Abstract
Aims
The Prospective Pakistan Registry of Echocardiographic Screening in Asymptomatic Pregnant Women (PRESAP) is a registry designed for the echocardiographic screening of structural heart disease (SHD). It offers a unique opportunity to compare the effectiveness of point-of-care ultrasound (POCUS) vs. routine transthoracic echocardiography (TTE) in this population. This sub-study of the PRESAP registry aimed to compare the detection of SHD in asymptomatic pregnant women using POCUS vs. routine TTE.
Methods and results
Between February 2023 and March 2024, pregnant women without known heart disease were enrolled in the PRESAP registry. Participants underwent a limited echocardiogram, using either POCUS or TTE. The primary outcome was the detection of abnormal findings, including left ventricular systolic dysfunction (LVSD), valvular heart disease (VHD), and congenital heart disease (CHD). Matching cohorts were created using propensity score matching, based on demographic factors, comorbid conditions, and gestational history. Among 18 401 patients, 9681 (52.6%) underwent POCUS. The 1:1 propensity-matched cohort included 4177 patients in each arm. Abnormal echocardiographic findings were detected in 4.4% (185) of the TTE group and 3% (124) of the POCUS group (P < 0.001). VHD (1.3% vs. 1.1%; P = 0.416) and CHD (0.4% vs. 0.6%; P = 0.093) were detected at similar rates by both methods, while LVSD was more frequently identified by TTE compared with POCUS (2.8% vs. 1.5%; P < 0.001).
Conclusion
POCUS may be considered as an alternative to TTE for screening for SHD in asymptomatic pregnant women, particularly in resource-limited settings. TTE was superior for LVSD, and POCUS was equally effective in identifying valvular and congenital heart disease.
Keywords: point-of-care ultrasound, transthoracic echocardiography, cardiovascular disease, pregnancy
Graphical Abstract
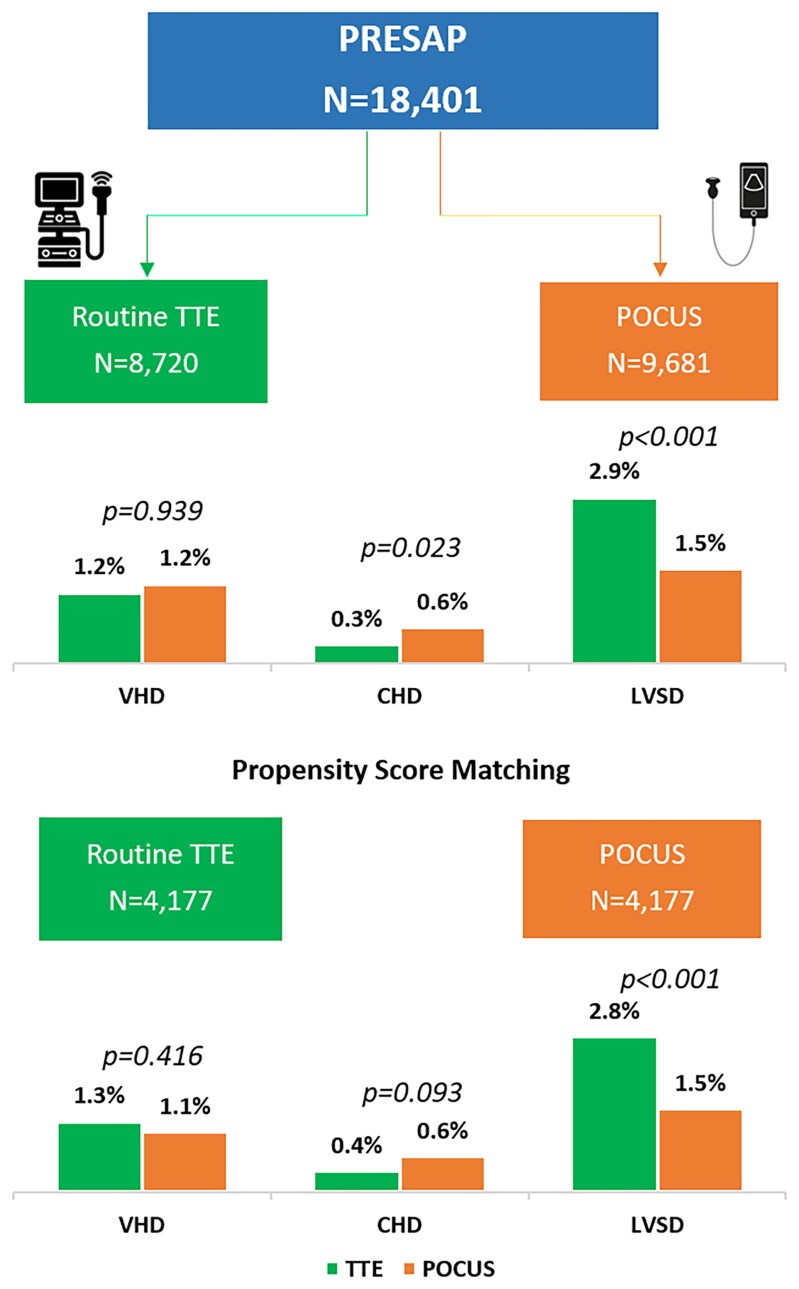
Graphical Abstract.
