Abstract
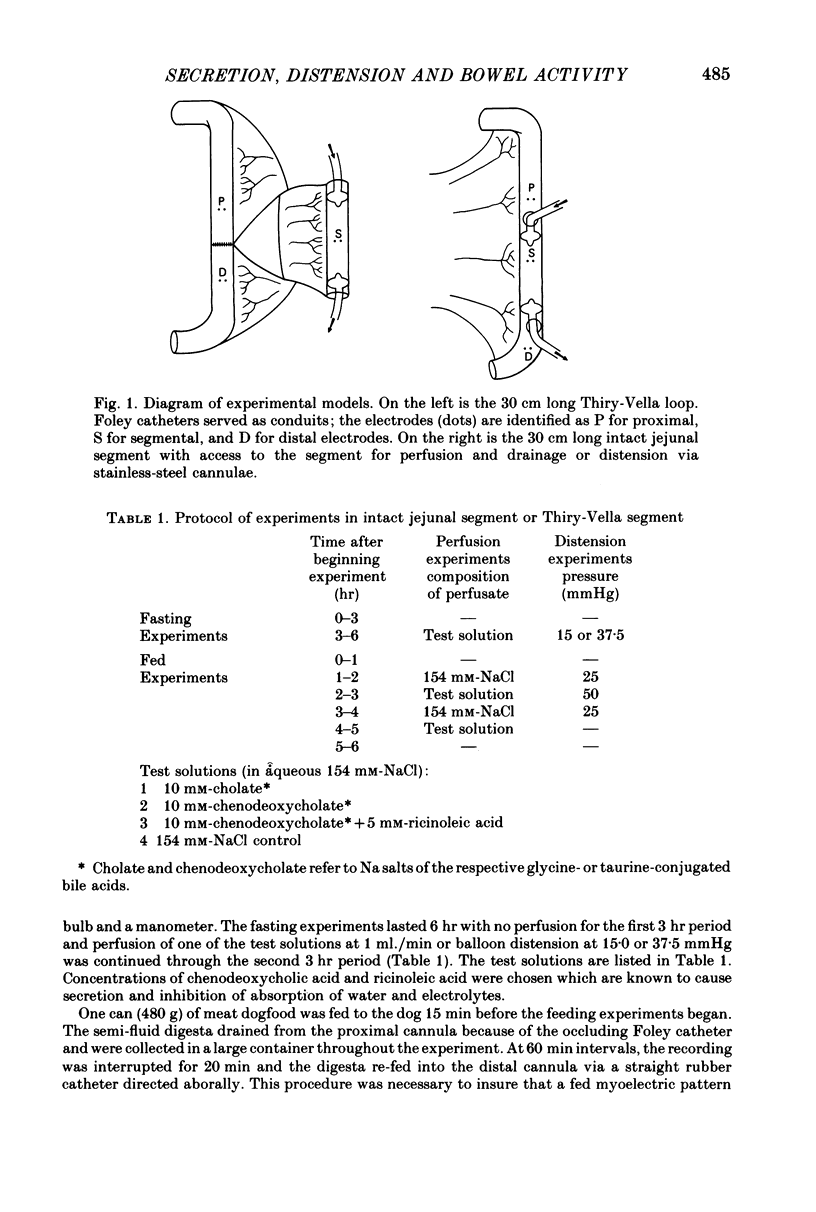
1. Defined jejunal segments were perfused with solutions of bile salts and of ricinoleic acid during fasting and after feeding in two groups of conscious dogs, one with the segment in continuity, and the other with a Thirty-Vella loop. Myoelectric activity was recorded from chronically implanted electrodes on the jejunal segment and also from the proximal and distal in situ bowel.
