Abstract
Dendritic cells (DCs) play a critical role in initiating antigen-specific immune responses, because they are able to capture exogenous antigens for presentation to naïve T cells on both MHC class I and II molecules. As such, DCs represent important elements in the development of vaccine therapy for cancer. Although DCs are known to present antigens from phagocytosed tumor cells or preprocessed peptides, we explored whether they might also present soluble recombinant NY-ESO-1, a well characterized cancer antigen. We compared the abilities of human monocyte-derived DCs and DCs derived in vitro from CD34-positive stem cells to present NY-ESO-1 epitopes to MHC class I-restricted cytotoxic T cells. Although monocyte-derived DCs did not efficiently crosspresent free NY-ESO-1 protein, IgG-immune complexes containing NY-ESO-1 were avidly presented after uptake by Fcγ receptors (FcγRII). In contrast, CD34-derived DCs were unable to process either soluble or immune complexed NY-ESO-1, although they efficiently presented preprocessed NY-ESO-1 peptides. This difference did not necessarily correlate with endocytic capacity. Although monocyte-derived DCs exhibited greater fluid-phase uptake than CD34-derived DCs, the two populations did not differ with respect to their surprisingly limited capacity for Fcγ receptor-mediated endocytosis. These results indicate that monocyte-derived DCs will be easier to load by using protein antigen in vitro than CD34-derived DCs, and that the latter population exhibits a restricted ability to crosspresent soluble exogenous antigens.
Dendritic cells (DCs) play a crucial role in the initiation of antigen-specific immune responses, exhibiting a variety of specializations that contribute to their efficiency as antigen-presenting cells (1, 2). One such specialization is the capacity to convert antigens captured by endocytosis into immunogenic peptides bound to MHC class I molecules. Thus, DCs are able to elicit CD8+ T cell responses to exogenous antigens, a pathway referred to as “crosspresentation” (3–6), in contrast to the classical pathway of MHC class I-restricted antigen presentation, in which peptides are derived from endogenously synthesized antigens.
The unique features of antigen presentation by DCs have generated considerable interest in their use as therapeutic vehicles, especially for vaccination. In recent years, a number of groups have successfully loaded both human and mouse DCs with a wide array of experimental antigens. The antigens have been delivered by using a variety of strategies, including synthetic peptides, viral vectors, apoptotic or necrotic cells, and RNA (3, 7). However, no consensus has yet emerged as to the most effective mode of antigen delivery or the most appropriate DC population to use for immunization. To a great extent, the lack of consensus reflects the small number of DC-antigen combinations that have thus far been examined in humans.
The development and evaluation of DC-based cancer vaccines are of particular interest for two reasons. First, they may provide the means to stimulate the immune system's natural response to the increasing number of tumor-specific antigens that are now becoming identified (8). Second, the analysis itself will afford a unique opportunity to evaluate and quantify fundamental features of the human immune response.
Of the tumor antigens characterized thus far, the cancer-testis antigen NY-ESO-1 is among the most promising (9). An abundant cytosolic protein of as-yet-unknown function, NY-ESO-1 elicits humoral and cellular immune responses in many cancer patients (10, 11). In vitro, human monocyte-derived antigen-presenting cells loaded with an immunogenic NY-ESO-1 peptide can stimulate antigen-specific CD8+ T cells (12). In addition, human monocyte-derived DCs (mo-DCs) can also process and crosspresent NY-ESO-1 after phagocytosis of NY-ESO-1+ myeloma cells (13). It is interesting that presentation was greatly facilitated after phagocytosis of opsonized myeloma cells via mo-DC Fcγ receptors (FcγR).
Because recombinant NY-ESO-1 protein can be generated, it would in principle provide a useful and convenient source of immunogen. However, the endocytosis of soluble proteins by DCs is most often associated with presentation on MHC class II molecules. By using a mouse DC cell line, Rodriguez et al. have recently found that soluble antigen (ovalbumin) can in fact be crosspresented on MHC class I, especially when internalized via FcγRs (14, 15). Here, we examine whether a similar approach can be used for the crosspresentation of NY-ESO-1 by human DCs, and we also compare the crosspresenting capacity of two well characterized human DC populations, mo-DCs and DCs differentiated from CD34+ bone marrow-derived stem cells.
Materials and Methods
Generation of DCs and Macrophages.
To generate mo-DCs, peripheral blood mononuclear cells (PBMC) were isolated from buffy-coated blood of healthy individuals, as described (16). CD14+ monocytes were enriched by negative selection by using magnetic beads (Dynal, Oslo) and then incubated in RPMI medium with 10% fetal calf serum, 1,000 units/ml of granulocyte/macrophage colony-stimulating factor (GM-CSF) (BD Pharmingen, San Diego) and 1,000 units/ml of IL-4 (BD Pharmingen). On day 5 or 6, immature mo-DCs were harvested, characterized by FACS, and used for antigen-presentation assays. DCs derived from CD34+ stem cells were produced exactly as described (17). Briefly, CD34+ cells were isolated by leukapheresis and positive selection on magnetic beads (Miltenyi Biotec, Auburn, CA) from blood collected from granulocyte-CSF-mobilized donors. Purified cells were cryoprotected and stored in liquid nitrogen. Thawed cells were grown for 6–7 days in defined serum-free medium to yield purified populations of CD1+ Langerin-positive DCs. Macrophages were produced by culturing PBMCs for 5 days without added GM-CSF or IL-4. The resulting cells were judged to be strongly positive for CD14 (17).