Prevalence of abnormal echocardiographic findings in unmatched and matched cohorts of TTE and POCUS.
Introduction
Cardiovascular diseases (CVDs) are a leading cause of maternal morbidity and mortality worldwide, accounting for nearly 15% of pregnancy-related deaths, with disproportionately higher rates in low- and middle-income countries (LMICs).1 Structural heart disease (SHD), including valvular heart disease (VHD), cardiomyopathies, and congenital heart disease (CHD), often remains undiagnosed during pregnancy due to asymptomatic presentation or symptom overlap with physiological changes of gestation.2,3 In Pakistan, where maternal mortality rates exceed global averages, the Prospective Pakistan Registry of Echocardiographic Screening in Asymptomatic Pregnant Women (PRESAP) recently demonstrated a 3.8% prevalence of SHD among 15 068 asymptomatic women, underscoring the critical need for scalable screening strategies.4,5 However, the comparative effectiveness of point-of-care ultrasound (POCUS) vs. routine transthoracic echocardiography (TTE) for detecting SHD in this population remains unaddressed—a gap with profound implications for LMICs.
The clinical challenge of diagnosing SHD during pregnancy is compounded by limited access to specialized cardiac care, particularly in regions like South Asia where socioeconomic disparities and infrastructural barriers restrict the use of conventional TTE. While TTE remains the gold standard for cardiac evaluation, its reliance on expensive equipment and trained sonographers limits scalability in resource-constrained antenatal clinics. POCUS, a portable and cost-effective alternative, has shown promise in emergency and primary care settings for rapid assessment of cardiac function.6,7 However, its diagnostic accuracy for detecting subtle SHD in pregnancy—where haemodynamic adaptations such as increased plasma volume and cardiac output may mask pathology—remains understudied.8 Prior validation studies in non-pregnant cohorts suggest POCUS achieves >90% concordance with TTE for major abnormalities,9,10 but data specific to antenatal screening are sparse.
The 2018 ESC guidelines for CVD management in pregnancy emphasize the importance of echocardiography for high-risk women, yet LMICs often lack protocols to implement such recommendations.2 PRESAP, the largest antenatal cardiac screening registry in Pakistan, provided a unique platform to address this disparity. While the parent study established the feasibility of routine echocardiographic screening, this sub-study leverages PRESAP’s infrastructure to directly compare POCUS and TTE in detecting SHD, including left ventricular systolic dysfunction (LVSD), VHD, and CHD.5 By evaluating diagnostic concordance and clinical utility in matched cohorts, we aim to inform context-appropriate screening strategies for LMICs, where early detection of SHD could mitigate adverse maternofoetal outcomes.11
Methods
Study design and population
This sub-study was conducted as part of the PRESAP, a prospective cohort study enrolling pregnant women without known heart disease who attended routine antenatal care at the National Institute of Cardiovascular Diseases (NICVD) in Karachi, Pakistan, from February 2023 to March 2024. Participants underwent a limited transthoracic echocardiogram using either POCUS or a standard machine (TTE) based on availability in the screening area that day. Women were screened once during their pregnancy, with exclusion criteria including prior echocardiographic screening at earlier antenatal visits. Baseline demographic, clinical characteristics, and obstetric history were collected from medical records and structured interviews. Data were securely entered into the REDCap database.
Echocardiographic screening protocol
All participants underwent a standardized limited 2D transthoracic echocardiogram. TTE was performed by trained sonographers using General Electric ultrasound systems, while POCUS was conducted using Butterfly POCUS devices. The echocardiographic protocol included parasternal long-axis, parasternal short-axis, apical four-chamber, and apical two-chamber views. Colour Doppler imaging was performed across the mitral and aortic valves, along with continuous wave and pulse wave Doppler assessments of the left ventricular outflow tract to evaluate for flow gradients. Data from POCUS were uploaded to a secure, cloud-based server accessible to dedicated faculty for quality assurance. Examples of TTE and POCUS are shown in Supplementary data online, Figures 1–4 and Supplementary data online, Videos 1–4.
Evaluation of cardiac function and structural abnormalities
Left ventricular systolic function was assessed visually by two independent readers, with discrepancies adjudicated by a third reviewer. Left ventricular ejection fraction (EF) was calculated using the biplane Simpson’s method. LVSD was categorized following the American Society of Echocardiography guidelines: severe (EF ≤ 30%), moderate (EF 30–40%), mild (EF 41–55%), and normal (>55%). Significant LVSD was defined as EF < 55%. Patients identified with more than mild VHD or suspected CHD on initial screening underwent comprehensive TTE for further evaluation.
Management and follow-up
Participants diagnosed with SHD were referred to an adjacent cardio-obstetrics clinic for further management, following standard-of-care guidelines. These patients were longitudinally followed within the cardio-obstetrics registry to monitor outcomes.
Statistical analysis
The comparative data analyses were performed using IBM SPSS version 21. Continuous variables were expressed as mean ± standard deviation (SD) or median with interquartile range (IQR) for skewed distributions. Categorical variables were summarized as frequencies and percentages. Baseline characteristics and echocardiographic findings were compared between the POCUS and TTE groups using χ2 tests for categorical variables and independent sample t-tests or Mann–Whitney U tests for continuous variables. Statistical significance was defined as P < 0.05.
