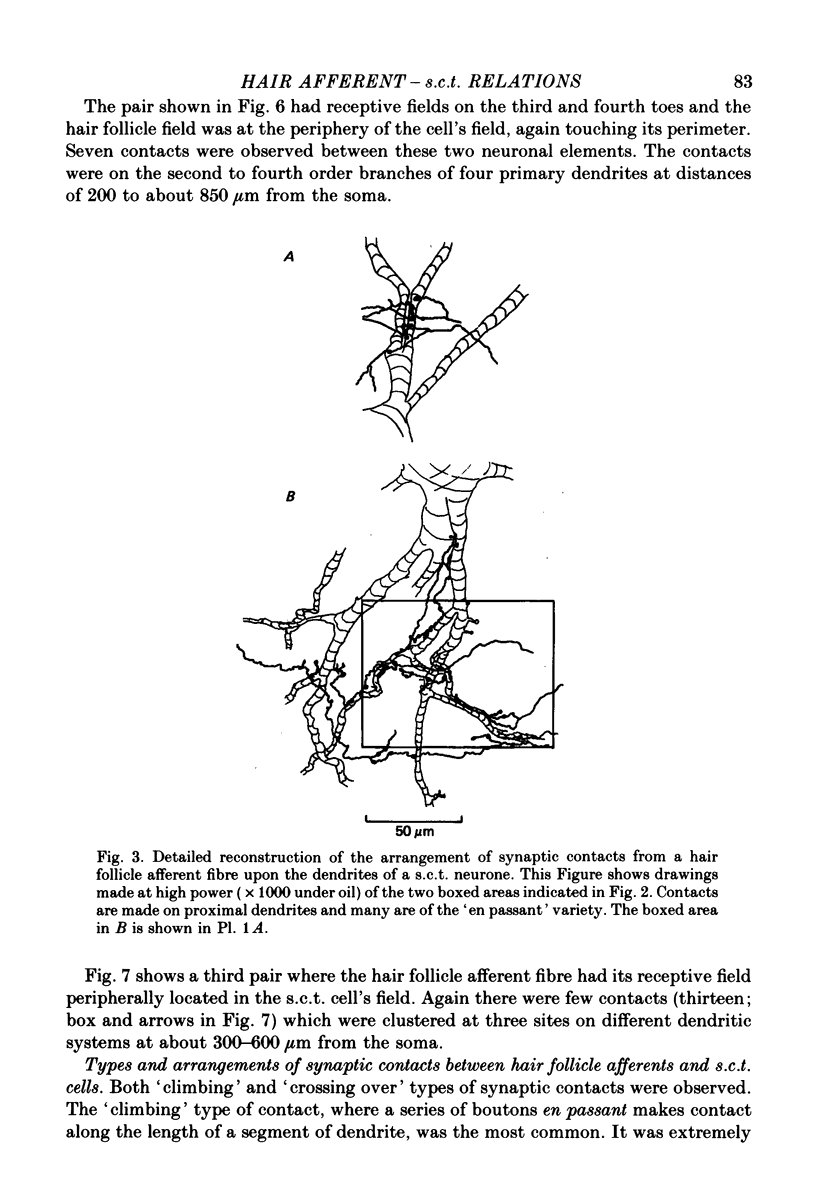
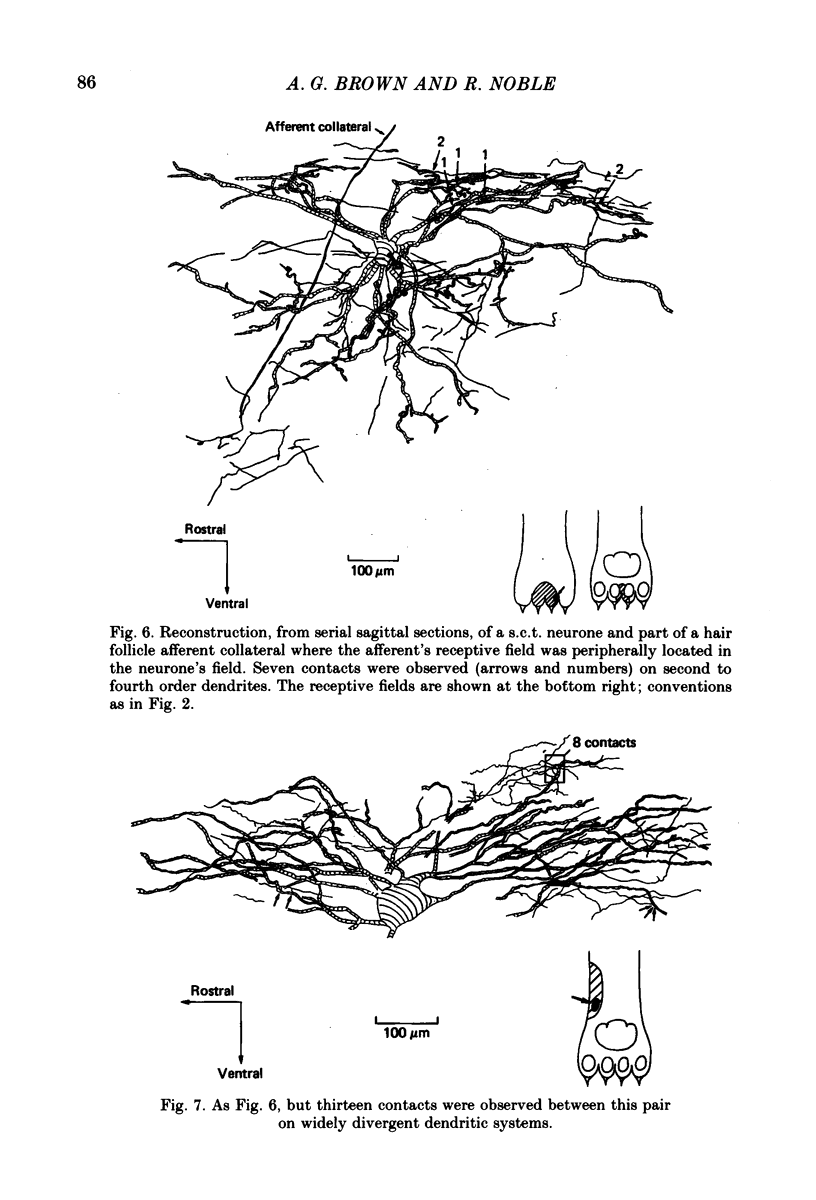
Abstract
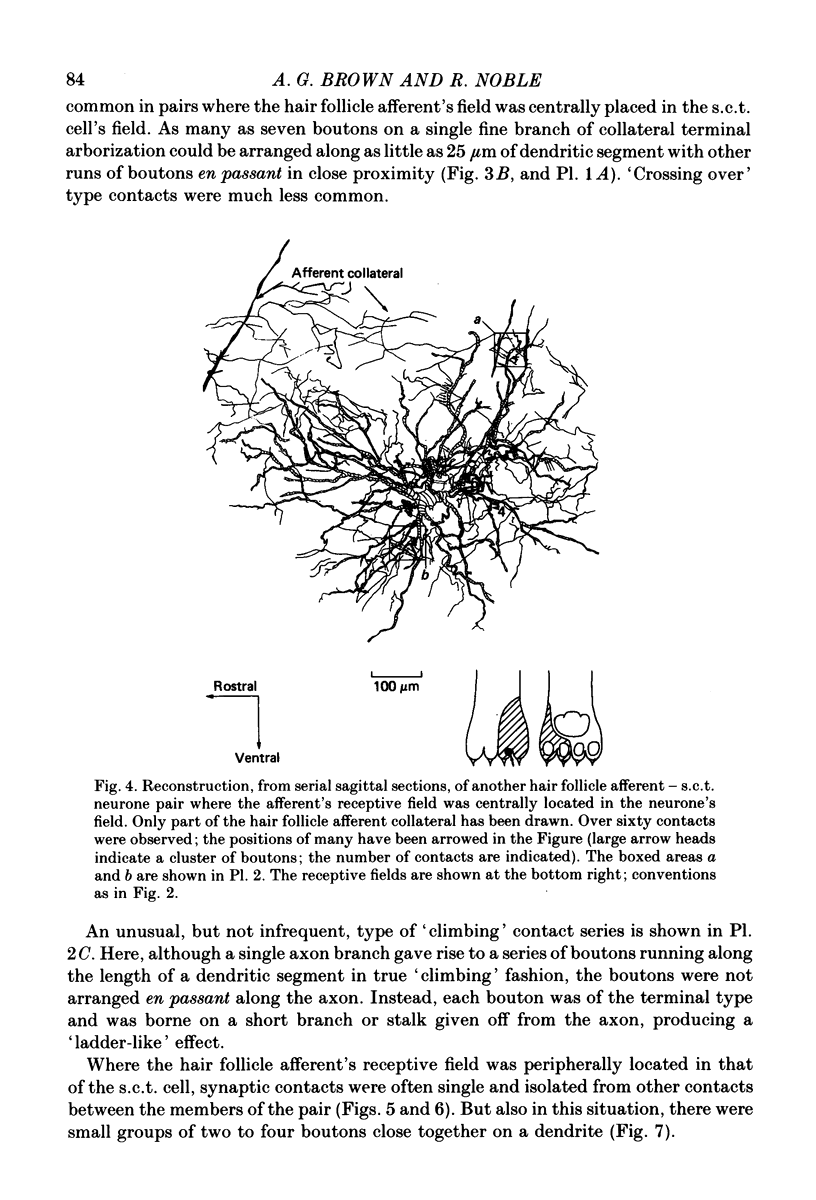
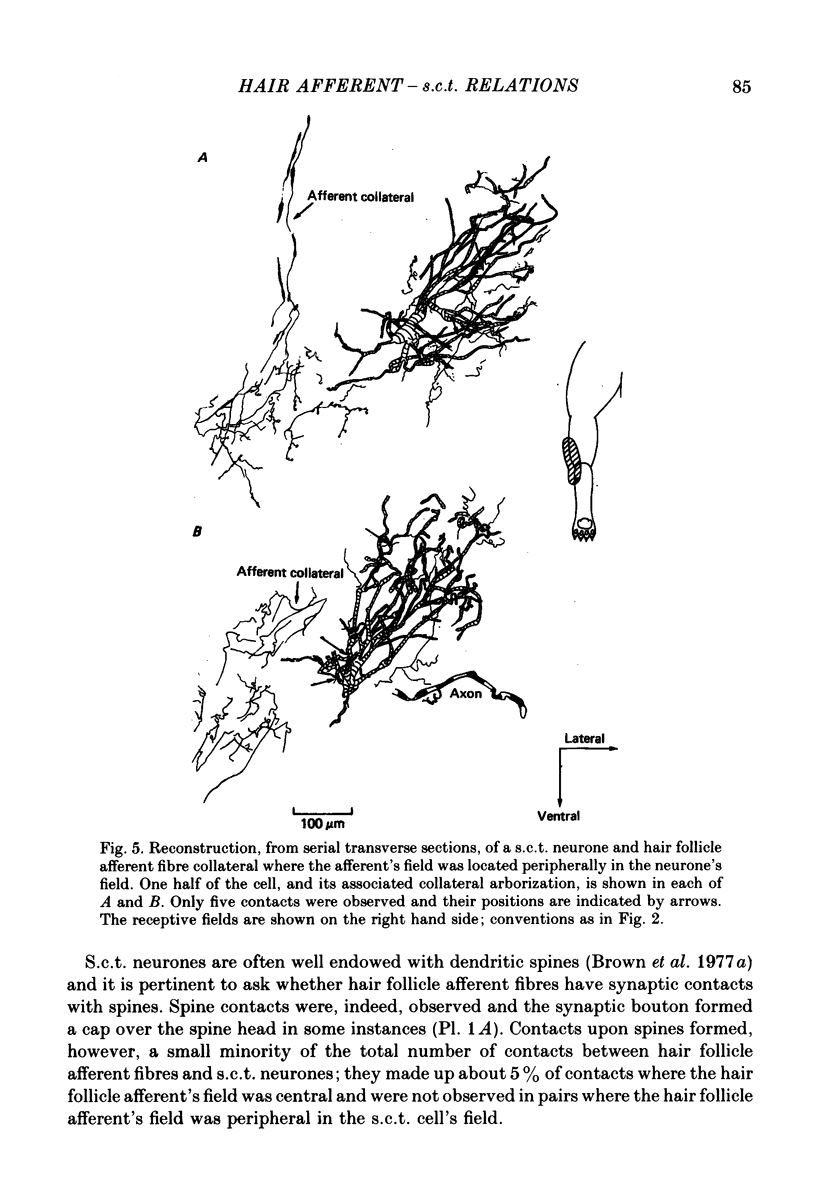
1. Relationships between the terminal arborizations of hair follicle afferent fibres and dendritic trees of spinocervical tract (s.c.t.) neurones were studied using intra-axonal and intracellular injections of horseradish peroxidase in chloralose-anaesthetized, paralysed cats. 2. Seventeen afferent-neurone pairs were successfully stained and their receptive fields determined. Ten of the pairs had s.c.t. neurones with a field containing that of the hair follicle afferent and seven pairs had separate fields on the hind limb. 3. Where the afferent fibre's field was outside the neurone's field there were no indications of synaptic contacts between the two neuronal elements. 