CD8+ T Cell Lines.
HLA-A2- and Cw3-restricted, NY-ESO-1-specific CD8+ T cell lines were generated from melanoma patient NW29 (18). Peripheral blood leukocytes were stimulated by antigen-presenting cells infected with a recombinant adenovirus expressing NY-ESO-1, as described (18). The HLA-A2-restricted NY-ESO-1-specific CD8+ T cell clone, clone 5, was generated by limiting dilution from tumor infiltrating lymphocytes of a melanoma patient (19).
Recombinant Proteins.
NY-ESO-1 and SSX2 proteins were expressed in Escherichia coli as full-length proteins with a six-histidine tag at the amino terminus (10). The proteins were purified from washed and solubilized inclusion bodies by nickel chelate affinity chromatography (Chelating Sepharose FF; Amersham Pharmacia Biotech) by using a pH gradient. NY-ESO-1 and SSX2 proteins were eluted in 8 M urea, 100 mM phosphate, and 10 mM Tris at pH 4.5. The purified proteins were reactive with anti-NY-ESO-1 and anti-SSX2 monoclonal antibodies by Western blot analysis; purity was >80% by SDS/PAGE.
Antibodies.
Monoclonal antibodies against NY-ESO-1, ES121 IgG, and E978 IgG were prepared from mice immunized with recombinant NY-ESO-1 protein (10). A Fab fragment was prepared from E978 IgG by papain cleavage. A polyclonal antibody against NY-ESO-1 was also affinity-purified from rabbit antisera. Purified or FITC-labeled anti-CD32 antibodies, anti-CD16-PE, anti-HLA-DR-cychrome, and purified anti-CD107a (LAMP-1) antibodies were purchased from BD Pharmingen. Anti-CD83-PE antibody was from Immunotech (Marseille, France), and polyclonal rabbit anti-biotin antibody was from Rockland (Gilbertsville, PA).
Peptides.
HLA-A2-restricted NY-ESO-1 peptide, p157–165 (SLLMWITQC) and HLA-Cw3, p92–100 (LAMPFATPM) were selected to analyze the CD8+ T cell responses to NY-ESO-1 (11, 18). All peptides were synthesized by Multiple Peptide Systems (San Diego), with a purity of >86% as determined by reverse-phase HPLC.
Antigen Loading and Endocytosis.
NY-ESO-1 protein and either monoclonal antibody ES121 or E978 were mixed at 4:1 molar ratio in serum-free RPMI medium 1640 and incubated at 37°C for 30 min. Antibody concentrations used ranged from 1 to 30 μg/ml. In some experiments, complexes were formed by using various dilutions of a human immune antiserum to NY-ESO-1. For endocytosis experiments, the immune complexes were also formed by using recombinant NY-ESO-1 together with an affinity-purified rabbit anti-NY-ESO-1 antibody. For antigen-presentation experiments, DCs (mo-DCs and cd34-DCs, on day 5–6 of culture) were incubated with free peptide, NY-ESO-1 protein, NY-ESO-1 immune complexes, or antibody alone for 12 hr prior to T cell assay. For endocytosis experiments, DCs were incubated with immune complexes, free NY-ESO-1 protein, or FITC–dextran (0.5 mg/ml) for various periods of time at 0 or 37°C. Internalized immune complexes were visualized by using an anti-rabbit-Alexa488 antibody (Molecular Probes).
ELISPOT Assay.
ELISPOT assays were performed as previously described (20). Briefly, 1 × 103 CD8+ T cells and 5 × 104 DCs were incubated for 20 hr in RPMI medium 1640 lacking IL-2 and serum. After this incubation, the plates were developed by using an enzyme-linked colorimetric assay. After washing, dark-violet spots were quantified by counting under a microscope. Cell-free antigen controls were routinely included in every experiment to define background. Effector-to-target cell ratios were optimized and fixed at 1:50 for all experiments shown.
Results
Phenotype of Human mo-Derived DCs.
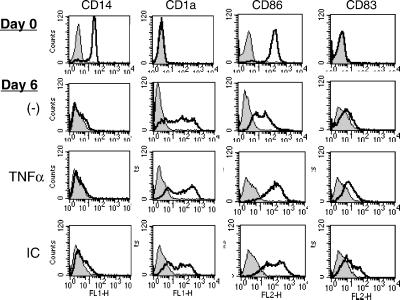
To investigate the ability of mo-DCs to crosspresent NY-ESO-1, mo-DCs were prepared from healthy donors and characterized by flow cytometry. As shown in Fig. 1, the starting monocyte population (day 0) exhibited high surface expression of CD14 and CD86 (B7.2), with low expression of CD1a and CD83, as expected (21, 22). After 6 days of culture in GM-CSF and IL-4, CD14 expression had completely disappeared, whereas expression of the DC marker CD1a had increased. CD86 had decreased significantly, and CD83 remained nearly negative, consistent with an immature DC phenotype. Furthermore, these immature mo-DCs exhibited a marked capacity for endocytosis of FITC–dextran (see below). After exposure for 1 day to the proinflammatory cytokine tumor necrosis factor α (TNFα), the cells adopted a more mature phenotype, increasing the expression of both CD86 and CD83; MHC class II levels also increased (not shown). TNFα treatment also markedly reduced the cells' capacity for FITC–dextran endocytosis (see below). Interestingly, exposure of the immature mo-DCs to NY-ESO-1-containing immune complexes (ICs) also induced a partially mature phenotype (Fig. 1), although it was not clear whether this effect was due to FcR binding or to trace amounts of lipopolysaccharide possibly contaminating the NY-ESO-1 recombinant protein. At these concentrations, free NY-ESO-1 protein did not induce maturation, but as an IC, it (together with any contaminant) would be concentrated at the cell surface >1,000-fold by binding to FcγR. In any event, immature mo-DCs (day 6) were used for all subsequent experiments.