Considering the potential confounding effects of the baseline characteristics, propensity matching was performed to form matching cohorts for TTE and POCUS. Continuous variables with more than 5% missing values were dropped from the matching analysis, while variables with less than 5% missing values were imputed with either mean or median depending on the nature of distribution. Matching parameters included age, systolic and diastolic blood pressure, heart rate, parity, history of neonatal death, history of spontaneous abortion, number of gestations, hypertension, diabetes, pregnancy induced hypertension, and trimester. We implemented propensity score matching using python through calliper matching approach. Propensity scores were estimated using logistic regression model, the calliper was set at 10% of standard deviation of the propensity score, k-nearest neighbours method was adopted to get three matching controls for each case, and closest observation was picked among three to maintain 1:1 ratio of cases to controls.
Results
Baseline characteristics
A total of 18 401 pregnant women were enrolled in the study, with 9681 (52.6%) undergoing a limited echocardiogram using POCUS and 8720 (47.4%) undergoing limited TTE using standard machines. The mean age of the cohort was 26.2 ± 5.1 years, with participants in the POCUS group being slightly younger compared with those in the TTE group (26.1 ± 5.1 vs. 26.3 ± 5.0 years, P = 0.001). A higher proportion of primiparous women was observed in the POCUS group (33.2%) compared with the TTE group (31%). Most participants were in their third trimester at the time of screening (71.1%). Hypertension and diabetes mellitus were slightly more prevalent in the POCUS group (3.4% and 2.4%) compared with the TTE group (0.6% and 0.3%). These baseline characteristics are summarized in Table 1.
Table 1.
Distribution of baseline demographic and clinical characteristics for the study sample stratified by ECHO method
| Total | ECHO | P-value | ||
|---|---|---|---|---|
| TTE | POCUS | |||
| Total | 18 401 | 8720 (47.4%) | 9681 (52.6%) | |
| Mean age (years) | 26.2 ± 5.1 | 26.3 ± 5 | 26.1 ± 5.1 | 0.001 |
| ≤20 years | 2626 (14.3%) | 1147 (13.2%) | 1479 (15.3%) | 0.001 |
| 21–30 years | 12 686 (68.9%) | 6084 (69.8%) | 6602 (68.2%) | |
| 31–40 years | 3010 (16.4%) | 1450 (16.6%) | 1560 (16.1%) | |
| 41–50 years | 79 (0.4%) | 39 (0.4%) | 40 (0.4%) | |
| Parity | ||||
| Primiparous | 5892 (32%) | 2892 (33.2%) | 3000 (31%) | 0.002 |
| Multiparous | 12 509 (68%) | 5828 (66.8%) | 6681 (69%) | |
| History of neonatal death | 1141 (6.2%) | 254 (2.9%) | 887 (9.2%) | <0.001 |
| Number of gestation | ||||
| 1.0 | 18 222 (99%) | 8690 (99.7%) | 9532 (98.5%) | <0.001 |
| 2.0 | 159 (0.9%) | 29 (0.3%) | 130 (1.3%) | |
| 3.0 | 20 (0.1%) | 1 (0%) | 19 (0.2%) | |
| Trimester | ||||
| First trimester | 273 (1.5%) | 173 (2%) | 100 (1%) | <0.001 |
| Second trimester | 5040 (27.4%) | 2509 (28.8%) | 2531 (26.1%) | |
| Third trimester | 13 088 (71.1%) | 6038 (69.2%) | 7050 (72.8%) | |
| Mean systolic blood pressure (mmHg) | 109.6 ± 11.1 | 109.4 ± 11 | 109.8 ± 11.1 | 0.012 |
| Mean diastolic blood pressure (mmHg) | 71.6 ± 9.3 | 71.4 ± 9.4 | 71.8 ± 9.3 | 0.002 |
| Mean heart rate (bpm) | 88.8 ± 8.3 | 89.1 ± 7.9 | 88.4 ± 8.6 | <0.001 |
| Diabetes mellitus | ||||
| Non-diabetic | 18 139 (98.6%) | 8693 (99.7%) | 9446 (97.6%) | <0.001 |
| Diabetic | 262 (1.4%) | 27 (0.3%) | 235 (2.4%) | |
| Hypertension | ||||
| Non-hypertensive | 18 024 (98%) | 8672 (99.4%) | 9352 (96.6%) | <0.001 |
| Hypertensive | 377 (2%) | 48 (0.6%) | 329 (3.4%) | |
| History of spontaneous abortion | 3856 (21%) | 1402 (16.1%) | 2454 (25.3%) | <0.001 |
| Pregnancy induced hypertension | ||||
| No | 18 152 (98.6%) | 8637 (99%) | 9515 (98.3%) | <0.001 |
| Yes | 249 (1.4%) | 83 (1%) | 166 (1.7%) | |
| Anaemia | ||||
| Normal | 6482 (35.2%) | 2609 (29.9%) | 3873 (40%) | <0.001 |
| Anaemia | 9978 (54.2%) | 4242 (48.6%) | 5736 (59.3%) | |
| NA | 1941 (10.5%) | 1869 (21.4%) | 72 (0.7%) | |
| Mean haemoglobin (g/dL) | 10.1 ± 1.5 | 10 ± 1.5 | 10.1 ± 1.6 | <0.001 |
Echocardiographic findings
Abnormal echocardiographic findings were identified in 664 women (3.6%), with a significantly higher detection rate in the TTE group compared with the POCUS group (4.3% vs. 3.0%, P < 0.001). LVSD was more frequently diagnosed using TTE compared with POCUS (2.9% vs. 1.5%, P < 0.001). The prevalence of VHD was similar between the two groups, with both reporting a prevalence of 1.2% (P = 0.939). Among cases of VHD, rheumatic origin accounted for the majority of lesions (0.8%), while non-rheumatic valvular disease was less common (0.4%). CHD was detected more commonly using POCUS compared with routine TTE (0.6% vs. 0.3%, P = 0.023), with atrial septal defects being the most frequently identified congenital lesions (Table 2).
