–6313. doi: 10.1073/pnas.75.12.6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam D. M., Steinman L. The uptake of ( - 3 H) aminobutyric acid in the goldfish retina. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2777–2781. doi: 10.1073/pnas.68.11.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam D. M., Su Y. Y., Swain L., Marc R. E., Brandon C., Wu J. Y. Immunocytochemical localisation of L-glutamic acid decarboxylase in the goldfish retina. Nature. 1979 Apr 5;278(5704):565–567. doi: 10.1038/278565a0. [DOI] [PubMed] [Google Scholar]
- Lam D. M. Synaptic chemistry of identified cells in the vertebrate retina. Cold Spring Harb Symp Quant Biol. 1976;40:571–579. doi: 10.1101/sqb.1976.040.01.053. [DOI] [PubMed] [Google Scholar]
- MACNICHOL E. J., SVAETICHIN G. Electric responses from the isolated retinas of fishes. Am J Ophthalmol. 1958 Sep;46(3 Pt 2):26–46. doi: 10.1016/0002-9394(58)90053-9. [DOI] [PubMed] [Google Scholar]
- Marc R. E., Stell W. K., Bok D., Lam D. M. GABA-ergic pathways in the goldfish retina. J Comp Neurol. 1978 Nov 15;182(2):221–244. doi: 10.1002/cne.901820204. [DOI] [PubMed] [Google Scholar]
- Miyazaki S. I., Ohmori H., Sasaki S. Action potential and non-linear current-voltage relation in starfish oocytes. J Physiol. 1975 Mar;246(1):37–54. doi: 10.1113/jphysiol.1975.sp010879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S. I., Ohmori H., Sasaki S. Potassium rectifications of the starfish oocyte membrane and their changes during oocyte maturation. J Physiol. 1975 Mar;246(1):55–78. doi: 10.1113/jphysiol.1975.sp010880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S. I., Takahashi K., Tsuda K. Electrical excitability in the egg cell membrane of the tunicate. J Physiol. 1974 Apr;238(1):37–54. doi: 10.1113/jphysiol.1974.sp010509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S. I., Takahashi K., Tsuda K., Yoshii M. Analysis of non-linearity observed in the current-voltage relation of the tunicate embryo. J Physiol. 1974 Apr;238(1):55–77. doi: 10.1113/jphysiol.1974.sp010510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka K. I., Rushton W. A. The generation and spread of S-potentials in fish (Cyprinidae). J Physiol. 1967 Sep;192(2):437–461. doi: 10.1113/jphysiol.1967.sp008308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahashi T. Chemicals as tools in the study of excitable membranes. Physiol Rev. 1974 Oct;54(4):813–889. doi: 10.1152/physrev.1974.54.4.813. [DOI] [PubMed] [Google Scholar]
- Normann R. A., Perlman I. Signal transmission from red cones to horizontal cells in the turtle retina. J Physiol. 1979 Jan;286:509–524. doi: 10.1113/jphysiol.1979.sp012634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton A. L., Spekreijse H., Wolbarsht M. L., Wagner H. G. Receptive field organization of the S-potential. Science. 1968 May 31;160(3831):1021–1022. doi: 10.1126/science.160.3831.1021. [DOI] [PubMed] [Google Scholar]
- O'Bryan P. M. Properties of the depolarizing synaptic potential evoked by peripheral illumination in cones of the turtle retina. J Physiol. 1973 Nov;235(1):207–223. doi: 10.1113/jphysiol.1973.sp010385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolino M., Gerschenfeld H. M. Characteristics and ionic processes involved in feedback spikes of turtle cones. Proc R Soc Lond B Biol Sci. 1980 Jan 17;206(1165):439–463. doi: 10.1098/rspb.1980.0007. [DOI] [PubMed] [Google Scholar]
- Piccolino M., Neyton J., Gerschenfeld H. M. Synaptic mechanisms involved in responses of chromaticity horizontal cells of turtle retina. Nature. 1980 Mar 6;284(5751):58–60. doi: 10.1038/284058a0. [DOI] [PubMed] [Google Scholar]
- Pinto L. H., Pak W. L. Light-induced changes in photoreceptor membrane resistance and potential in Gecko retinas. I. Preparations treated to reduce lateral interactions. J Gen Physiol. 1974 Jul;64(1):26–48. doi: 10.1085/jgp.64.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto L. H., Pak W. L. Light-induced changes in photoreceptor membrane resistance and potential in Gecko retinas. II. Preparations with active lateral interactions. J Gen Physiol. 1974 Jul;64(1):49–69. doi: 10.1085/jgp.64.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarthy P. V., Lam D. M. Biochemical studies of isolated glial (Müller) cells from the turtle retina. J Cell Biol. 1978 Sep;78(3):675–684. doi: 10.1083/jcb.78.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarthy P. V., Lam D. M. Endogenous levels of neurotransmitter candidates in photoreceptor cells of the turtle retina. J Neurochem. 1979 Feb;32(2):455–461. doi: 10.1111/j.1471-4159.1979.tb00371.x. [DOI] [PubMed] [Google Scholar]
- Stell W. K., Lightfoot D. O. Color-specific interconnections of cones and horizontal cells in the retina of the goldfish. J Comp Neurol. 1975 Feb 15;159(4):473–502. doi: 10.1002/cne.901590404. [DOI] [PubMed] [Google Scholar]
- TOMITA T. Electrical activity in the vertebrate retina. J Opt Soc Am. 1963 Jan;53:49–57. doi: 10.1364/josa.53.000049. [DOI] [PubMed] [Google Scholar]
- Toyoda J. I., Tonosaki K. Effect of polarisation of horizontal cells on the on-centre bipolar cell of carp retina. Nature. 1978 Nov 23;276(5686):399–400. doi: 10.1038/276399a0. [DOI] [PubMed] [Google Scholar]
- Trifonov I. U. Izuchenie sinapticheskoi peredachi mezhdu fotoretseptorom i gorizontal'noi kletkoi pri pomoshchi élektricheskikh razdrazhenii setchatki. Biofizika. 1968 Sep-Oct;13(5):809–817. [PubMed] [Google Scholar]
- Waloga G., Pak W. L. Ionic mechanism for the generation of horizontal cell potentials in isolated axolotl retina. J Gen Physiol. 1978 Jan;71(1):69–92. doi: 10.1085/jgp.71.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin F. S. Anomalous rectification in horizontal cells. J Physiol. 1975 Jan;244(3):639–657. doi: 10.1113/jphysiol.1975.sp010817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin F. S., Dowling J. E. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969 May;32(3):339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]
- Witkovsky P., Dowling J. E. Synaptic relationships in the plexiform layers of carp retina. Z Zellforsch Mikrosk Anat. 1969;100(1):60–82. doi: 10.1007/BF00343821. [DOI] [PubMed] [Google Scholar]
- Yamada E., Ishikawa T. The fine structure of the horizontal cells in some vertebrate retinae. Cold Spring Harb Symp Quant Biol. 1965;30:383–392. doi: 10.1101/sqb.1965.030.01.038. [DOI] [PubMed] [Google Scholar]