Fig 1.
Phenotype of monocyte-derived DCs. Monocytes isolated by magnetic bead selection from PBMCs were harvested immediately (day 0) cultured with GM-CSF and IL-4 for 5 days. At this time, TNFα (10 ng/ml) or NY-ESO-1-containing immune complexes (1 μg/ml as protein) were added and the cells harvested 24 hr later. The cells were then analyzed by flow cytometry by using the indicated antibodies.
Antigen Presentation by mo-Derived DCs.
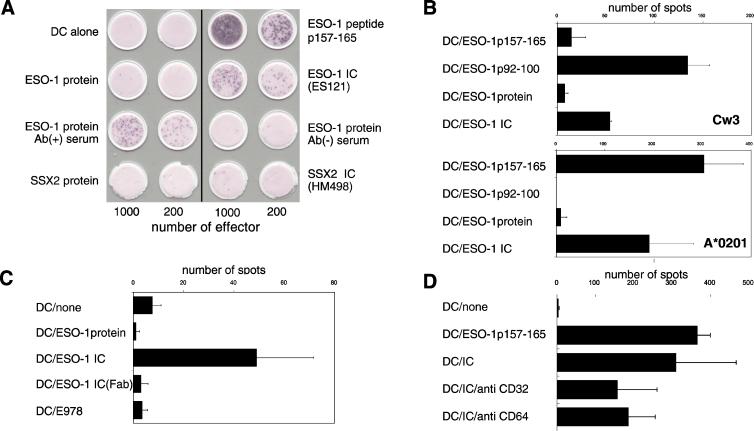
To determine whether mo-DCs could generate peptide–MHC class I complexes, cultures exposed to either free NY-ESO-1 protein or the NY-ESO-1 ICs were assayed by ELISPOT by using antigen-specific CD8+ T cell lines. The T cells were isolated from an immune melanoma patient, and cell lines recognizing NY-ESO-1 peptides restricted by the HLA-A2 or HLA-Cw3 haplotypes were selected in vitro (18). As shown in Fig. 2A, significant responses were observed by using mo-DCs (HLA-A*0201) exposed to NY-ESO-1-containing ICs formed by using an immune patient antiserum or the anti-NY-ESO-1 monoclonal antibody ES121 IgG. Although the degree of response was less than that seen with the immunogenic NY-ESO-1 HLA-A2 peptide (p157–165; Fig. 2A Top Right), it was nearly 200-fold greater than that observed by using mo-DCs alone or DCs exposed to free NY-ESO-1 protein, NY-ESO-1 incubated together with control antiserum, or with an irrelevant recombinant protein (SSX2, either free or as an IC together with the anti-SSX2 monoclonal antibody HM498 IgG). Only NY-ESO-1 incubated with sera from patients having anti-NY-ESO-1 reactivity elicited ELISPOT signals.
Fig 2.
Presentation of NY-ESO-1-derived epitopes to antigen-specific CD8+ T cells by monocyte-derived DCs exposed to NY-ESO-1-containing immune complexes. Monocyte-derived DCs were prepared from a HLA-A2 (A*0201)-positive healthy donor and used as antigen-presenting cells for different NY-ESO-1-specific CD8+ T cells. (A) ELISPOT assay using mo-DCs exposed in medium 12 hr (DC alone) or NY-ESO-1 synthetic peptide (p157–165 at 10 μg/ml) (Upper), free NY-ESO-1 protein (1 μg/ml), or NY-ESO-1 immune complexes prepared by using the ES121 monoclonal antibody (1 μg/ml of NY-ESO-1 protein, 1 μg/ml of IgG), NY-ESO-1 immune complexes prepared by using serum from a melanoma patient with antibody for ESO-1 (1 μg/ml of NY-ESO-1 protein, 1/1,000 dilution of sera) or with serum from antibody-negative patient, free recombinant SSX2 protein (1 μg/ml; negative control), and/or SSX2 immune complex generated by using the monoclonal antibody HM498 (1 μg/ml of SSX2 protein, 1 μg/ml of IgG). Shown are excised filters stained for γ-interferon secretion. Numbers at bottom refer to the number of CD8+ T cells added per well. (B) mo-DCs incubated with the HLA-A2-restricted NY-ESO-1 peptide p157–165, the HLA-Cw3-restricted peptide p92–100, free NY-ESO-1 protein (1 μg/ml) or NY-ESO-1 ICs (as in A). Data in Top represent number of positive ELISPOT spots using Cw3-restricted CTLs expanded from a melanoma patient; bottom using the A2-restricted CTL “clone 5.” (C) ELISPOT assay performed as above comparing presentation of peptide to clone 5 by mo-DCs alone, exposed to free NY-ESO-1 protein, NY-ESO-1 ICs, NY-ESO-1 ICs prepared by using a Fab fragment of the monoclonal antibody E978 (1 μg/ml) or exposed to mAb E978. For all experiments, mo-DCs were collected on day 5 and incubated with peptide, protein, or immune complexes overnight before ELISPOT assay. (D) ELISPOT assay performed as above except (where indicated) NY-ESO-1-IC-containing wells also contained 10 μg/ml or monoclonal IgGs against CD32 (FcγRII) or CD64 (FcγRI).