Table 2.
Distribution of echocardiographic findings for the study sample stratified by ECHO method
| Total | ECHO | P-value | ||
|---|---|---|---|---|
| TTE | POCUS | |||
| Total | 18 401 | 8720 (47.4%) | 9681 (52.6%) | |
| Abnormal ECHO findings | 664 (3.6%) | 378 (4.3%) | 286 (3%) | <0.001 |
| Valvular lesions | 227 (1.2%) | 107 (1.2%) | 120 (1.2%) | 0.939 |
| Non-rheumatic | 75 (0.4%) | 33 (0.4%) | 42 (0.4%) | 0.799 |
| Rheumatic | 152 (0.8%) | 74 (0.8%) | 78 (0.8%) | |
| Mitral | 120 (0.7%) | 61 (0.7%) | 59 (0.6%) | 0.745 |
| Aortic | 2 (0%) | 1 (0%) | 1 (0%) | |
| Multi-valve | 30 (0.2%) | 12 (0.1%) | 18 (0.2%) | |
| Congenital heart disease | 83 (0.5%) | 29 (0.3%) | 54 (0.6%) | 0.023 |
| Atrial septal defect (ASD) | 35 (42.2%) | 10 (34.5%) | 25 (46.3%) | 0.299 |
| Ventricular septal defect (VSD) | 22 (26.5%) | 8 (27.6%) | 14 (25.9%) | 0.870 |
| Patent ductus arteriosus (PDA) | 7 (8.4%) | 5 (17.2%) | 2 (3.7%) | 0.034 |
| Tetralogy of Fallot (TOF) | 10 (12%) | 4 (13.8%) | 6 (11.1%) | 0.720 |
| Pulmonary valve stenosis (PS) | 7 (8.4%) | 2 (6.9%) | 5 (9.3%) | 0.712 |
| Coarctation of aorta (COA) | 4 (4.8%) | 0 (0%) | 4 (7.4%) | 0.133 |
| Left ventricular dysfunction | 394 (2.1%) | 251 (2.9%) | 143 (1.5%) | <0.001 |
| Ejection fraction | ||||
| Severely reduced (≤30%) | 97 (0.5%) | 57 (0.7%) | 40 (0.4%) | <0.001 |
| Moderately reduced (31–40%) | 170 (0.9%) | 123 (1.4%) | 47 (0.5%) | |
| Mildly reduced (41–55%) | 127 (0.7%) | 71 (0.8%) | 56 (0.6%) | |
| Normal (>55%) | 18 007 (97.9%) | 8469 (97.1%) | 9538 (98.5%) | |
| Other abnormal ECHO findings | 43 (0.2%) | 15 (0.2%) | 28 (0.3%) | >0.999 |
Other abnormal ECHO findings included pericardial effusion, dilated coronary sinus, dilated RA/RV, non-obstructive HCM, dextrocardia, dilated pulmonary artery, interatrial septum aneurysm, and congenital complete heart block.
Propensity score matching
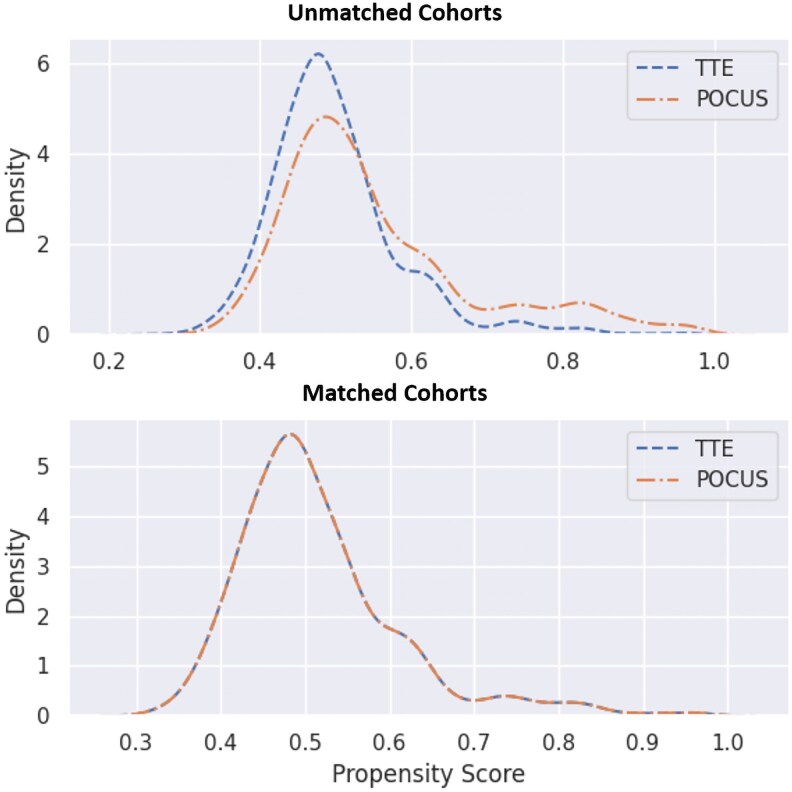
To account for potential confounding factors, a propensity score matching analysis was performed, resulting in a matched cohort of 8354 women (4177 in each group). After matching, the baseline demographic and clinical characteristics were well-balanced between the groups (Table 3 and Figure 1).