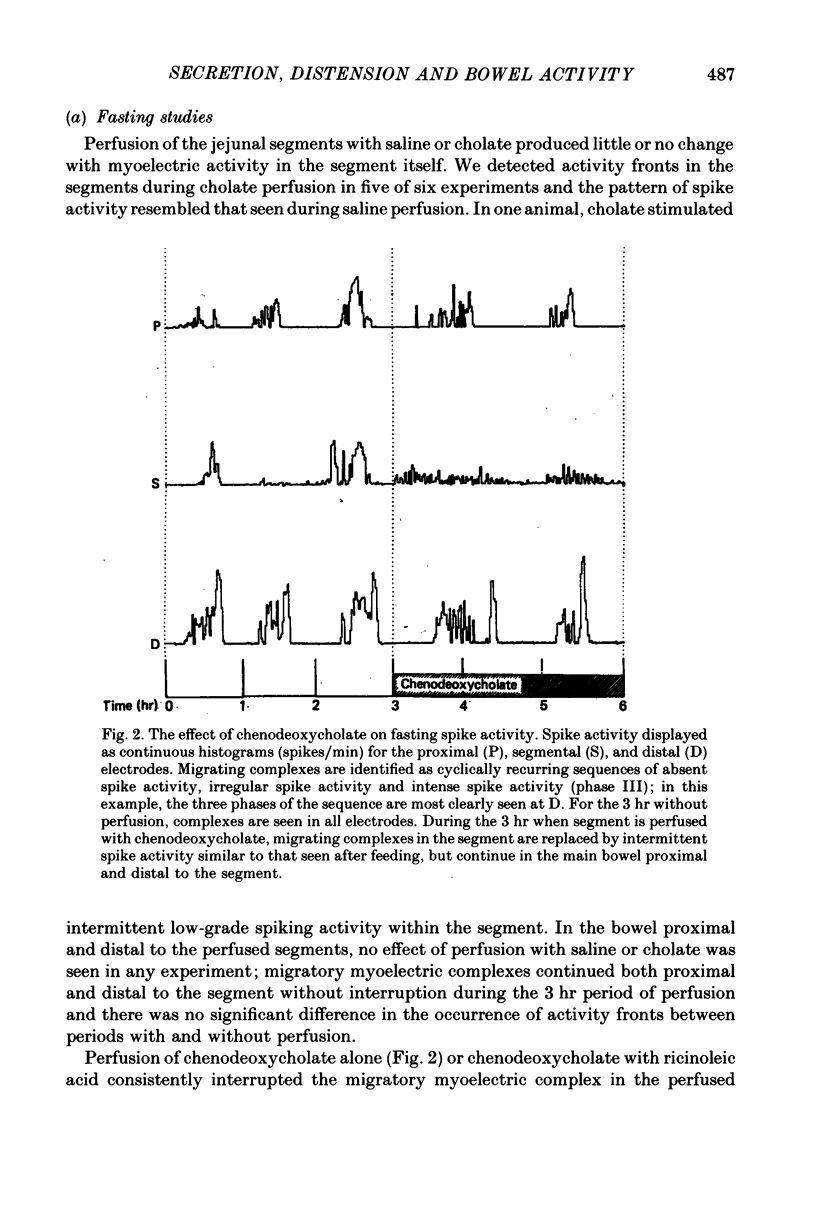
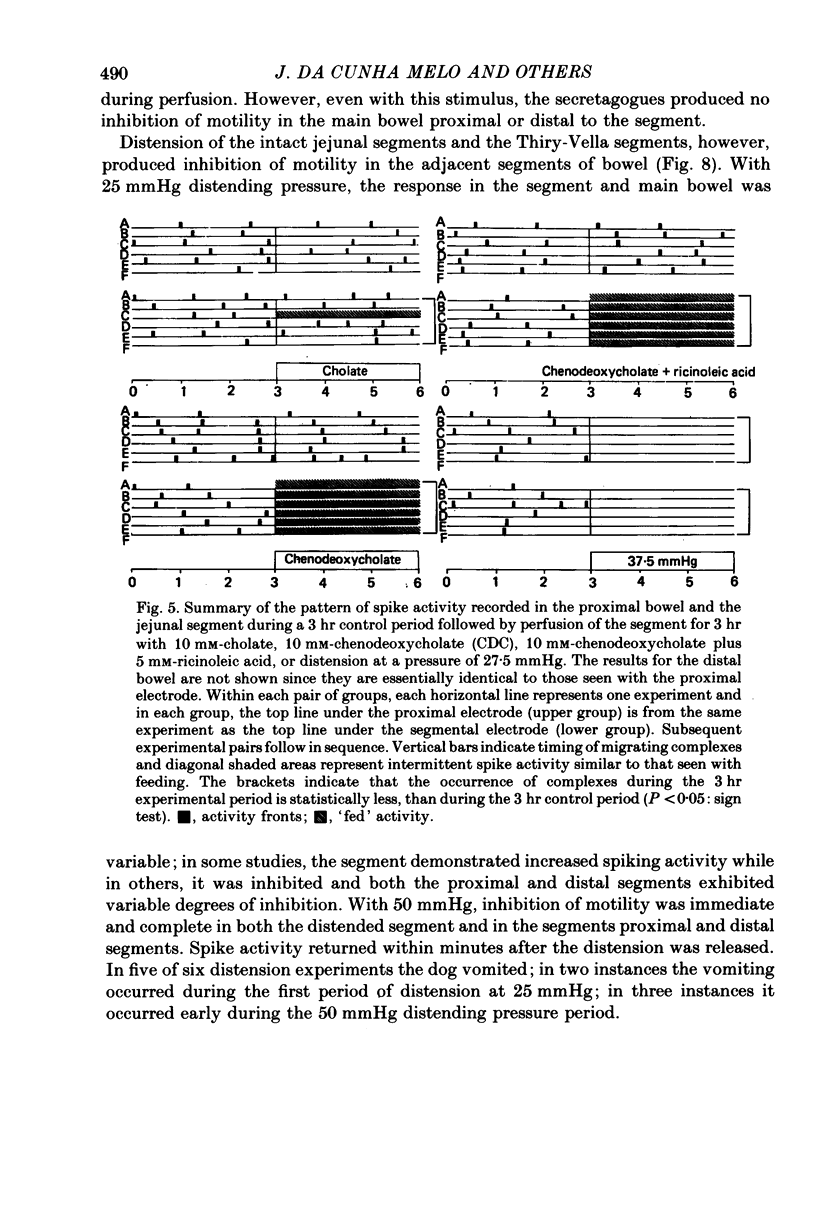
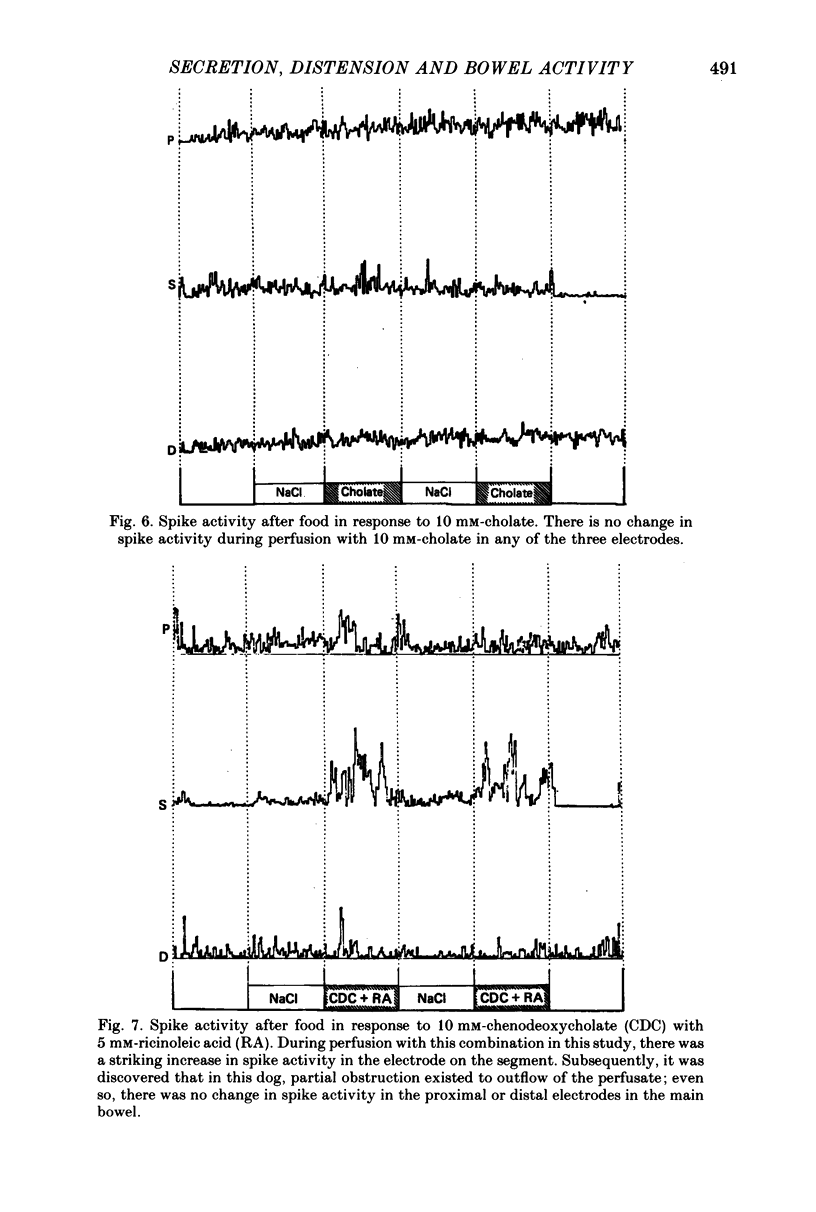
2. The results in both groups were identical. During fasting, migrating complexes were present in the segment, but were replaced