To determine whether crosspresentation of a second epitope restricted by a different HLA haplotype might also occur under these conditions, we next examined the ability of NY-ESO-1-pulsed mo-DCs to stimulate HLA-Cw3-restricted CD8+ T cell lines (Fig. 2B). The response was specific to the Cw3-restricted peptide (p92–100) as opposed to the HLA-A2-restricted peptide (p157–165). Little response was observed when the mo-DCs were incubated with free NY-ESO-1 protein, but incubation with NY-ESO-1 ICs produced a significant ELISPOT signal (∼50 spots compared to 125 spots for the specific peptide) (Fig. 2B Upper). To exclude the possibility that the ELISPOT signal was at least partly due to NK cell activity, we next used an HLA-A2-restricted T cell clone, clone 5 (19). As expected, this T cell clone exhibited high reactivity to both peptide- and IC-loaded mo-DCs (Fig. 2B Lower).
The enhanced crosspresentation of ESO-1 epitopes was found to reflect the involvement of FcγRs rather than some other effect, such as epitope protection by the bound antibody. As shown in Fig. 2C, incubating mo-DCs with NY-ESO-1 protein complexed with a Fab fragment of an anti-NY-ESO-1 antibody did not lead to recognition by CD8+ T cells over background. Similarly, crosspresentation of NY-ESO-1 ICs could be at least partially inhibited by antibodies to FcγRII (CD32) and to a lesser extent FcγRI (CD64) (Fig. 2D).
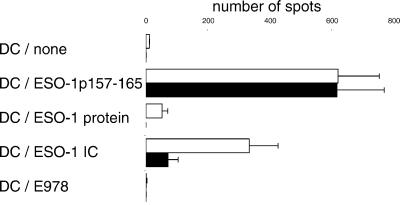
Crosspresentation is thought to occur in DCs when antigen internalized by endocytosis somehow escapes from endosomes or lysosomes and gains access to the cytosol, thus to the proteasome. After cleavage, the resulting peptides are translocated via transporter associated with antigen processing translocators into the endoplasmic reticulum, where they can be loaded onto MHC class I molecules as in the “conventional” endogenous pathway (13, 14). To confirm that this was the mechanism responsible for the presentation of exogenous NY-ESO-1, mo-DCs were exposed to NY-ESO-1 ICs in the presence or absence of the proteasome inhibitor lactacystin (23, 24). As shown in Fig. 3, lactacystin had no effect on the presentation of the p157–165 peptide to CD8+ T cells but did inhibit the presentation of NY-ESO-1 to nearly background levels. Thus, it appears that crosspresentation of NY-ESO-1 by mo-DCs is proteasome-dependent.
Fig 3.
Lactacystin inhibits crosspresentation of a NY-ESO-1 epitope. mo-DCs were harvested at day 5 and then cultured for an additional 12 hr in the presence or absence of lactacystin (10 μM) in normal growth medium or medium containing the NY-ESO-1 peptide p157–165 (10 μg/ml), free NY-ESO-1 (1 μg/ml), NY-ESO-1 ICs (using the monoclonal antibody E978 at 1 μg/ml), or the control protein SSX2. ELISPOT assays using clone 5 were performed as above. Open bars, no lactacystin; solid bars, lactacystin added.
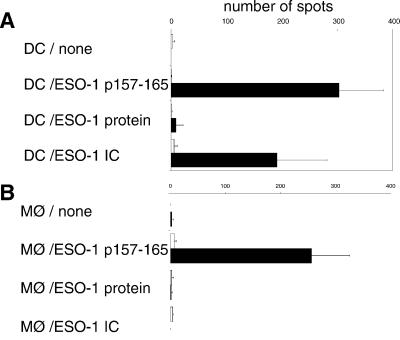
Macrophages Do Not Crosspresent NY-ESO-1 Immune Complexes.
Although crosspresentation is widely thought to be a function restricted to DCs, there are data suggesting that macrophages can also display this activity (4). Because macrophages are strongly FcγR-positive, we next asked whether these cells might also present exogenous NY-ESO-1 when internalized as ICs. Macrophages were differentiated by adherence from PBMCs isolated from healthy HLA-A*0201-positive or -negative donors. In a paired experiment, they were then tested for the ability to crosspresent free NY-ESO-1 protein, NY-ESO-1 ICs, or immunogenic peptide. As shown in Fig. 4, although macrophages (from A*0201-positive donors) were capable of presenting peptide to A2-restricted CD8+ T cells, no ELISPOT signal was detected over background when the macrophages were allowed to internalize free NY-ESO-1 or NY-ESO-1 ICs for 12 hr. In contrast, mo-DCs exhibited abundant crosspresentation of NY-ESO-1 in ICs in an HLA-A2-restricted fashion.
Fig 4.