Table 3.
Distribution of baseline demographic and clinical characteristics for the study sample stratified by matched cohorts of ECHO method
| Total | ECHO | P-value | ||
|---|---|---|---|---|
| TTE | POCUS | |||
| Total | 8354 | 4177 | 4177 | |
| Mean age (years) | 26.1 ± 5.1 | 26.2 ± 5 | 26 ± 5.1 | 0.210 |
| ≤20 years | 1225 (14.7%) | 589 (14.1%) | 636 (15.2%) | 0.338 |
| 21–30 years | 5744 (68.8%) | 2908 (69.6%) | 2836 (67.9%) | |
| 31–40 years | 1353 (16.2%) | 663 (15.9%) | 690 (16.5%) | |
| 41–50 years | 32 (0.4%) | 17 (0.4%) | 15 (0.4%) | |
| Parity | ||||
| Primiparous | 2741 (32.8%) | 1340 (32.1%) | 1401 (33.5%) | 0.155 |
| Multiparous | 5613 (67.2%) | 2837 (67.9%) | 2776 (66.5%) | |
| History of neonatal death | 372 (4.5%) | 187 (4.5%) | 185 (4.4%) | 0.916 |
| Number of gestation | ||||
| 1.0 | 8295 (99.3%) | 4149 (99.3%) | 4146 (99.3%) | 0.406 |
| 2.0 | 54 (0.6%) | 27 (0.6%) | 27 (0.6%) | |
| 3.0 | 5 (0.1%) | 1 (0%) | 4 (0.1%) | |
| Trimester | ||||
| First trimester | 115 (1.4%) | 65 (1.6%) | 50 (1.2%) | 0.305 |
| Second trimester | 2388 (28.6%) | 1179 (28.2%) | 1209 (28.9%) | |
| Third trimester | 5851 (70%) | 2933 (70.2%) | 2918 (69.9%) | |
| Mean systolic blood pressure (mmHg) | 109.5 ± 10.9 | 109.5 ± 10.9 | 109.6 ± 10.9 | 0.533 |
| Mean diastolic blood pressure (mmHg) | 71.5 ± 9.2 | 71.4 ± 9.4 | 71.7 ± 9 | 0.093 |
| Mean heart rate (bpm) | 89 ± 8 | 88.9 ± 7.5 | 89.1 ± 8.6 | 0.200 |
| Diabetes mellitus | ||||
| Non-diabetic | 8314 (99.5%) | 4154 (99.4%) | 4160 (99.6%) | 0.342 |
| Diabetic | 40 (0.5%) | 23 (0.6%) | 17 (0.4%) | |
| Hypertension | ||||
| Non-hypertensive | 8275 (99.1%) | 4137 (99%) | 4138 (99.1%) | 0.910 |
| Hypertensive | 79 (0.9%) | 40 (1%) | 39 (0.9%) | |
| History of spontaneous abortion | 1700 (20.3%) | 860 (20.6%) | 840 (20.1%) | 0.587 |
| Pregnancy induced hypertension | ||||
| No | 8241 (98.6%) | 4127 (98.8%) | 4114 (98.5%) | 0.218 |
| Yes | 113 (1.4%) | 50 (1.2%) | 63 (1.5%) | |
Figure 1.
The distribution of propensity score in unmatched and matched cohorts of TTE and POCUS.
Findings in matched cohorts
The prevalence of abnormal echocardiographic findings remained significantly higher in the TTE group compared with the POCUS group (4.4% vs. 3.0%, P < 0.001). Similar to the unmatched analysis, LVSD was more frequently identified with TTE compared with POCUS (2.8% vs. 1.6%, P < 0.001). However, after matching, the difference in CHD (0.4% vs. 0.6%, P = 0.093) and VHD (1.3% vs. 1.1%, P = 0.416) detection between the two groups was similar (Table 4 and Graphical Abstract).
Table 4.