4. Synaptic contacts were always observed (at the light microscope level) for the ten pairs with the hair afferent's receptive field contained within the s.c.t. cell's field. Contacts were always made by the branches of only a single collateral from the hair follicle afferent fibre. The numbers and locations of synaptic contacts were related to the relative positions of the receptive field: where the hair follicle afferent's field was centrally placed there were many (forty to sixty) contacts on proximal dendrites; where the hair follicle afferent's field was peripherally placed in the s.c.t. cell's receptive field there were few contacts (two to thirteen) and these were peripherally placed on the dendritic tree. Where the primary afferent fibre had a centrally placed field contacts upon dendritic spines were observed. 5. The results are discussed in terms of the synthesis of receptive fields and the organization of neuronal connexions within the mammalian spinal cord.
Full text
PDF


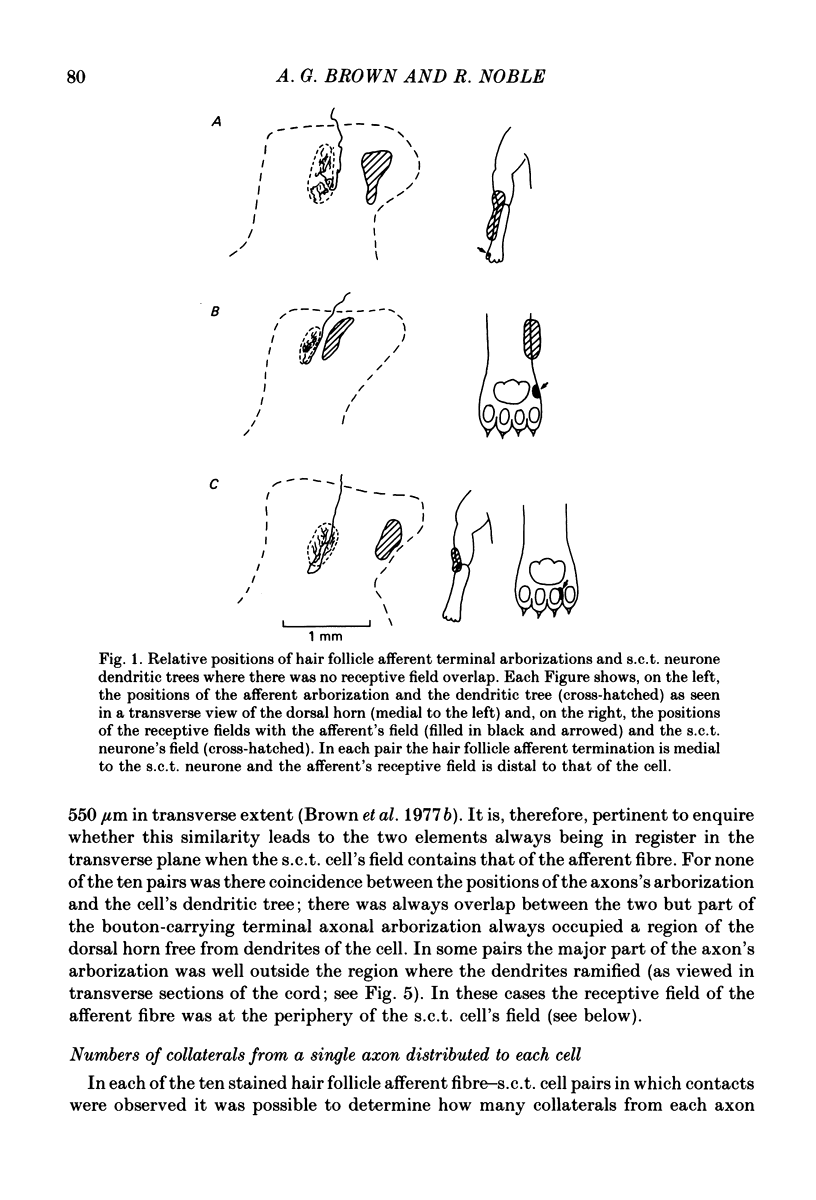

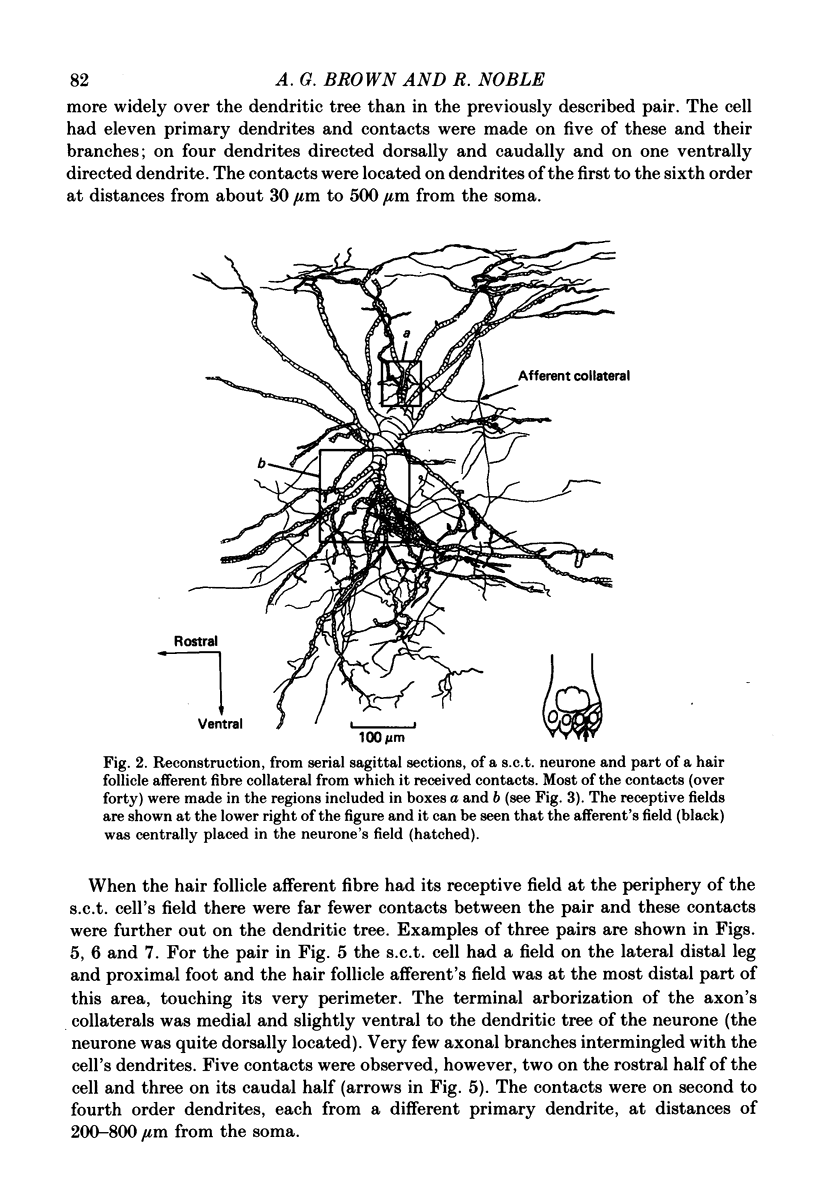




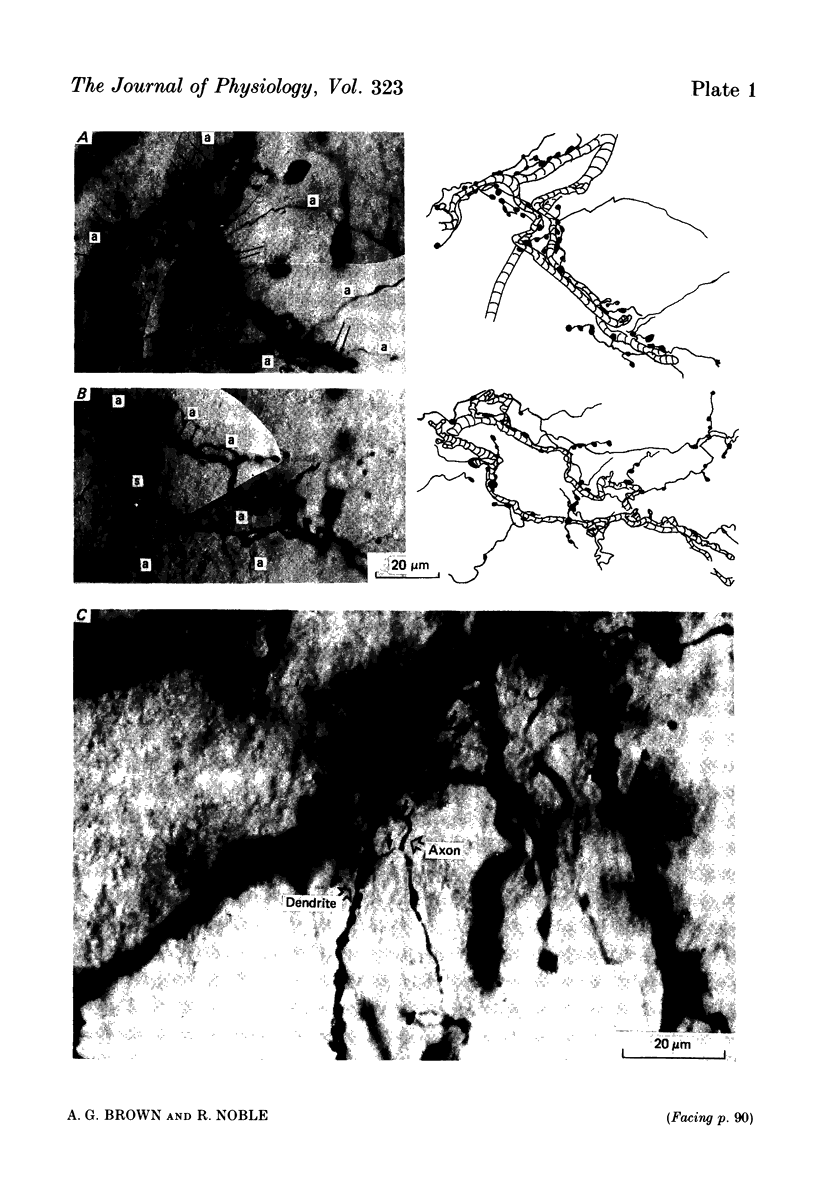
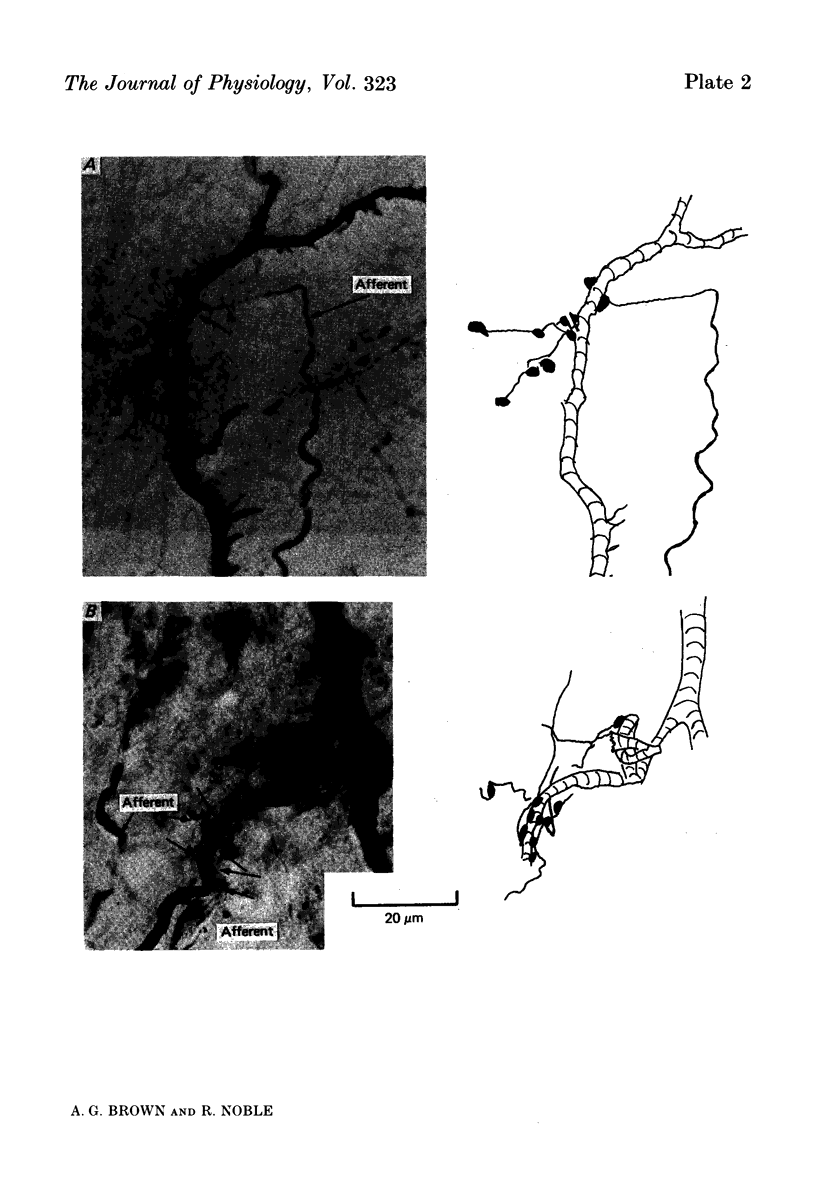

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P., Silfvenius H., Sundberg S. H., Sveen O. A comparison of distal and proximal dendritic synapses on CAi pyramids in guinea-pig hippocampal slices in vitro. J Physiol. 1980 Oct;307:273–299. doi: 10.1113/jphysiol.1980.sp013435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbaum A. I., Wall P. D. Chronic changes in the response of cells in adult cat dorsal horn following partial deafferentation: the appearance of responding cells in a previously non-responsive region. Brain Res. 1976 Nov 5;116(2):181–204. doi: 10.1016/0006-8993(76)90899-4. [DOI] [PubMed] [Google Scholar]
