Abstract
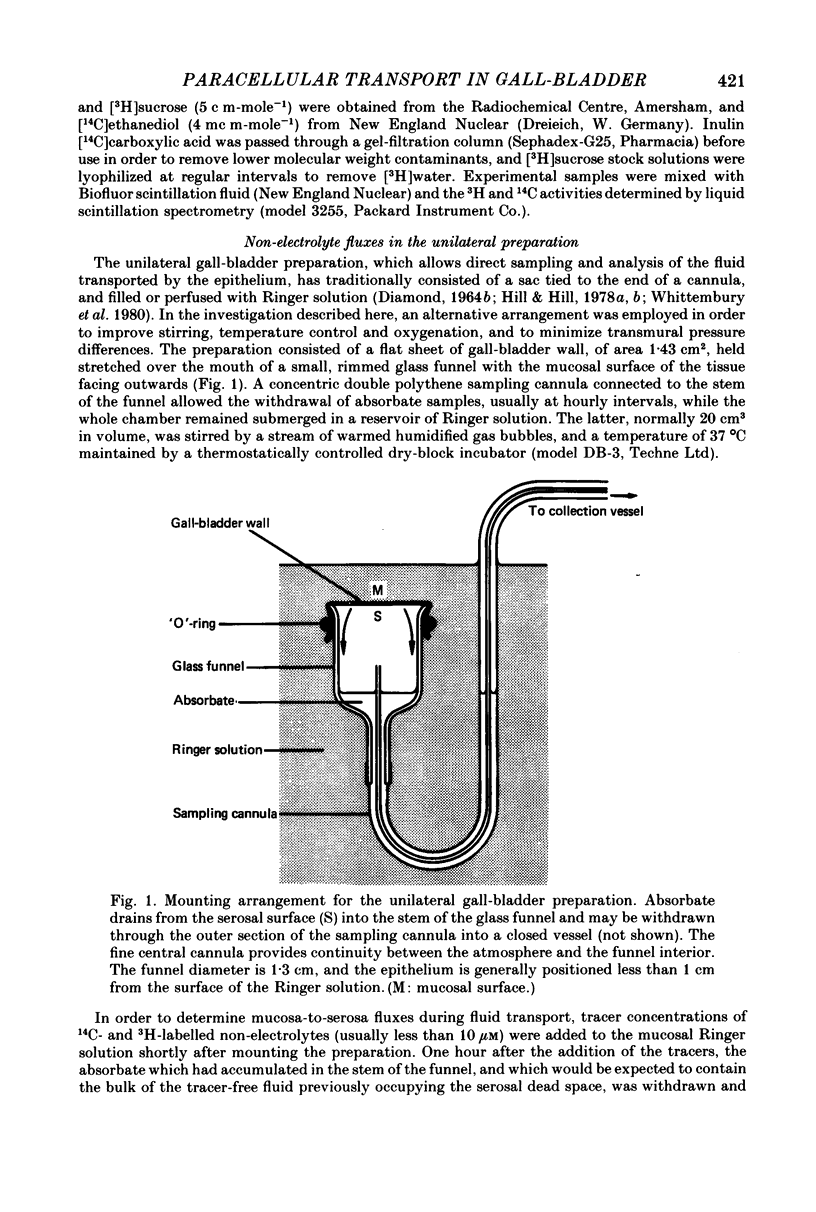
1. Mucosa-to-serosa fluxes of seven polar non-electrolytes were determined during isotonic fluid transport across the unilateral rabbit gall-bladder preparation in an attempt to estimate the contribution of the paracellular pathway to the total transepithelial water flow.
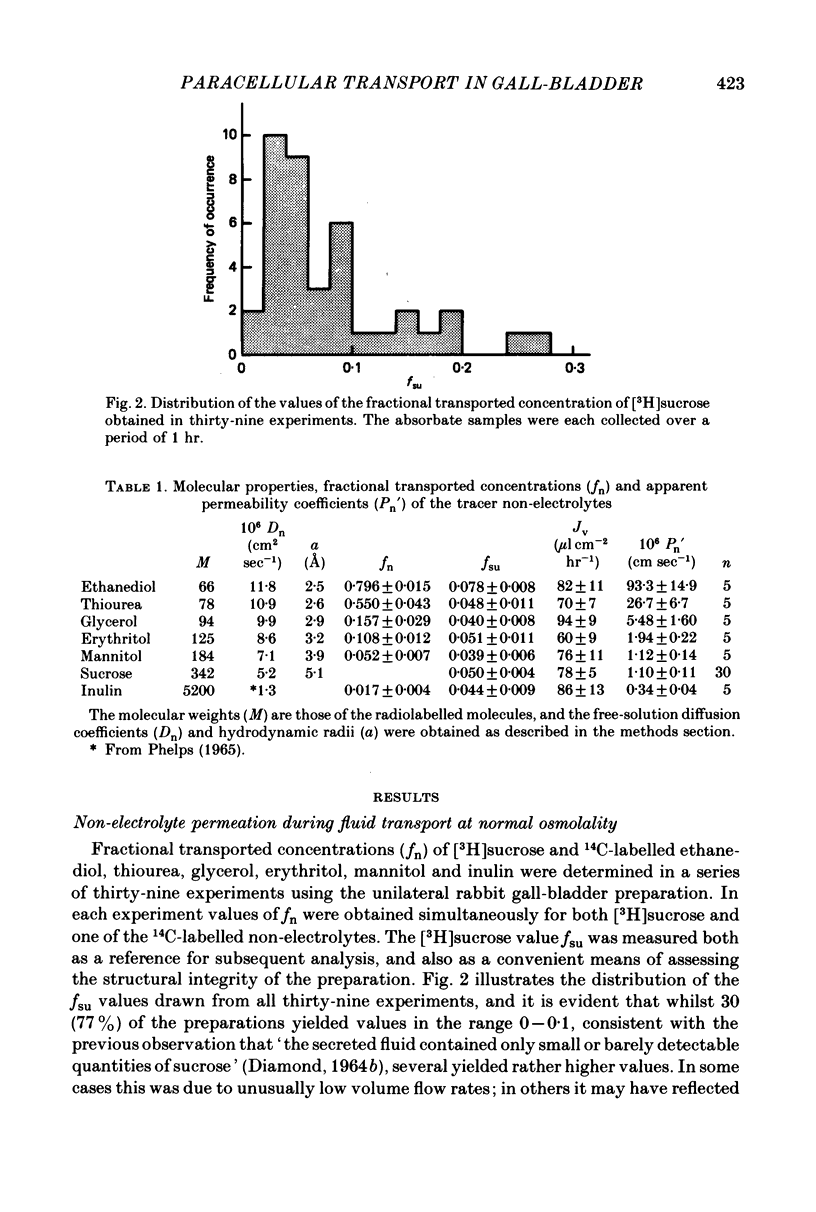