Distribution of echocardiographic findings for the study sample stratified by matched cohorts of ECHO method
| Total | ECHO | P-value | ||
|---|---|---|---|---|
| TTE | POCUS | |||
| Total | 8354 | 4177 | 4177 | |
| Abnormal ECHO findings | 309 (3.7%) | 185 (4.4%) | 124 (3%) | <0.001 |
| Valvular lesions | 98 (1.2%) | 53 (1.3%) | 45 (1.1%) | 0.416 |
| Non-rheumatic | 26 (0.3%) | 13 (0.3%) | 13 (0.3%) | 0.639 |
| Rheumatic | 72 (0.9%) | 40 (1%) | 32 (0.8%) | |
| Mitral | 56 (0.7%) | 32 (0.8%) | 24 (0.6%) | 0.563 |
| Aortic | 0 (0%) | 0 (0%) | 0 (0%) | |
| Multi-valve | 16 (0.2%) | 8 (0.2%) | 8 (0.2%) | |
| Congenital heart disease | 43 (0.5%) | 16 (0.4%) | 27 (0.6%) | 0.093 |
| Atrial septal defect (ASD) | 22 (51.2%) | 6 (37.5%) | 16 (59.3%) | 0.168 |
| Ventricular septal defect (VSD) | 9 (20.9%) | 5 (31.3%) | 4 (14.8%) | 0.200 |
| Patent ductus arteriosus (PDA) | 2 (4.7%) | 2 (12.5%) | 0 (0%) | 0.060 |
| Tetralogy of Fallot (TOF) | 3 (7%) | 1 (6.3%) | 2 (7.4%) | 0.885 |
| Pulmonary valve stenosis (PS) | 7 (16.3%) | 2 (12.5%) | 5 (18.5%) | 0.605 |
| Coarctation of aorta (COA) | 1 (2.3%) | 0 (0%) | 1 (3.7%) | 0.436 |
| Left ventricular dysfunction | 180 (2.2%) | 117 (2.8%) | 63 (1.5%) | <0.001 |
| Ejection fraction | ||||
| Severely reduced (≤30%) | 48 (0.6%) | 31 (0.7%) | 17 (0.4%) | <0.001 |
| Moderately reduced (31–40%) | 77 (0.9%) | 56 (1.3%) | 21 (0.5%) | |
| Mildly reduced (41–55%) | 55 (0.7%) | 30 (0.7%) | 25 (0.6%) | |
| Normal (>55%) | 8174 (97.8%) | 4060 (97.2%) | 4114 (98.5%) | |
| Other abnormal ECHO findings | 23 (0.3%) | 11 (0.3%) | 12 (0.3%) | 0.835 |
Other abnormal ECHO findings included pericardial effusion, dilated coronary sinus, dilated RA/RV, non-obstructive HCM, dextrocardia, dilated pulmonary artery, interatrial septum aneurysm, and congenital complete heart block.
Discussion
This sub-study from the PRESAP registry demonstrates that limited echocardiography using POCUS may be used as an alternative to standard machines (TTE) for detecting SHD in asymptomatic pregnant women, particularly in resource-limited settings. While POCUS showed comparable diagnostic accuracy for VHD and CHD, TTE was able to detect more LVSD, perhaps picking up subtler changes and also because of the ease of performing biplane measurements on TTE compared with POCUS. This is the first and the largest study evaluating the use of POCUS compared with standard TTE to detect SHD. These findings are important as they align with global efforts to optimize antenatal cardiovascular screening in LMICs, where maternal mortality rates remain disproportionately high due to undiagnosed SHD.
The comparable detection rates of VHD (1.2% vs. 1.2%, P = 0.939) and CHD (0.6% vs. 0.3%, P = 0.023) between POCUS and TTE underscore the utility of portable ultrasound in identifying structural abnormalities requiring urgent intervention, such as rheumatic mitral stenosis or atrial septal defects. The portability of POCUS may facilitate broader screening access in antenatal clinics with limited infrastructure, potentially addressing disparities in cardiac care highlighted in the original PRESAP cohort. However, the lower detection of LVSD by POCUS (1.5% vs. 2.9%, P < 0.001) suggests that TTE remains critical for assessing subtle systolic dysfunction, which demands precise quantification of EF via Simpson’s biplane method. These findings also align with prior studies showing that handheld devices may underestimate EF in settings with high cardiac output states, such as pregnancy.
After propensity score matching, the difference in CHD detection between POCUS and TTE attenuated (0.4% vs. 0.6%, P = 0.093), suggesting that baseline demographic disparities—such as younger age and higher primiparity in the POCUS group—initially influenced outcomes. This reinforces the importance of standardized screening protocols to minimize confounding in heterogeneous populations. The sustained superiority of TTE for LVSD detection post-matching (2.8% vs. 1.5%, P < 0.001) underscores its role in identifying high-risk women who may benefit from guideline-directed therapies to mitigate adverse foetomaternal outcomes.
Our findings complement recent data from LMICs, including a large Indian registry where echocardiographic screening identified SHD in 2.5% of pregnant women, with rheumatic VHD constituting two-thirds of cases.12 The higher prevalence of LVSD in PRESAP (2.2% vs. 0.46% in India) may reflect regional differences in risk factors such as anaemia and multiparity, both prevalent in Pakistan.4,5 Notably, anaemia—present in 61.5% of women with LVSD in PRESAP—has been linked to subclinical myocardial injury, exacerbating systolic dysfunction in pregnancy.
The study’s focus on real-world clinical settings adds to its relevance, particularly in resource-limited environments where POCUS could be transformative. However, several limitations warrant consideration. The single-centre, non-randomized design may limit generalizability to other populations with differing healthcare contexts. The reliance on self-reported symptoms to define ‘asymptomatic’ status introduces potential bias, as subtle cardiovascular symptoms may be underreported. POCUS’s operator dependency raises concerns about diagnostic consistency, highlighting the need for standardized training; further, the experience and training of the operators performing and interpreting POCUS and TTE may have influenced the findings. Moreover, the lack of longitudinal outcome data limits the ability to assess the prognostic impact of early SHD detection. Lastly, potential interobserver variability in echocardiographic interpretations, especially with POCUS where biplane Simpson is difficult to do, may influence diagnostic accuracy.