- Brown A. G. Effects of descending impulses on transmission through the spinocervical tract. J Physiol. 1971 Dec;219(1):103–125. doi: 10.1113/jphysiol.1971.sp009652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Fyffe R. E. Direct observations on the contacts made between Ia afferent fibres and alpha-motoneurones in the cat's lumbosacral spinal cord. J Physiol. 1981;313:121–140. doi: 10.1113/jphysiol.1981.sp013654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Fyffe R. E. Form and function of dorsal horn neurones with axons ascending the dorsal columns in cat. J Physiol. 1981 Dec;321:31–47. doi: 10.1113/jphysiol.1981.sp013970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Iggo A. A quantitative study of cutaneous receptors and afferent fibres in the cat and rabbit. J Physiol. 1967 Dec;193(3):707–733. doi: 10.1113/jphysiol.1967.sp008390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Kirk E. J., Martin H. F., 3rd Descending and segmental inhibition of transmission through the spinocervical tract. J Physiol. 1973 May;230(3):689–705. doi: 10.1113/jphysiol.1973.sp010212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Martin H. F., 3rd Activation of descending control of the spinocervical tract by impulses ascending the dorsal columns and relaying through the dorsal column nuclei. J Physiol. 1973 Dec;235(2):535–550. doi: 10.1113/jphysiol.1973.sp010402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Noble R. Relationships between hair follicle afferent fibres and spinocervical tract neurones in the cat [proceedings]. J Physiol. 1979 Nov;296:38P–39P. [PubMed] [Google Scholar]
- Brown A. G., Rose P. K., Snow P. J. Dendritic trees and cutaneous receptive fields of adjacent spinocervical tract neurones in the cat. J Physiol. 