Macrophages do not crosspresent NY-ESO-1 immune complexes. mo-DCs and CD14+ macrophages were compared for their abilities to present exogenous NY-ESO-1 either as free protein or as an immune complex (as above) to the HLA-A2-restricted CD8+ T cell clone 5. ELISPOT data are shown. A contains data from mo-DCs; B contains data from macrophages. The NY-ESO-1 peptide was used for this experiment (p157–165, 1 μg/ml). Solid bars represent HLA-A*0201-positive mo-DCs or macrophages; open bars represent HLA-mismatched controls.
DCs Derived from CD34+ Precursors Do Not Crosspresent Exogenous NY-ESO-1.
DCs comprise a heterogeneous array of cells whose phenotypes and lineages can vary considerably. Although much work on human DCs has focused on those derived from PBMCs, it is also possible to generate DC populations in vitro from CD34+ precursors (17, 25). When CD34+ cells are harvested from granulocyte-CSF-mobilized donors, growth in medium containing various cytokines including transformed growth factor β yield an immature DC population with many of the characteristics of epidermal Langerhans cells, a DC population that may be particularly relevant to immunization. To determine whether these CD34-derived DCs (cd34-DC) were capable of crosspresentation in vitro, we examined their ability to stimulate NY-ESO-1-specific CD8+ T cells.
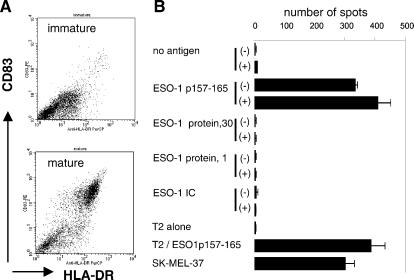
cd34-DCs were grown from HLA-A2-positive donors and either maintained in culture as immature cells or induced to mature by disruption of proliferating clusters in the presence of heat-killed E. coli (17). Mature cells exhibited a characteristic increase in the surface expression of both MHC class II molecules and CD83 (Fig. 5A). Addition of the A2-restricted peptide (p157–165) to either immature or mature cells resulted in a robust ELISPOT signal, with mature cd34-DCs being slightly more effective (Fig. 5B). At constant effector/target cell ratios of 1:50, peptide presentation was similar to that observed by using peptide-pulsed T2 cells or SK-MEL-37 that express NY-ESO-1 endogenously. Addition of free NY-ESO-1 protein either at 30 or 1 μg/ml did not elicit any CD8+ T cell reactivity whether incubated with immature or mature cd34-DCs. Surprisingly, however, the cells also failed to crosspresent NY-ESO-1 epitopes even if delivered to the cd34-DCs as an immune complex, despite the fact that under exactly the same conditions, NY-ESO-1 ICs were effectively crosspresented by mo-DCs (see above).
Fig 5.
CD34-derived DCs fail to crosspresent NY-ESO-1. Immature cd34-DCs were prepared and cultured overnight in the presence of the indicated antigens or peptides with or without the addition of heat-killed E. coli to induce maturation (17). A illustrates the surface expression by flow cytometry of HLA-DR and CD83, showing the ability of the E. coli to induce maturation (i.e., enhanced expression of both of these markers). B shows the results of a series of ELISPOT assays using clone 5 as the NY-ESO-1-specific T cell clone. Immature (−) or stimulated (+) cd34-DCs were cultured overnight in medium containing no addition, the HLA-A2-restricted peptide p157–165, free NY-ESO-1 protein (30 or 1 μg/ml), or NY-ESO-1 ICs (1 μg/ml of NY-ESO-1 protein). As positive controls, the ability of T2 cells (A*0201) to present p157–165 was measured, as was the ability of the melanoma cell line SK-MEL-37 to present endogenous NY-ESO-1.
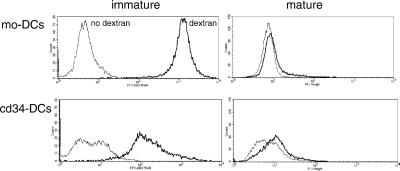
To determine whether the difference in crosspresentation between mo-DCs and cd34-DCs reflected differences in endocytosis between the two cell types, we next assayed the internalization of both fluid-phase and FcγR-bound ligands. As found previously, immature mo-DCs internalized large amounts of FITC–dextran, a marker of fluid macropinocytosis and mannose receptor-mediated endocytosis (26). Maturation resulted in down-regulation of FITC–dextran uptake to an amount ∼5% of that observed in immature cells (Fig. 6 Upper). In contrast, even immature cd34-DCs internalized relatively small amounts of FITC–dextran, <10% of that internalized by immature mo-DCs (Fig. 6 Lower). This amount was diminished still further (∼10-fold) on maturation. Similar results were obtained by using horseradish peroxidase as a probe (not shown). Thus, although cd34-DCs exhibited a relatively limited capacity for fluid-phase uptake of dextran, this alone could not explain their failure to crosspresent, because even mo-DCs failed to efficiently stimulate CD8+ T cells when allowed to internalize free NY-ESO-1.
Fig 6.
Endocytosis of fluid-phase FITC–dextran by monocyte- and CD34-derived immature DCs. Immature or mature DCs were incubated in medium containing FITC–dextran (40,000 molecular weight; 1.0 mg/ml) for 1 hr at 37°C. After washing, samples were analyzed by flow cytometry. Immature mo-DCs accumulated >10-fold more FITC–dextran than did cd34-DCs. Maturation reduced uptake by both cell populations to nearly control values. Dark line, cells exposed to FITC–dextran; thin line, no dextran control.