Moving forward, the scalability of POCUS will depend on robust training programmes, quality assurance, and potentially leveraging tele-echocardiography for remote interpretation.13 Future studies should explore hybrid screening models combining POCUS for initial assessment and TTE for confirmatory evaluation of LVSD. Additionally, cost-effectiveness analyses are needed to guide policy decisions in LMICs, where allocating limited resources to scalable screening tools could reduce maternal mortality.
Conclusion
POCUS may be considered as an alternative to limited TTE for screening SHD in asymptomatic pregnant women, particularly in resource-limited settings. While standard TTE may offer superior detection of LVSD, POCUS is equally effective in identifying valvular and CHD. These findings support the integration of POCUS into routine antenatal care in high prevalence areas to facilitate early detection and management of SHD in pregnant women.
Clinical perspectives
Clinical competencies
Ability to evaluate the use of screening TTE and POCUS for the detection of structural CVD in pregnant women.
Translational outlook
In a large cohort of pregnant women being screened for structural CVD, POCUS was found to be as effective as TTE in diagnosing valvular and CHD though TTE was slightly better for picking up subtle decreases in LV dysfunction.
Supplementary Material
Acknowledgements
The authors wish to acknowledge the Association of Pakistani-Descent Cardiologists of North America (APCNA) for donating some of the POCUS devices used.
Contributor Information
Sabha Bhatti, Department of Adult Cardiology, National Institute of Cardiovascular Diseases (NICVD), Rafiqui (H.J.) Shaheed Road, Karachi 75510, Pakistan; Department of Cardiology, Rutgers Robert Wood Johnson University Hospital, New Brunswick, NJ 08901, USA.
Shakeela Naz, Department of Adult Cardiology, National Institute of Cardiovascular Diseases (NICVD), Rafiqui (H.J.) Shaheed Road, Karachi 75510, Pakistan.
Sumyia Gurmani, Department of Adult Cardiology, National Institute of Cardiovascular Diseases (NICVD), Rafiqui (H.J.) Shaheed Road, Karachi 75510, Pakistan.
Wajid Hussain, Department of Adult Cardiology, National Institute of Cardiovascular Diseases (NICVD), Rafiqui (H.J.) Shaheed Road, Karachi 75510, Pakistan.
Uzma Atif, Department of Adult Cardiology, National Institute of Cardiovascular Diseases (NICVD), Rafiqui (H.J.) Shaheed Road, Karachi 75510, Pakistan.
Shazia Ahmed, Department of Adult Cardiology, National Institute of Cardiovascular Diseases (NICVD), Rafiqui (H.J.) Shaheed Road, Karachi 75510, Pakistan.
Kiran Zahra, Department of Adult Cardiology, National Institute of Cardiovascular Diseases (NICVD), Rafiqui (H.J.) Shaheed Road, Karachi 75510, Pakistan.
Haleema Yasmin, Department of Obstetrics and Gynecology, Jinnah Postgraduate Medical Centre (JPMC), Karachi, Pakistan.
Ali Khan, Department of Medicine, Brown University, Providence, RI, USA.
Musa Karim, Department of Adult Cardiology, National Institute of Cardiovascular Diseases (NICVD), Rafiqui (H.J.) Shaheed Road, Karachi 75510, Pakistan.
Abdul Hakeem, Department of Adult Cardiology, National Institute of Cardiovascular Diseases (NICVD), Rafiqui (H.J.) Shaheed Road, Karachi 75510, Pakistan.
Supplementary data
Supplementary data are available at European Heart Journal – Imaging Methods and Practice online.
Author contributions
S.B., S.N., S.G., and H.Y. contributed to the concept and design of the study; S.B. and M.K. contributed to the analysis and interpretation of the data; S.B., S.N., S.G., W.H., S.A., K.Z., and U.A. collected the data and drafted the manuscript; and S.B., A.K., and A.H. critically analysed for content. All authors approved the final draft to the manuscript.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of the National Institute of Cardiovascular Diseases (NICVD), Karachi, Pakistan (approval number: IRB-33/2023). Verbal informed consent was obtained from all the patients regarding their participation in the study and publication of data while maintaining confidentiality and anonymity. Due to observational nature of the study, IRB waived the written consent and verbal consent was approved by the IRB.
Funding
None declared.
Data availability
Data and material will be available upon request.