1980 Mar;300:429–440. doi: 10.1113/jphysiol.1980.sp013170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Rose P. K., Snow P. J. The morphology of hair follicle afferent fibre collaterals in the spinal cord of the cat. J Physiol. 1977 Nov;272(3):779–797. doi: 10.1113/jphysiol.1977.sp012073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Rose P. K., Snow P. J. The morphology of spinocervical tract neurones revealed by intracellular injection of horseradish peroxidase. J Physiol. 1977 Sep;270(3):747–764. doi: 10.1113/jphysiol.1977.sp011980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor M., Merrill E. G., Wall P. D. Dorsal horn cells that respond to stimulation of distant dorsal roots. J Physiol. 1977 Sep;270(2):519–531. doi: 10.1113/jphysiol.1977.sp011966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor M., Wall P. D. Reorganisation of spinal cord sensory map after peripheral nerve injury. Nature. 1978 Nov 2;276(5683):75–76. doi: 10.1038/276075a0. [DOI] [PubMed] [Google Scholar]
- Hanker J. S., Yates P. E., Metz C. B., Rustioni A. A new specific, sensitive and non-carcinogenic reagent for the demonstration of horseradish peroxidase. Histochem J. 1977 Nov;9(6):789–792. doi: 10.1007/BF01003075. [DOI] [PubMed] [Google Scholar]
- Mark R. F. Chemospecific synaptic repression as a possible memory store. Nature. 1970 Jan 10;225(5228):178–179. doi: 10.1038/225178b0. [DOI] [PubMed] [Google Scholar]
- Mark R. F. Selective innervation of muscle. Br Med Bull. 1974 May;30(2):122–126. doi: 10.1093/oxfordjournals.bmb.a071181. [DOI] [PubMed] [Google Scholar]
- Mendell L. M., Sassoon E. M., Wall P. D. Properties of synaptic linkage from long ranging afferents onto dorsal horn neurones in normal and deafferented cats. J Physiol. 1978 Dec;285:299–310. doi: 10.1113/jphysiol.1978.sp012572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill E. G., Wall P. D. Factors forming the edge of a receptive field: the presence of relatively ineffective afferent terminals. J Physiol. 1972 Nov;226(3):825–846. doi: 10.1113/jphysiol.1972.sp010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pubols L. M., Goldberger M. E. Recovery of function in dorsal horn following partial deafferentation. J Neurophysiol. 1980 Jan;43(1):102–117. doi: 10.1152/jn.1980.43.1.102. [DOI] [PubMed] [Google Scholar]
- Snow P. J., Rose P. K., Brown A. G. Tracing axons and axon collaterals of spinal neurons using intracellular injection of horseradish peroxidase. Science. 1976 Jan 23;191(4224):312–313. doi: 10.1126/science.54936. [DOI] [PubMed] [Google Scholar]
- Wall P. D. The presence of ineffective synapses and the circumstances which unmask them. Philos Trans R Soc Lond B Biol Sci. 1977 Apr 26;278(961):361–372. doi: 10.1098/rstb.1977.0048. [DOI] [PubMed] [Google Scholar]