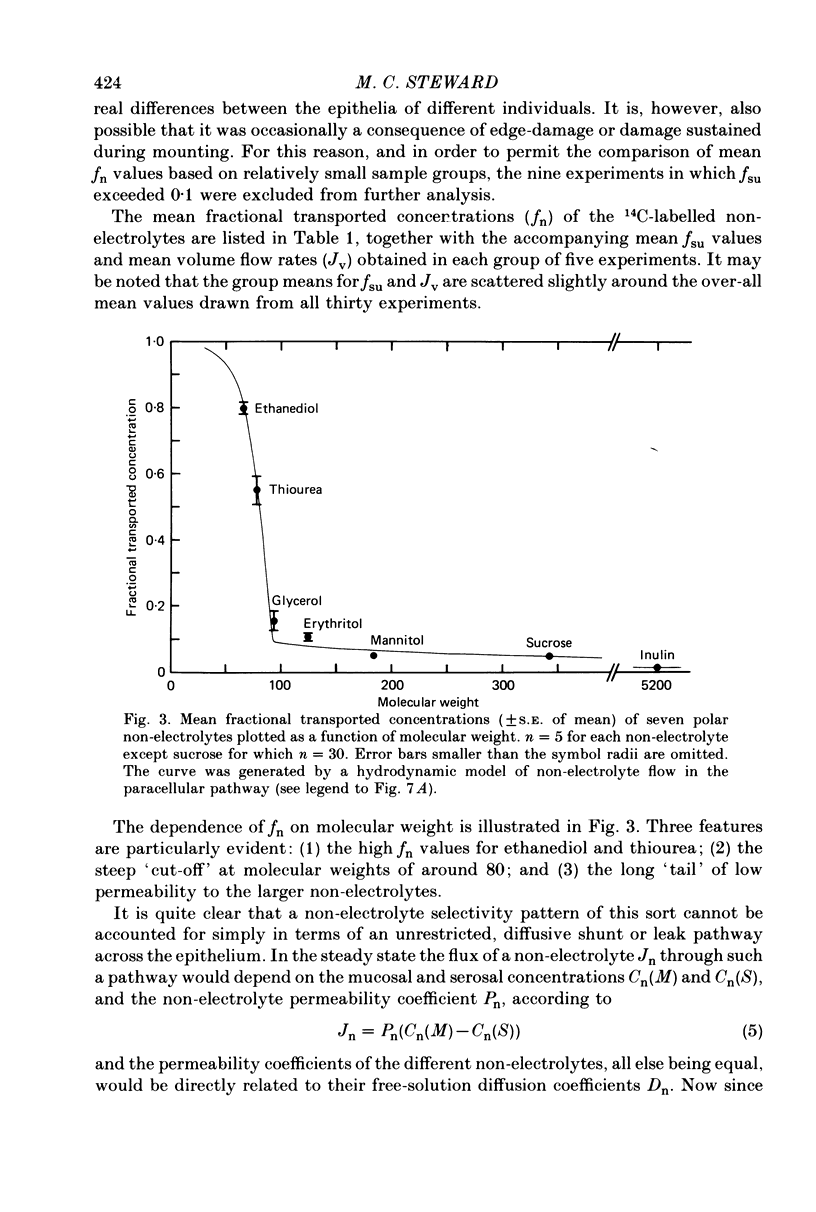
2. 3H- and 14C-labelled non-electrolyte tracers appeared in the transported fluid at fractions (fn) of their mucosal concentration which were inversely related to molecular size: ethanediol, 0·80; thiourea, 0·55; glycerol, 0·16; erythritol, 0·11; mannitol, 0·05; sucrose, 0·05; inulin, 0·02. The mean volume flow rate was 78 μl. cm-2 hr-1.