Lead author biography

Prof. Sabha Bhatti, MD FACC FASE FASNC Professor Cardiology NICVD Director Cardiac Imaging NICVD Program Director Cardiac Imaging Program NICVD Adjunct faculty, Rutgers Robert Wood Johnson Medical School, NJ, USA
After completing my training and working in the USA for 10 years, I moved to Pakistan 2 years ago for family reasons and to work in the public sector to help serve the very under privileged patients here. I immediately noted that cardiac imaging was very underdeveloped as a subspecialty here in Pakistan. In addition, there are so many neglected diseases such as valve diseases, specifically rheumatic heart disease, and cardiomyopathies such as peripartum cardiomyopathy. Patients with these conditions often present very late due to lack of access to care and awareness. Given my background in cardiac imaging and my interest in valve disease and cardiomyopathies, I helped start the NICVD valve centre and also started the cardio-obstetrics service line (all free of cost to the patient with patients coming from all over Pakistan) in addition to an Imaging Fellowship and a school of Sonography. Rheumatic heart disease patients should be diagnosed much earlier (in the asymptomatic latent phase) so that they can be started on secondary prophylaxis with PenG. Screening in schools may not capture these young females since they usually get married early and drop out. Based on this, we decided to screen for cardiovascular disease (CVD) in the antenatal clinics. In a pilot study that we already started earlier this year, all patients in an antenatal clinic were screened with a limited echo (using portable but not handheld echo machines) regardless of symptoms. We found that many patients had undiagnosed CVD. These patients were started on treatment and followed in our Cardio-obstetrics Clinic. We would like to expand this pilot to using point-of-care ultrasound and carry this out in more than just a one hospital setting.
Prior Research Experience
2022 Valve Registry: A centre-based registry collecting data on all patients referred for management of severe valve disease. Several ongoing projects such as mitral stenosis scoring systems, 2D versus 3D areas, and anticoagulation strategies. RHD screening in schools and antenatal clinics in underserved areas
2023: CardioObstetrics Registry of all CVD in pregnancy. Includes RHD screening
2023: CardioOncology Registry
2019–22: Co-PI on several structural interventional trials at Rutgers RWJ, cardio-oncology studies
2020–21: Echo findings in patients with severe COVID-19 at Rutgers RWJ
2012–17: Echo and MRI-based studies in cardio-oncology, specifically multiple myeloma and amyloidosis
2008–11: Cardiac MRI strain imaging in patients post stem cell transplant
2006–08: Nuclear cardiology VA study of prognostic value of SPECT in CKD
2004–05: Full-time 1-year research fellow position at Aga Khan Hospital in Nairobi
References
- 1. World Health Organization . Trends in Maternal Mortality 2000 to 2020: Estimates by WHO, UNICEF, UNFPA, World Bank Group and UNDESA/Population Division: WHO Press, World Health Organization. Geneva, Switzerland; 2023. [Google Scholar]
- 2. Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, Blomström-Lundqvist C, Cifkova R, De Bonis M et al. 2018 ESC guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J 2018;39:3165–241. [DOI] [PubMed] [Google Scholar]
- 3. Davis MB, Arany Z, McNamara DM, Goland S, Elkayam U. Peripartum cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol 2020;75:207–21. [DOI] [PubMed] [Google Scholar]
- 4. Bhutta ZA, Hafeez A, Rizvi A, Ali N, Khan A, Ahmad F et al. Reproductive, maternal, newborn, and child health in Pakistan: challenges and opportunities. Lancet 2013;381:2207–18. [DOI] [PubMed] [Google Scholar]
- 5. Bhatti S, Naz S, Gurmani S, Yasmin H, Atif U, Karim M et al. Prospective Pakistan registry of echocardiographic screening in asymptomatic pregnant women. JACC Adv 2024;3:101215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Spencer KT, Kimura BJ, Korcarz CE, Pellikka PA, Rahko PS, Siegel RJ. Focused cardiac ultrasound: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr 2013;26:567–81. [DOI] [PubMed] [Google Scholar]
- 7. Kimura BJ, Shaw DJ, Amundson SA, Phan JN, Blanchard DG, DeMaria AN. Cardiac limited ultrasound examination techniques to augment the bedside cardiac physical examination. J Ultrasound Med 2015;34:1683–90. [DOI] [PubMed] [Google Scholar]
- 8. Sanghavi M, Rutherford JD. Cardiovascular physiology of pregnancy. Circulation 2014;130:1003–8. [DOI] [PubMed] [Google Scholar]
- 9. Ragusa M, Gaudrea-Simard M, Ruller S, Kaka S, Evans J. The use of cardiac point of care ultrasound (pocus) versus inpatient transthoracic echocardiogram (TTE) in a large tertiary care centre: a comparability and appropriateness study. Can J Cardiol 2024;40:S124–5. [Google Scholar]
- 10. Hammadah M, Ponce C, Sorajja P, Cavalcante JL, Garcia S, Gössl M. Point-of-care ultrasound: closing guideline gaps in screening for valvular heart disease. Clin Cardiol 2020;43:1368–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Elkayam U, Akhter MW, Singh H, Khan S, Bitar F, Hameed A et al. Pregnancy-associated cardiomyopathy: clinical characteristics and a comparison between early and late presentation. Circulation 2005;111:2050–5. [DOI] [PubMed] [Google Scholar]
- 12. Patel A, Ranard LS, Aranoff N, Rahim H, Vanukuru R, Tangalapally S et al. Use of routine echocardiographic screening for structural heart disease in at-risk pregnant women in India. Cardiovascular Imaging 2021;14:692–3. [DOI] [PubMed] [Google Scholar]
- 13. Fortuni F, Ciliberti G, De Chiara B, Conte E, Franchin L, Musella F et al. Advancements and applications of artificial intelligence in cardiovascular imaging: a comprehensive review. Eur Heart J Imaging Methods Pract 2024;2:qyae136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and material will be available upon request.