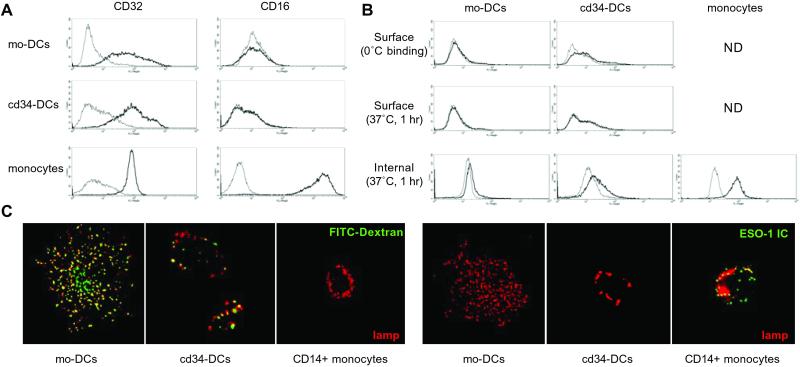
The ability of both DC types to internalize NY-ESO-1-ICs was then determined. We first examined the expression of FcγRs by mo-DCs and cd34-DCs, specifically FcγRII (CD32) and FcγRIII (CD16), the receptor classes generally associated with IC endocytosis (27). As shown in Fig. 7A, both cell types exhibited moderate but heterogeneous expression of CD32, with cd34-DCs on average expressing slightly higher levels. The amounts were comparable to CD32 found on CD14+ human monocytes, which, however, exhibited a far more uniform pattern of expression. The monocytes also expressed high levels of CD16, which in contrast was barely detectable on mo-DCs and cd34-DCs (Fig. 7A).
Fig 7.
Binding and endocytosis of NY-ESO-1-containing immune complexes by monocyte- and CD34-derived DCs. (A) Surface FcγRs were determined by flow cytometry on mo-DCs, cd34-DCs, and CD14+ monocytes by using antibodies to FcγRII (CD32) and FcγRIII (CD16). (B) ICs formed by combining recombinant NY-ESO-1 and affinity-purified rabbit polyclonal anti-NY-ESO-1 antibody (30 μg IgG/ml) were incubated with immature mo-DCs, cd34-DCs, or CD14+ monocytes for 1 hr at 37°C. The cells were washed with PBS four times, fixed, and permeabilized by using 0.05% saponin to detect both surface and intracellular ICs. The cell-associated ICs were then visualized by polyclonal anti-rabbit-Alexa488 antibody prior to analysis by flow cytometry. Thick lines, ICs added; thin lines, no ICs added. (C) Immature mo-DCs, cd34-DCs, or CD14+ monocytes were exposed to FITC–dextran (green) or biotinylated NY-ESO-1 ICs for 1 hr at 37°C. The cells were then fixed, permeabilized, and stained for the ICs (green) or the lysosomal membrane marker lgp/lamp (red). Both DC types internalized FITC-dextran, delivering it to lamp-positive lysosomes (which thus appeared yellow); the DCs internalized little or no IC (cd34-DCs were barely over background). In contrast, the monocytes internalized little dextran but significant amounts of the NY-ESO-1 ICs.
Surprisingly, despite detectable levels of CD32, neither mo-DCs nor cd34-DCs exhibited efficient FcγR-mediated endocytosis. As shown in Fig. 7B, relatively little NY-ESO-1 IC was taken up after a 1-hr incubation at 37°C by either DC type. If anything, cd34-DCs accumulated slightly more of the FcγR-bound ligand than mo-DCs. More surprisingly, CD14+ monocytes, which were incapable of crosspresenting NY-ESO-1, were by far the most effective at NY-ESO-1 IC uptake. Similar results were obtained by using ICs formed by using multiply biotinylated NY-ESO-1 combined with an affinity-purified antibiotin antibody to increase the valency and thus the avidity of the resulting IC and obtained the same results as above (not shown).
To confirm the flow cytometry data, we next used fluorescence microscopy to directly visualize endocytosis by both DC types and CD14+ monocytes. After 1 hr at 37°C, the fluid-phase marker FITC–dextran (green) was clearly found in intracellular vesicles in both mo-DCs and cd34-DCs (Fig. 7C Left). Although the overall distribution of lysosomal glycoprotein/lysosome-associated membrane protein-positive lysosomal structures (red) was rather different in the two DC types, a significant fraction in both cases did contain the FITC–dextran (and thus appeared yellow). Interestingly, the monocytes internalized relatively small amounts of the dextran. The opposite result was observed for the ICs, however (Fig. 7C Right). After 1 hr, little if any of the FcγR ligand (green) was detected as having been internalized by either DC type, consistent with the FACS data. In contrast, the CD14+ monocytes accumulated relatively large amounts of the ICs (green) in intracellular vesicles, some of which had already reached lysosomal structures (red) during the 1-hr incubation. Thus, there was no correlation between the ability of cells to internalize the NY-ESO-1 ICs and their ability to crosspresent NY-ESO-1-derived peptides to CD8+ T cells.