by intermittent spike activity during chenodeoxycholate without and with ricinoleic acid perfusion. After food, when migrating complexes were replaced by intermittent spike activity, none of the solutions produced any consistent effect.
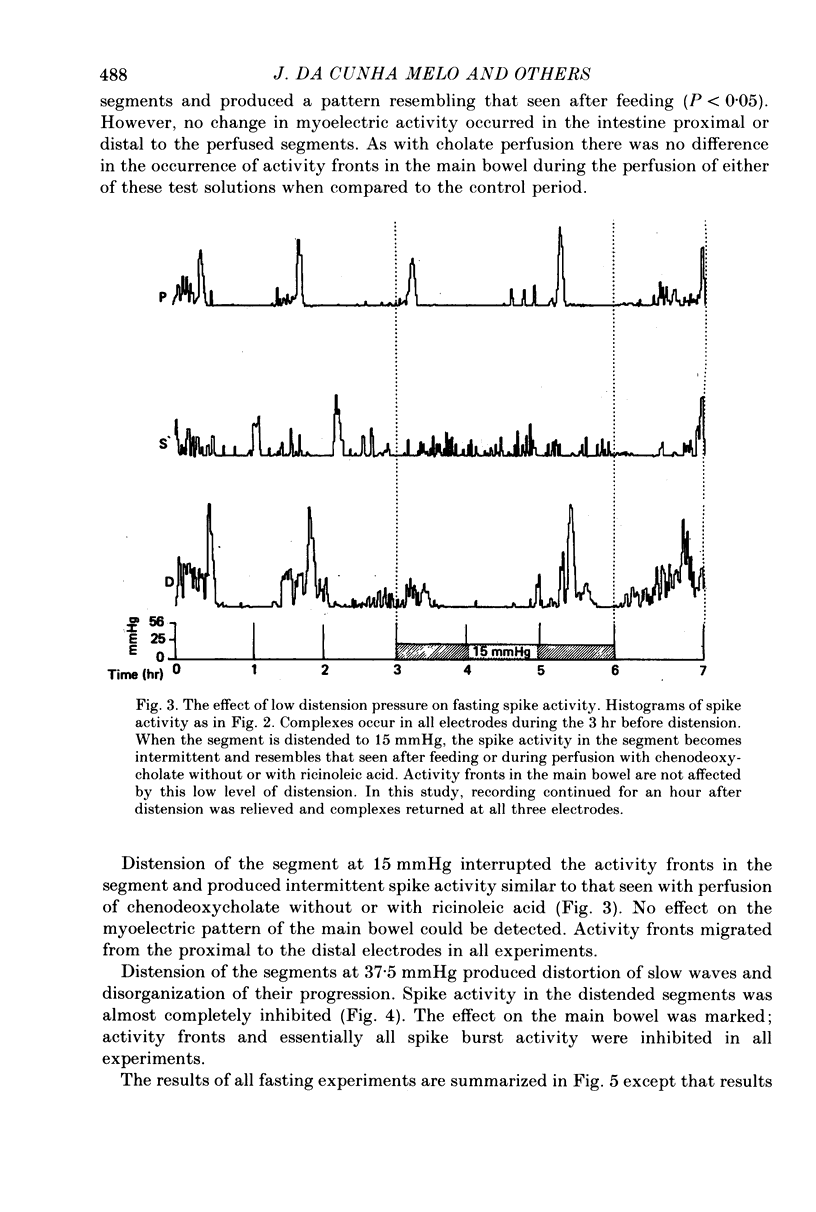
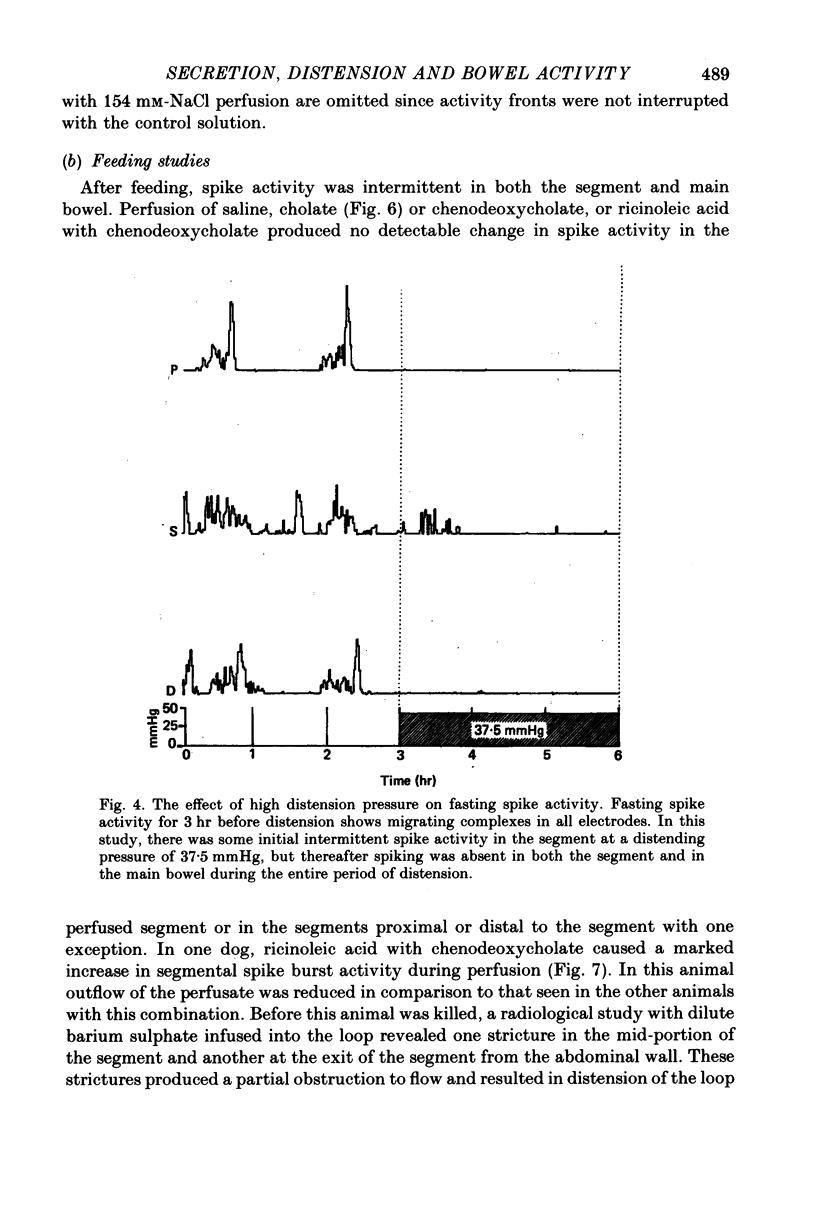
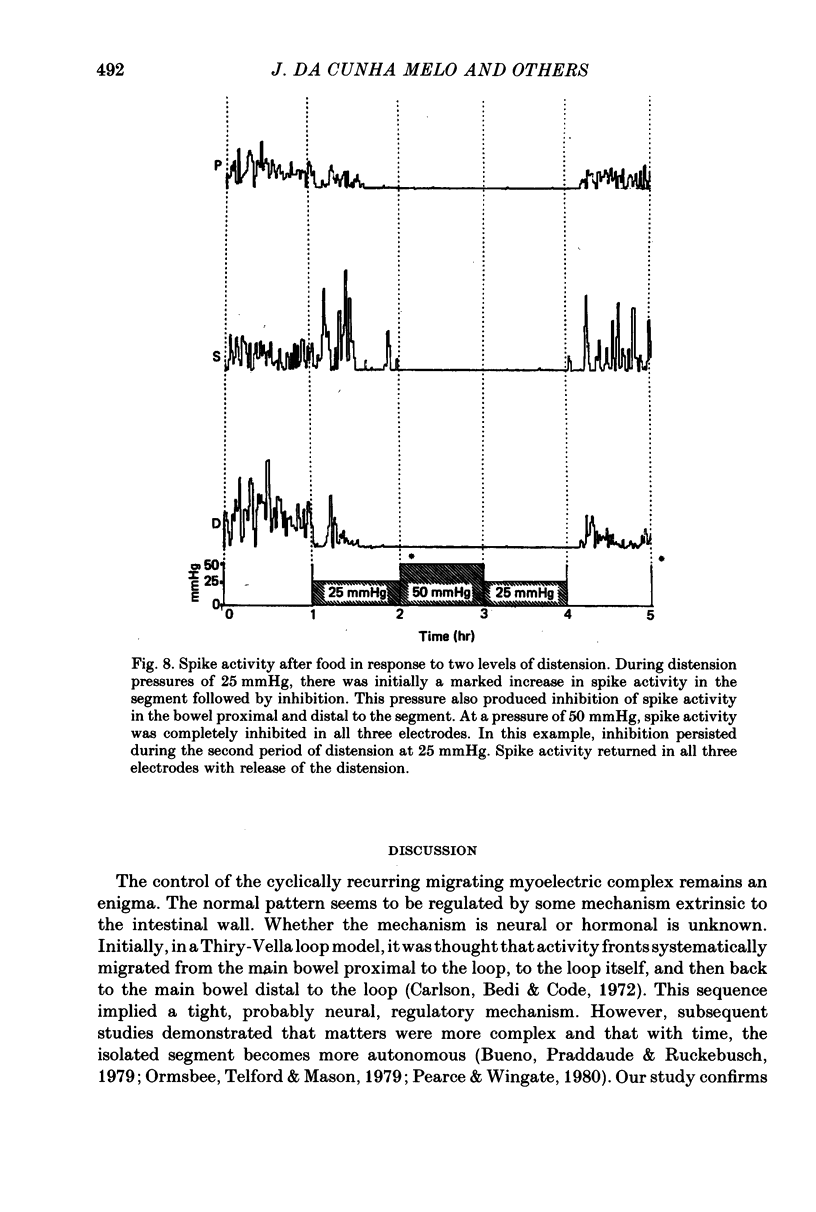
3. In fasted animals, low levels of distension (15 mmHg) interrupted the migrating complexes in the segment and induced intermittent spike activity which was similar to that seen with the secretagogues. The migrating complexes in the main bowel continued during distension. In fed animals, spike activity increased in the segment during distension at 25 mmHg and decreased in the main bowel. In both groups, distension of the segment to pressures between 37.5 and 50 mmHg abolished spike activity both in the distended segment and the main bowel in fasted and fed states, and, in fasted dogs, migrating complexes were also abolished.