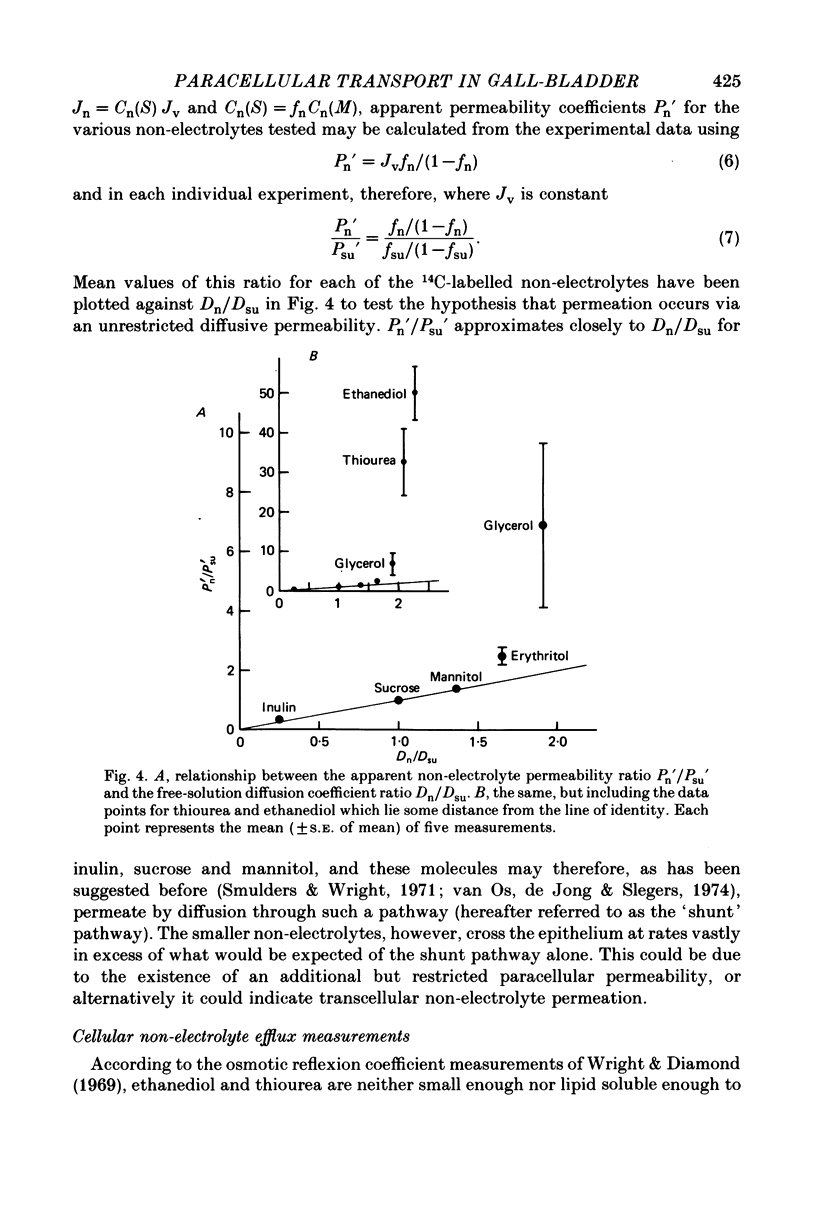
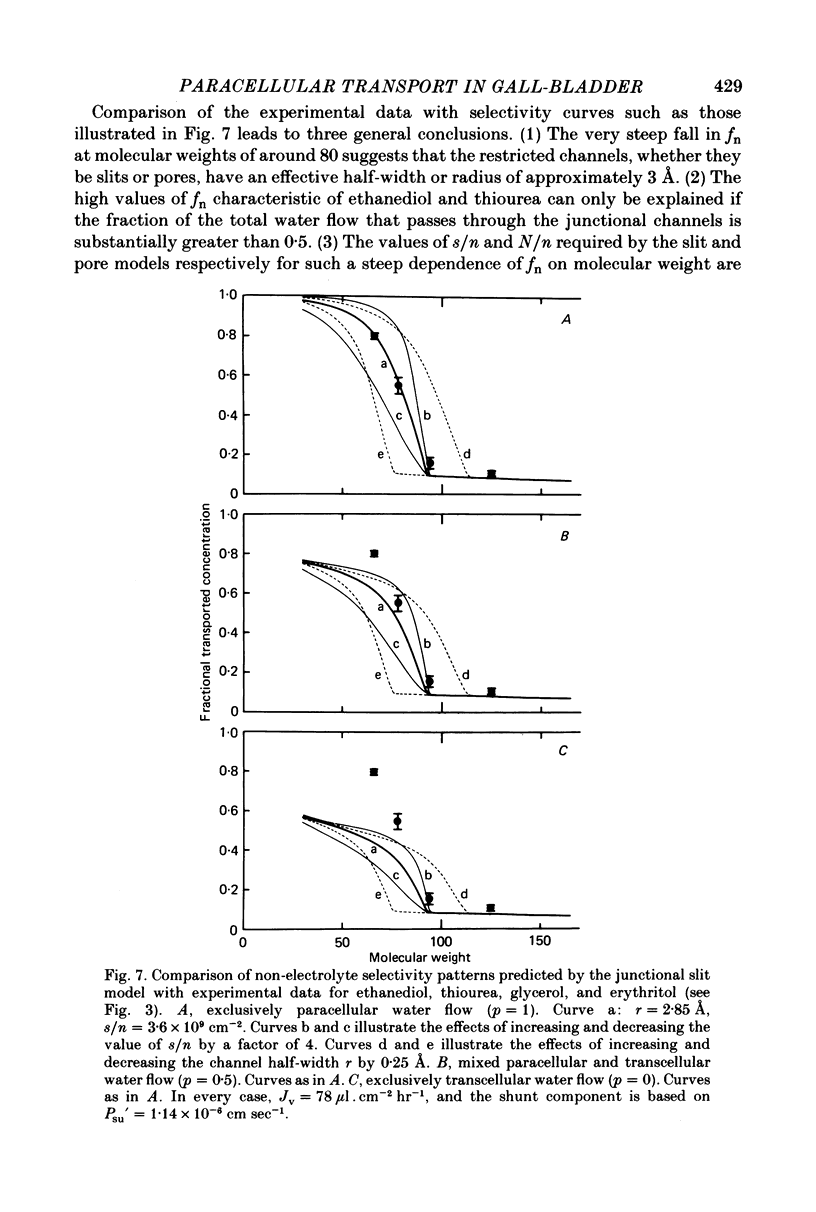
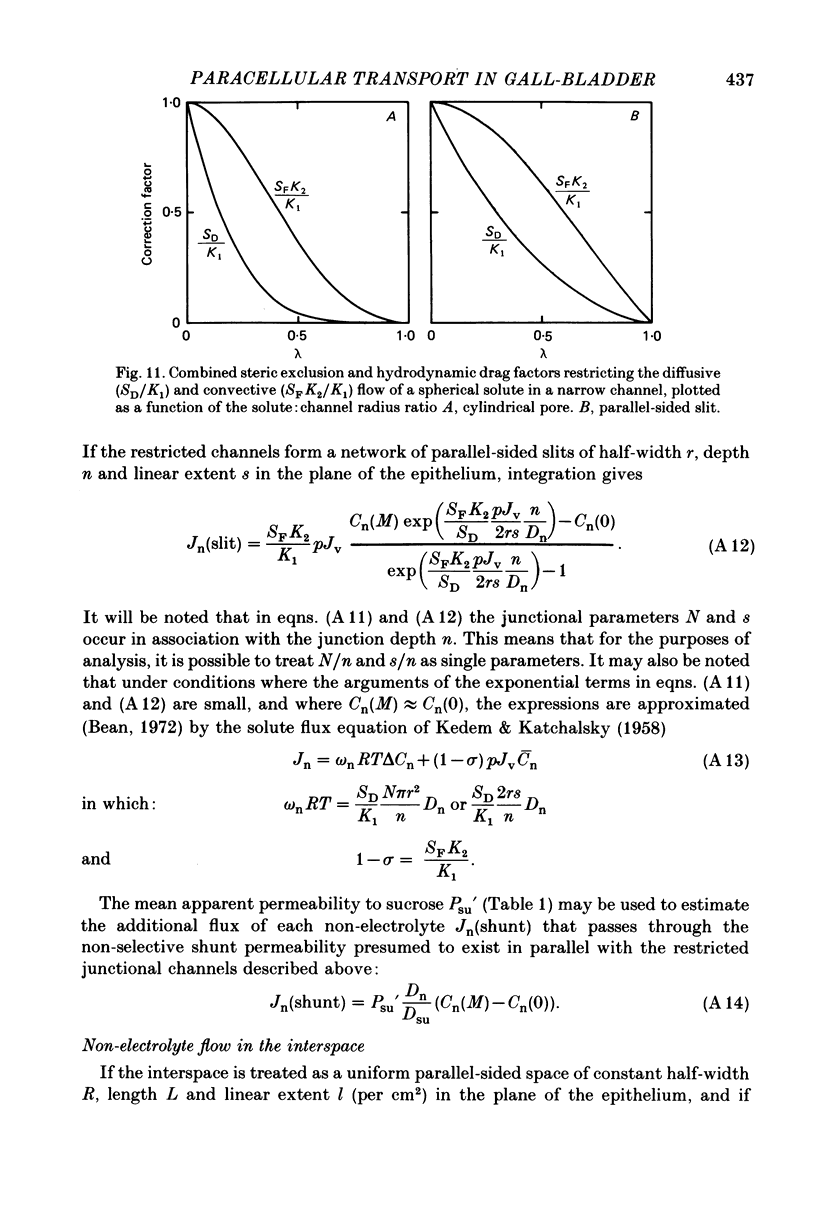
3. While the fluxes of the larger molecules were probably due to diffusion through a small but unrestricted paracellular `shunt' permeability, the high fn values obtained for the smaller molecules indicate the existence of a substantial paracellular permeability restricted to molecules smaller than erythritol.