Discussion
DCs comprise a rather heterogeneous group of cells that share a number of important functional and morphological features, including an exceptional capacity for T cell stimulation and the ability to “mature” in response to a variety of proinflammatory and microbial stimuli. Maturation typically results in an increase in T cell stimulatory capacity because of a marked increase in one or more MHC products, costimulatory molecules, and capacity for antigen processing (2). It is also accompanied by a characteristic cellular reorganization in which MHC class II molecules are recruited from intracellular compartments to the plasma membrane, concomitant with the extension of voluminous membrane folds and the down-regulation of macropinocytosis. Not all DCs are equivalent, however. They appear to derive from multiple lineages and, depending on their origin, site of residence, or type of maturation stimulus received, can program different T cell outcomes (1). Understanding the function of DCs in the immune response will require an understanding of the cellular biology underlying each of these events. Similarly, understanding how DCs can best be used for the development of therapeutic vaccines will require a better understanding of the properties of different DC populations.
We have demonstrated that it is possible to load human DCs in vitro with soluble recombinant NY-ESO-1 to generate immunogenic MHC class I complexes that can be recognized by antigen-specific CD8+ T cells. Crosspresentation was observed for two distinct NY-ESO-1 epitopes, restricted by different MHC class I alleles. We also found that delivering the NY-ESO-1 protein as an IgG-immune complex was far more efficient than delivering the antigen as free protein. The reasons for the increased efficiency are not yet clear. One possibility is that it reflects simply a more effective mode of NY-ESO-1 endocytosis relative to what can be taken up by nonspecific endocytosis. Indeed, receptor binding can increase uptake efficiency and antigen presentation >100-fold, at least in B cells, which have a limited capacity for fluid-phase endocytosis (28). It is also possible that binding of ligand to Fc receptors itself directs internalized antigens to intracellular sites suited for egress into the cytosol, or perhaps receptor binding itself helps trigger subsequent antigen release from endocytic organelles. Although crosspresentation of phagocytic and soluble antigens by DCs or DC-like cell lines has been shown by others to be enhanced by FcγR-mediated uptake (13, 14), the reasons for the enhancing effect remain to be determined. In this regard, it was surprising to learn that mo-DCs actually bound and internalized very low amounts of NY-ESO-1-containing immune complexes. This was not a property of the complexes themselves, because their binding and uptake could be easily observed by using CD14+ monocyte-macrophages. Rather, it appeared to be a reflection of the very low levels of CD16 and CD32 expression by immature mo-DCs. Thus, there was no correlation between the efficiency of FcγR-mediated uptake and crosspresentation.
We were also surprised to observe that cd34-DCs, although excellent at peptide presentation, were completely unable to crosspresent NY-ESO-1 even when delivered to cells as ICs. If anything, these cells appeared to express higher levels of FcγR (CD32) than mo-DCs, although the amount of antigen internalized remained very low. It is unclear as yet whether cd34-DCs express FcγRII or FcγRIII isoforms capable of rapid endocytosis. Our previous work has shown that differential mRNA splicing, for example, can result in the production of receptor cytoplasmic domains with markedly different capacities for clathrin-dependent endocytosis in mouse or human B cells vs. macrophages (28–31).
Because cd34- and mo-DCs appear to take up comparably low levels of NY-ESO-1-ICs, we suspect that their dramatically different capacities for crosspresentation reflect some other functional difference between these two cell types. Perhaps cd34-DCs require a specific and as-yet-uncharacterized type of stimulus to activate the crosspresentation pathway. In fact, our preliminary results using mouse bone marrow-derived DCs suggest that different maturation stimuli control the processing of exogenous antigens for presentation on MHC class I vs. class II molecules (19). On the other hand, the likely lineage differences between cd34-DCs and mo-DCs may produce DCs with different capacities for crosspresentation, at least when assayed in vitro. One cannot conclude from our data that Langerhans cells in the epidermis, which are phenotypically related to the cd34-DCs used here (17), are incapable of crosspresenting exogenous antigen in situ. We also cannot exclude definitively the possibility that differences among donors are at least partly responsible for the inability of cd34-DCs to crosspresent exogenous NY-ESO-1, although cells collected from three different G-CSF-mobilized donors were evaluated with similar results. Nevertheless, when designing strategies for the ex vivo loading of antigen into DCs, mo-DCs would at present appear to be the more efficacious vehicle.
Acknowledgments
We gratefully acknowledge the contributions made by a variety of laboratory colleagues to the development of this work, particularly members of the combined Mellman–Warren group. We also are indebted to the Ludwig Institute for Cancer Research and to the National Institutes of Health for their generous support.