4. These results demonstrate that the inhibitory intestino-intestinal reflex is mediated through extrinsic nerves and does not require an intact myenteric plexus, whereas the altered myoelectric activity induced by secretagogues is a local effect and does not spread to adjacent bowel through either intrinsic or extrinsic neural pathways. It seems likely that the local motor effect of secretagogues is a result of net secretion, producing distension to pressures below the threshold required to activate the intestino-intestinal reflex.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ammon H. V., Thomas P. J., Phillips S. F. Effects of oleic and ricinoleic acids on net jejunal water and electrolyte movement. Perfusion studies in man. J Clin Invest. 1974 Feb;53(2):374–379. doi: 10.1172/JCI107569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atchison W. D., Stewart J. J., Bass P. A unique distribution of laxative-induced spike potentials from the small intestine of the dog. Am J Dig Dis. 1978 Jun;23(6):513–520. doi: 10.1007/BF01072695. [DOI] [PubMed] [Google Scholar]
- Bueno L., Praddaude F., Ruckebusch Y. Propagation of electrical spiking activity along the small intestine: intrinsic versus extrinsic neural influences. J Physiol. 1979 Jul;292:15–26. doi: 10.1113/jphysiol.1979.sp012835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson G. M., Bedi B. S., Code C. F. Mechanism of propagation of intestinal interdigestive myoelectric complex. Am J Physiol. 1972 Apr;222(4):1027–1030. doi: 10.1152/ajplegacy.1972.222.4.1027. [DOI] [PubMed] [Google Scholar]
- Christensen J., Freeman B. W. Circular muscle electromyogram in the cat colon: local effect of sodium ricinoleate. Gastroenterology. 1972 Dec;63(6):1011–1015. [PubMed] [Google Scholar]
- Cline W. S., Lorenzsonn V., Benz L., Bass P., Olsen W. A. The effects of sodium ricinoleate on small intestinal function and structure. J Clin Invest. 1976 Aug;58(2):380–390. doi: 10.1172/JCI108482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eeckhout C., De Wever I., Vantrappen G., Janssens J. Local disorganization of interdigestive migrating complex by perfusion of a Thiry-Vella loop. Am J Physiol. 1980 Jun;238(6):G509–G513. doi: 10.1152/ajpgi.1980.238.6.G509. [DOI] [PubMed] [Google Scholar]
- Mathias J. R., Martin J. L., Burns T. W., Carlson G. M., Shields R. P. Ricinoleic acid effect on the electrical activity of the small intestine in rabbits. J Clin Invest. 1978 Mar;61(3):640–644. doi: 10.1172/JCI108975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow M. P., Simmonds W. J. The effect of bile salts on contractility and calcium depletion of polarized and depolarized smooth muscle. J Pharmacol Exp Ther. 1965 Nov;150(2):208–215. [PubMed] [Google Scholar]
- Stewart J. J., Bass P. Effect of intravenous C-terminal octapeptide of cholecystokinin and intraduodenal ricinoleic acid on contractile activity of the dog intestine. Proc Soc Exp Biol Med. 1976 Jun;152(2):213–217. doi: 10.3181/00379727-152-39363. [DOI] [PubMed] [Google Scholar]
- Stewart J. J., Bass P. Effects of ricinoleic and oleic acids on the digestive contractile activity of the canine small and large bowel. Gastroenterology. 1976 Mar;70(3):371–376. [PubMed] [Google Scholar]
- Stewart J. J., Gaginella T. S., Olsen W. A., Bass P. Inhibitory actions of laxatives on motility and water and electrolyte transport in the gastrointestinal tract. J Pharmacol Exp Ther. 1975 Feb;192(2):458–467. [PubMed] [Google Scholar]
- Teem M. V., Phillips S. F. Perfusion of the hamster jejunum with conjugated and unconjugated bile acids: inhibition of water absorption and effects on morphology. Gastroenterology. 1972 Feb;62(2):261–267. [PubMed] [Google Scholar]
- Wingate D. L., Phillips S. F., Hofmann A. F. Effect of glycine-conjugated bile acids with and without lecithin on water and glucose absorption in perfused human jejunum. J Clin Invest. 1973 May;52(5):1230–1236. doi: 10.1172/JCI107290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingate D., Barnett T., Green R., Armstrong-James M. Automated high-speed analysis of gastrointestinal myoelectric activity. Am J Dig Dis. 1977 Mar;22(3):243–251. doi: 10.1007/BF01072284. [DOI] [PubMed] [Google Scholar]
- Wingate D., Green R., Symes J., Pilot M. Interpretation of fluctuation of transmural potential difference across the proximal small intestine. Gut. 1974 Jul;15(7):515–520. doi: 10.1136/gut.15.7.515. [DOI] [PMC free article] [PubMed] [Google Scholar]