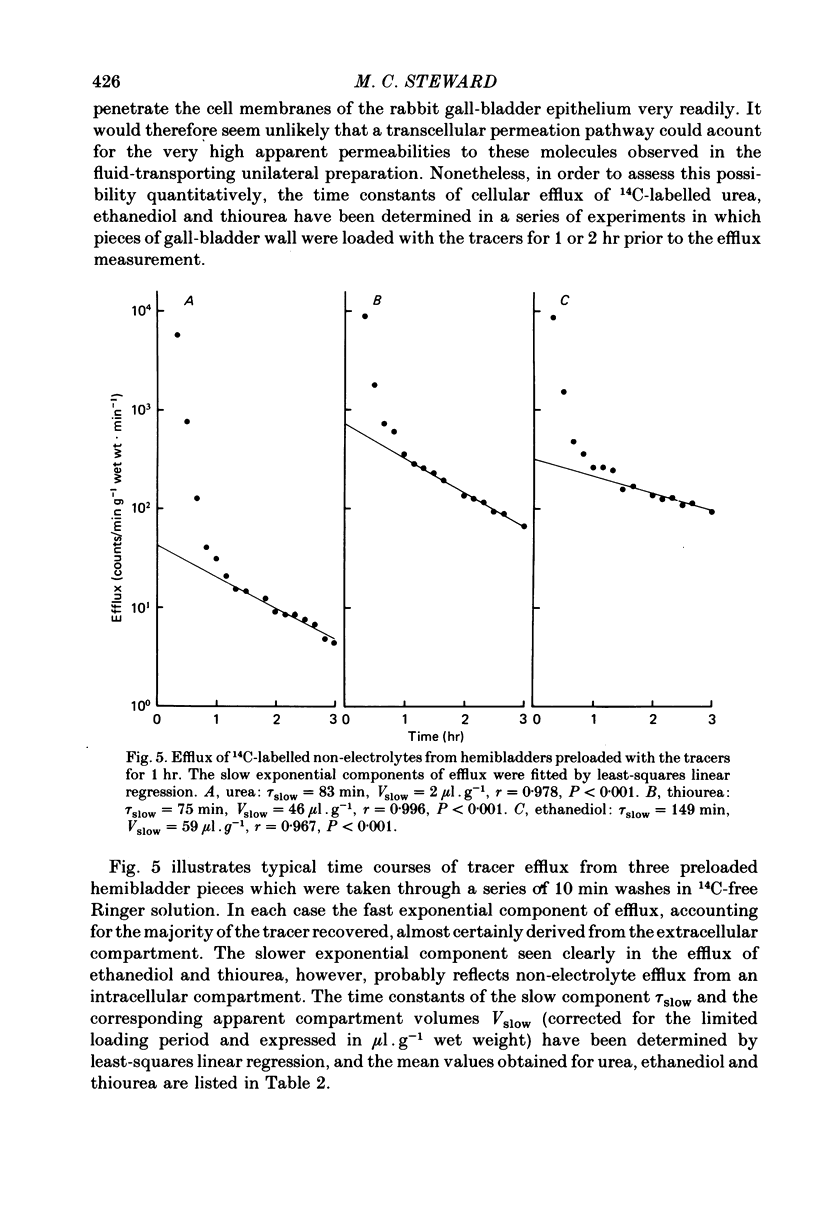
4. Upper limits to the transcellular ethanediol and thiourea permeabilities, estimated from the time constants of tracer efflux from preloaded epithelial cells, were too low to account for more than a very small fraction of the transepithelial fluxes observed in the unilateral preparation.