Abbreviations
DC, dendritic cell
IC, immune complex
GM-CSF, granulocyte–macrophage colony-stimulating factor
mo-DC, monocyte-derived DC
PBMC, peripheral blood mononuclear cell
FcγR, Fcγ receptor
TNFα, tumor necrosis factor α
References
- 1.Banchereau J. & Steinman, R. M. (1998) Nature (London) 392, 245-252. [DOI] [PubMed] [Google Scholar]
- 2.Mellman I. & Steinman, R. M. (2001) Cell 106, 255-258. [DOI] [PubMed] [Google Scholar]
- 3.Albert M. L., Sauter, B. & Bhardwaj, N. (1998) Nature (London) 392, 86-89. [DOI] [PubMed] [Google Scholar]
- 4.Norbury C. C., Hewlett, L. J., Prescott, A. R., Shastri, N. & Watts, C. (1995) Immunity 3, 783-791. [DOI] [PubMed] [Google Scholar]
- 5.Kovacsovics-Bankowski M. & Rock, K. L. (1995) Science 267, 243-246. [DOI] [PubMed] [Google Scholar]
- 6.Bevan M. J. (1976) J. Exp. Med. 143, 1283-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boczkowski D., Nair, S. K., Snyder, D. & Gilboa, E. (1996) J. Exp Med. 184, 465-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boon T. & Old, L. J. (1997) Curr. Opin. Immunol. 9, 681-683. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y. T., Scanlan, M. J., Sahin, U., Tureci, O., Gure, A. O., Tsang, S., Williamson, B., Stockert, E., Pfreundschuh, M. & Old, L. J. (1997) Proc. Natl. Acad. Sci. USA 94, 1914-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stockert E., Jager, E., Chen, Y. T., Scanlan, M. J., Gout, I., Karbach, J., Arand, M., Knuth, A. & Old, L. J. (1998) J. Exp. Med. 187, 1349-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jager E., Chen, Y. T., Drijfhout, J. W., Karbach, J., Ringhoffer, M., Jager, D., Arand, M., Wada, H., Noguchi, Y., Stockert, E., et al. (1998) J. Exp. Med. 187, 265-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jager E., Jager, D., Karbach, J., Chen, Y. T., Ritter, G., Nagata, Y., Gnjatic, S., Stockert, E., Arand, M., Old, L. J. & Knuth, A. (2000) J. Exp. Med. 191, 625-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhodapkar K. M., Krasovsky, J., Williamson, B. & Dhodapkar, M. V. (2002) J. Exp. Med. 195, 125-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez A., Regnault, A., Kleijmeer, M., Ricciardi-Castagnoli, P. & Amigorena, S. (1999) Nat. Cell. Biol. 1, 362-368. [DOI] [PubMed] [Google Scholar]
- 15.Norbury C. C., Chambers, B. J., Prescott, A. R., Ljunggren, H. G. & Watts, C. (1997) Eur. J. Immunol. 27, 280-288. [DOI] [PubMed] [Google Scholar]
- 16.O'Doherty U., Steinman, R. M., Peng, M., Cameron, P. U., Gezelter, S., Kopeloff, I., Swiggard, W. J., Pope, M. & Bhardwaj, N. (1993) J. Exp. Med. 178, 1067-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gatti E., Velleca, M. A., Biedermann, B. C., Ma, W., Unternaehrer, J., Ebersold, M. W., Medzhitov, R., Pober, J. S. & Mellman, I. (2000) J. Immunol. 164, 3600-3607. [DOI] [PubMed] [Google Scholar]
- 18.Gnjatic S., Nagata, Y., Jager, E., Stockert, E., Shankara, S., Roberts, B. L., Mazzara, G. P., Lee, S. Y., Dunbar, P. R., Dupont, B., et al. (2000) Proc. Natl. Acad. Sci. USA 97, 10917-10922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valmori D., Dutoit, V., Lienard, D., Rimoldi, D., Pittet, M. J., Champagne, P., Ellefsen, K., Sahin, U., Speiser, D., Lejeune, F., et al. (2000) Cancer Res. 60, 4499-4506. [PubMed] [Google Scholar]
- 20.Jager E., Nagata, Y., Gnjatic, S., Wada, H., Stockert, E., Karbach, J., Dunbar, P. R., Lee, S. Y., Jungbluth, A., Jager, D., et al. (2000) Proc. Natl. Acad. Sci. USA 97, 4760-4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cella M., Engering, A., Pinet, V., Pieters, J. & Lanzavecchia, A. (1997) Nature (London) 388, 782-787. [DOI] [PubMed] [Google Scholar]
- 22.Cella M., Salio, M., Sakakibara, Y., Langen, H., Julkunen, I. & Lanzavecchia, A. (1999) J. Exp. Med. 189, 821-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cerundolo V., Benham, A., Braud, V., Mukherjee, S., Gould, K., Macino, B., Neefjes, J. & Townsend, A. (1997) Eur. J. Immunol. 27, 336-341. [DOI] [PubMed] [Google Scholar]
- 24.Fenteany G., Standaert, R. F., Lane, W. S., Choi, S., Corey, E. J. & Schreiber, S. L. (1995) Science 268, 726-731. [DOI] [PubMed] [Google Scholar]
- 25.Strunk D., Rappersberger, K., Egger, C., Strobl, H., Kromer, E., Elbe, A., Maurer, D. & Stingl, G. (1996) Blood 87, 1292-1302. [PubMed] [Google Scholar]
- 26.Sallusto F., Cella, M., Danieli, C. & Lanzavecchia, A. (1995) J. Exp. Med. 182, 389-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ukkonen P., Lewis, V., Marsh, M., Helenius, A. & Mellman, I. (1986) J. Exp. Med. 163, 952-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amigorena S., Bonnerot, C., Drake, J. R., Choquet, D., Hunziker, W., Guillet, J. G., Webster, P., Sautes, C., Mellman, I. & Fridman, W. H. (1992) Science 256, 1808-1812. [DOI] [PubMed] [Google Scholar]
- 29.Stuart S. G., Trounstine, M. L., Vaux, D. J., Koch, T., Martens, C. L., Mellman, I. & Moore, K. W. (1987) J. Exp. Med. 166, 1668-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miettinen H. M., Rose, J. K. & Mellman, I. (1989) Cell 58, 317-327. [DOI] [PubMed] [Google Scholar]
- 31.Miettinen H. M., Matter, K., Hunziker, W., Rose, J. K. & Mellman, I. (1992) J. Cell Biol. 116, 875-888. [DOI] [PMC free article] [PubMed] [Google Scholar]