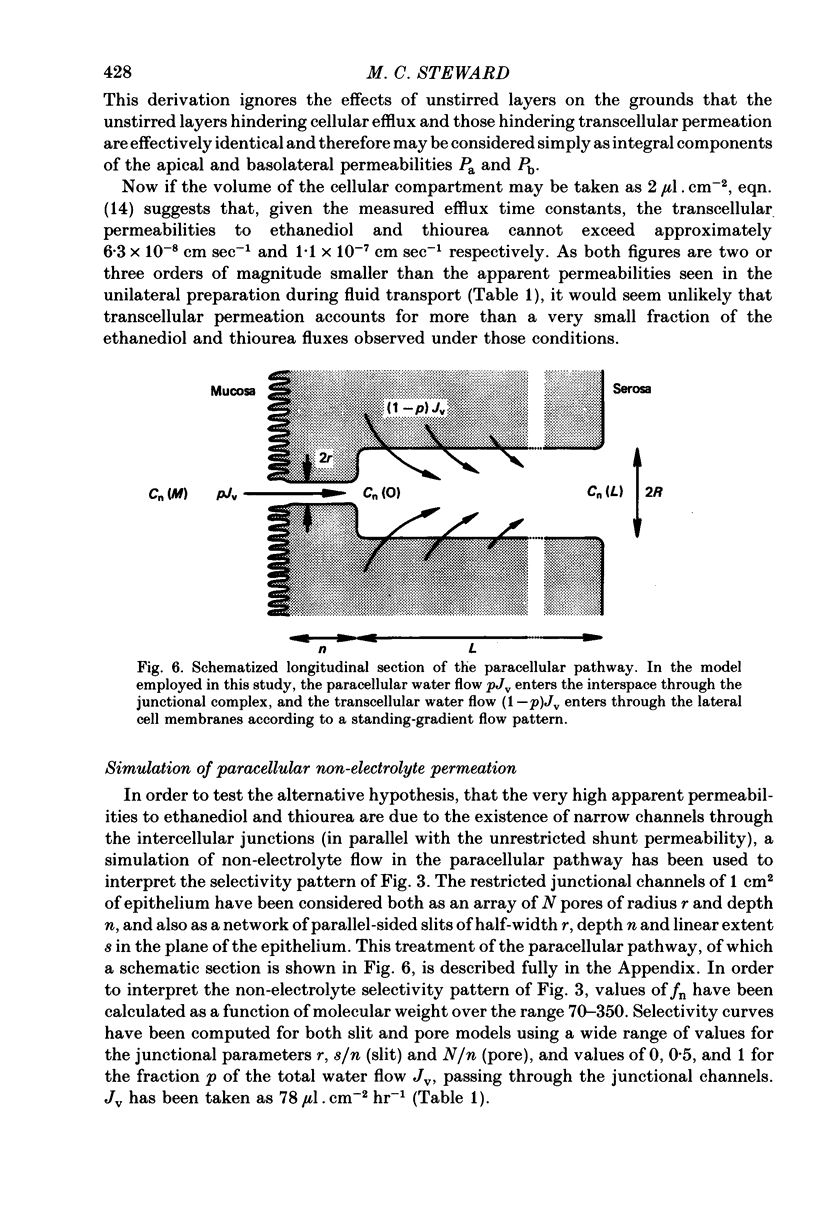
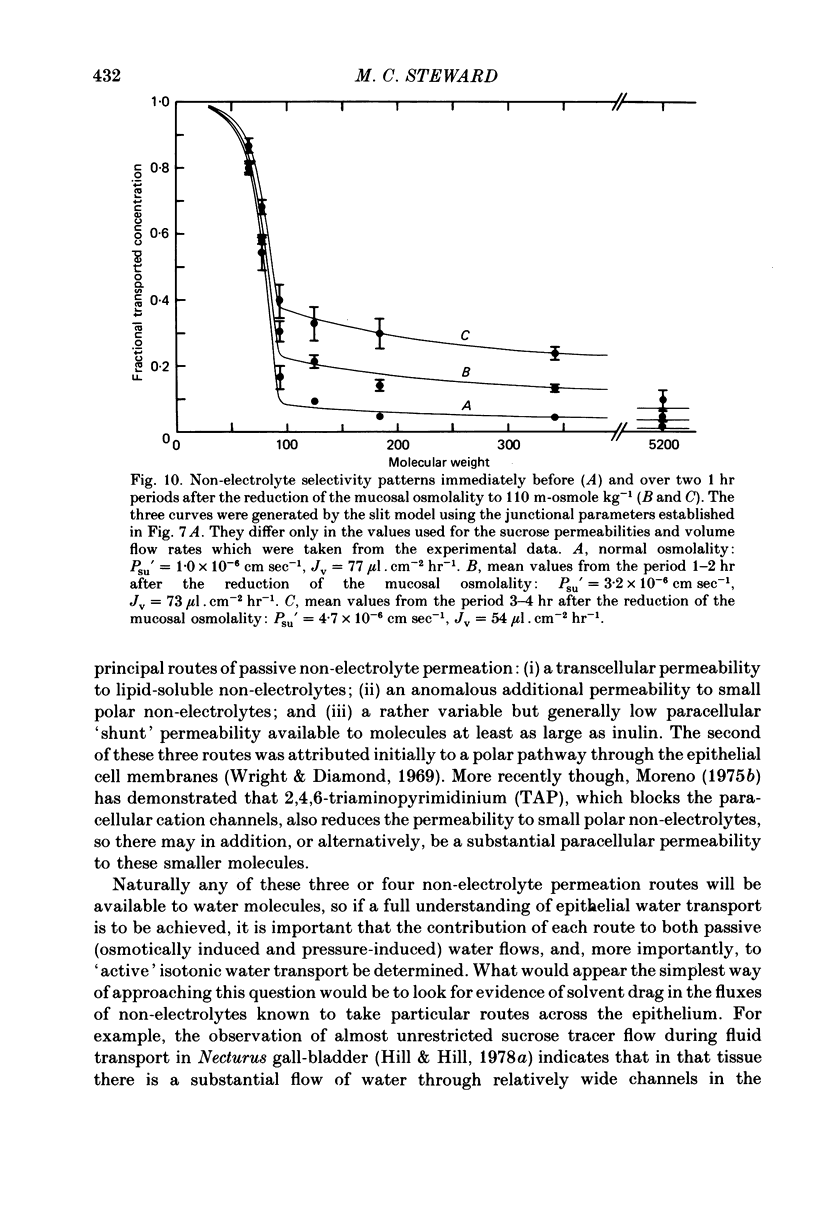
5. Comparison of the fn values with the predictions of a hydrodynamic model of paracellular permeation suggests that in order to account for the large fluxes of ethanediol and thiourea, considerably more than one half of the transepithelial water flow must follow the paracellular pathway.
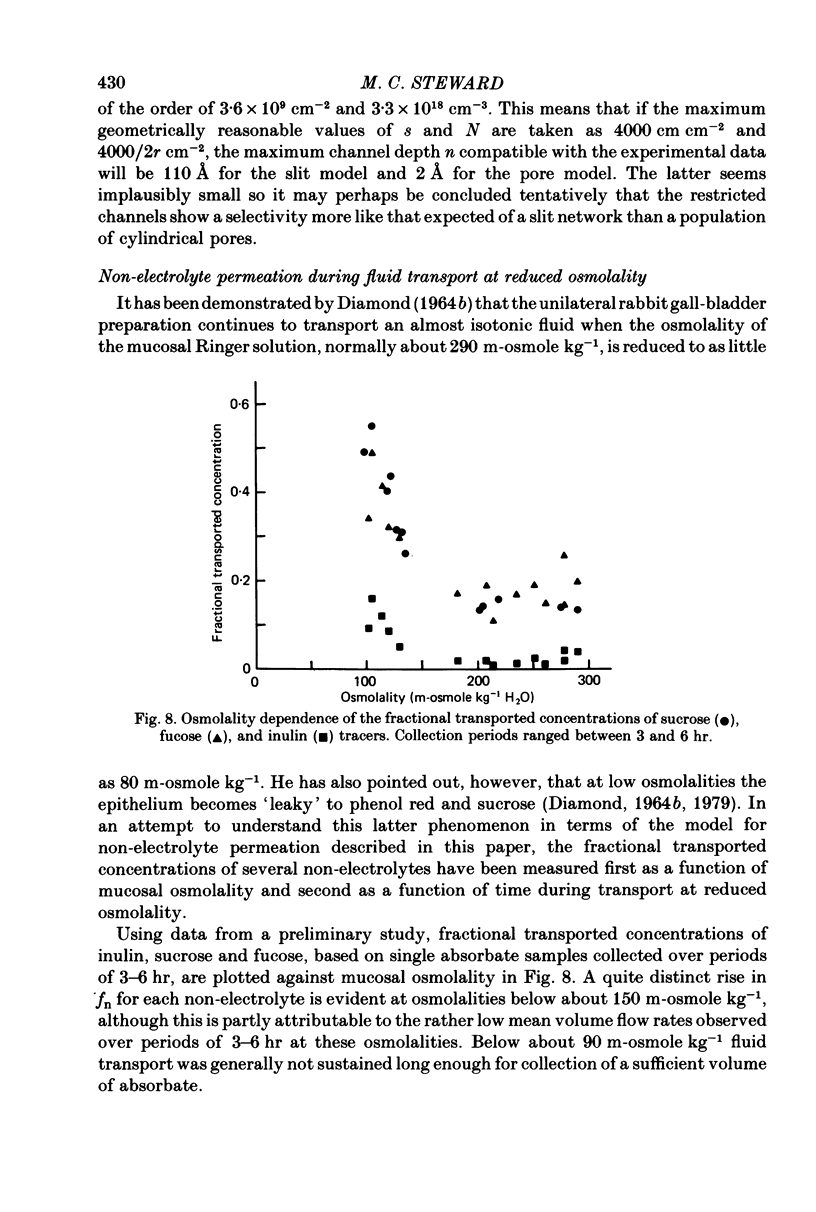
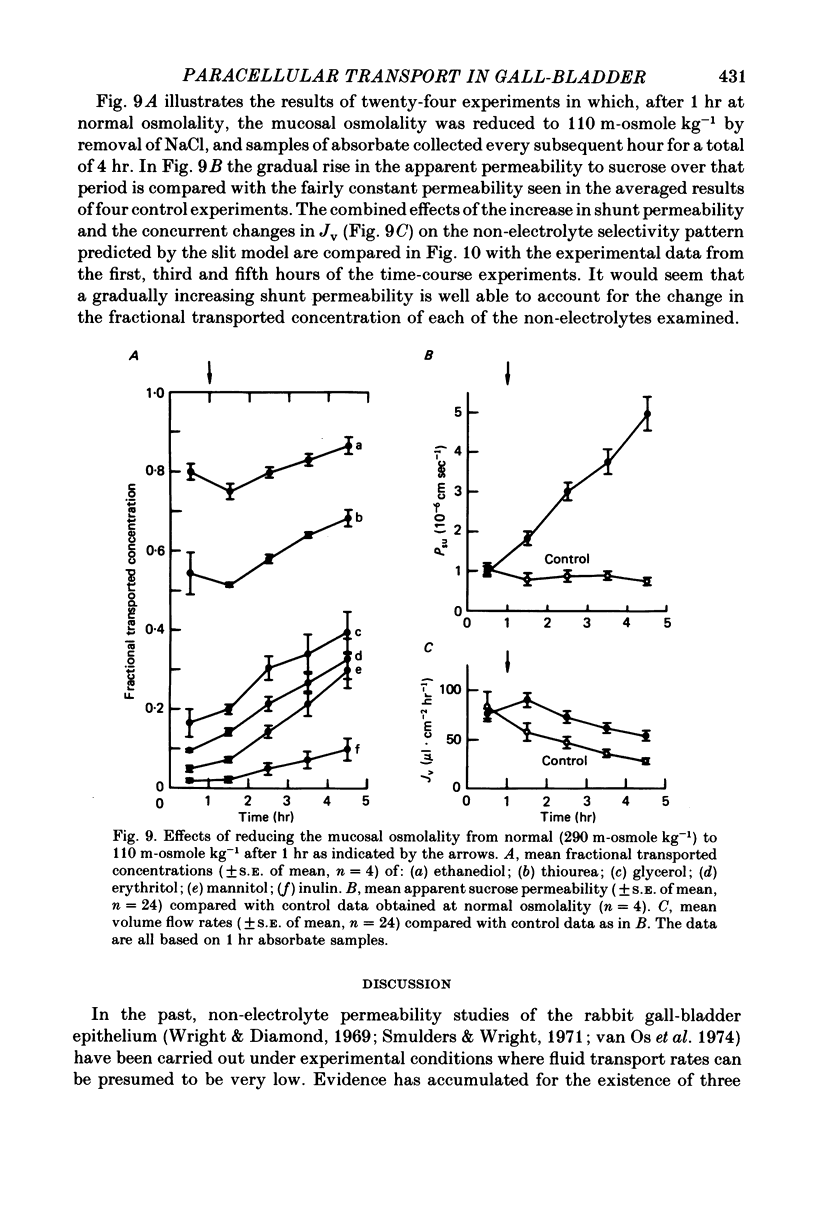
6. Following a reduction of the mucosal osmolality to 110 m-osmole kg-1, the apparent non-electrolyte permeability of the epithelium increased steadily over a period of 4 hr. This seems to reflect an increase in the shunt permeability rather than a change in the selectivity of the restricted permeability.
7. It is concluded that during isotonic fluid transport the bulk of the transepithelial water flow crossing the epithelium passes through paracellular channels of approximately 3 Å radius which are probably located in the intercellular junction.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry C. A., Boulpaep E. L. Nonelectrolyte permeability of the paracellular pathway in Necturus proximal tubule. Am J Physiol. 1975 Feb;228(2):581–595. doi: 10.1152/ajplegacy.1975.228.2.581. [DOI] [PubMed] [Google Scholar]
- Blom H., Helander H. F. Quantitative electron microscopical studies on in vitro incubated rabbit gallbladder epithelium. J Membr Biol. 1977 Oct 3;37(1):45–61. doi: 10.1007/BF01940923. [DOI] [PubMed] [Google Scholar]
- DIAMOND J. M. THE MECHANISM OF ISOTONIC WATER TRANSPORT. J Gen Physiol. 1964 Sep;48:15–42. doi: 10.1085/jgp.48.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIAMOND J. M. TRANSPORT OF SALT AND WATER IN RABBIT AND GUINEA PIG GALL BLADDER. J Gen Physiol. 1964 Sep;48:1–14. doi: 10.1085/jgp.48.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J. M., Bossert W. H. Standing-gradient osmotic flow. A mechanism for coupling of water and solute transport in epithelia. J Gen Physiol. 1967 Sep;50(8):2061–2083. doi: 10.1085/jgp.50.8.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J. M. Osmotic water flow in leaky epithelia. J Membr Biol. 1979 Dec 31;51(3-4):195–216. doi: 10.1007/BF01869084. [DOI] [PubMed] [Google Scholar]
- Hill A. E., Hill B. S. Sucrose fluxes and junctional water flow across Necturus gall bladder epithelium. Proc R Soc Lond B Biol Sci. 1978 Feb 23;200(1139):163–174. doi: 10.1098/rspb.1978.0013. [DOI] [PubMed] [Google Scholar]
- Hill A. Salt-water coupling in leaky epithelia. J Membr Biol. 1980 Oct 31;56(3):177–182. doi: 10.1007/BF01869474. [DOI] [PubMed] [Google Scholar]
- Hill B. S., Hill A. E. Fluid transfer by Necturus gall bladder epithelium as a function of osmolarity. Proc R Soc Lond B Biol Sci. 1978 Feb 23;200(1139):151–162. doi: 10.1098/rspb.1978.0012. [DOI] [PubMed] [Google Scholar]
- KEDEM O., KATCHALSKY A. Thermodynamic analysis of the permeability of biological membranes to non-electrolytes. Biochim Biophys Acta. 1958 Feb;27(2):229–246. doi: 10.1016/0006-3002(58)90330-5. [DOI] [PubMed] [Google Scholar]
- Moreno J. H. Blockage of gallbladder tight junction cation-selective channels by 2,4,6-triaminopyrimidinium (TAP). J Gen Physiol. 1975 Jul;66(1):97–115. doi: 10.1085/jgp.66.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno J. H. Routes of nonelectrolyte permeability in gallbladder. Effects of 2,4,6-triaminopyrimidinium (TAP). J Gen Physiol. 1975 Jul;66(1):117–128. doi: 10.1085/jgp.66.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHELPS C. F. THE PHYSICAL PROPERTIES OF INULIN SOLUTIONS. Biochem J. 1965 Apr;95:41–47. doi: 10.1042/bj0950041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHULTZ S. G., SOLOMON A. K. Determination of the effective hydrodynamic radii of small molecules by viscometry. J Gen Physiol. 1961 Jul;44:1189–1199. doi: 10.1085/jgp.44.6.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tormey J. M., Diamond J. M. The ultrastructural route of fluid transport in rabbit gall bladder. J Gen Physiol. 1967 Sep;50(8):2031–2060. doi: 10.1085/jgp.50.8.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittembury G., de Martínez C. V., Linares H., Paz-Aliaga A. Solvent drag of large solutes indicates paracellular water flow in leaky epithelia. Proc R Soc Lond B Biol Sci. 1980 Dec 31;211(1182):63–81. doi: 10.1098/rspb.1980.0158. [DOI] [PubMed] [Google Scholar]
- Wright E. M., Diamond J. M. Patterns of non-electrolyte permeability. Proc R Soc Lond B Biol Sci. 1969 Mar 18;171(1028):227–271. doi: 10.1098/rspb.1969.0021. [DOI] [PubMed] [Google Scholar]
- van Os C. H., de Jong M. D., Slegers J. F. Dimensions of polar pathways through rabbit gallbladder epithelium. The effect of phloretin on nonelectrolyte permeability. J Membr Biol. 1974;15(4):363–382. doi: 10.1007/BF01870095. [DOI] [PubMed] [Google Scholar]


