Abstract
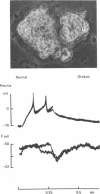
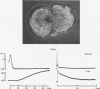
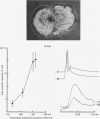
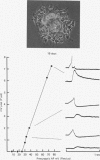
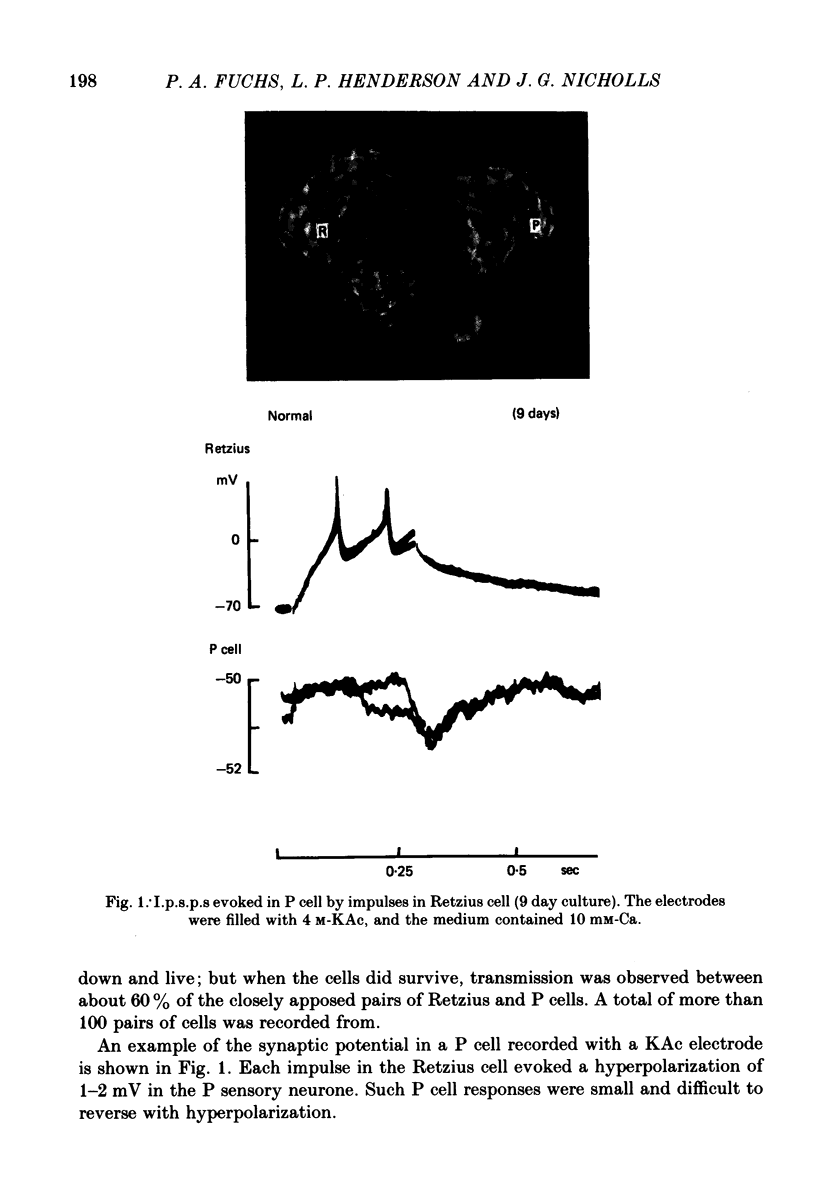
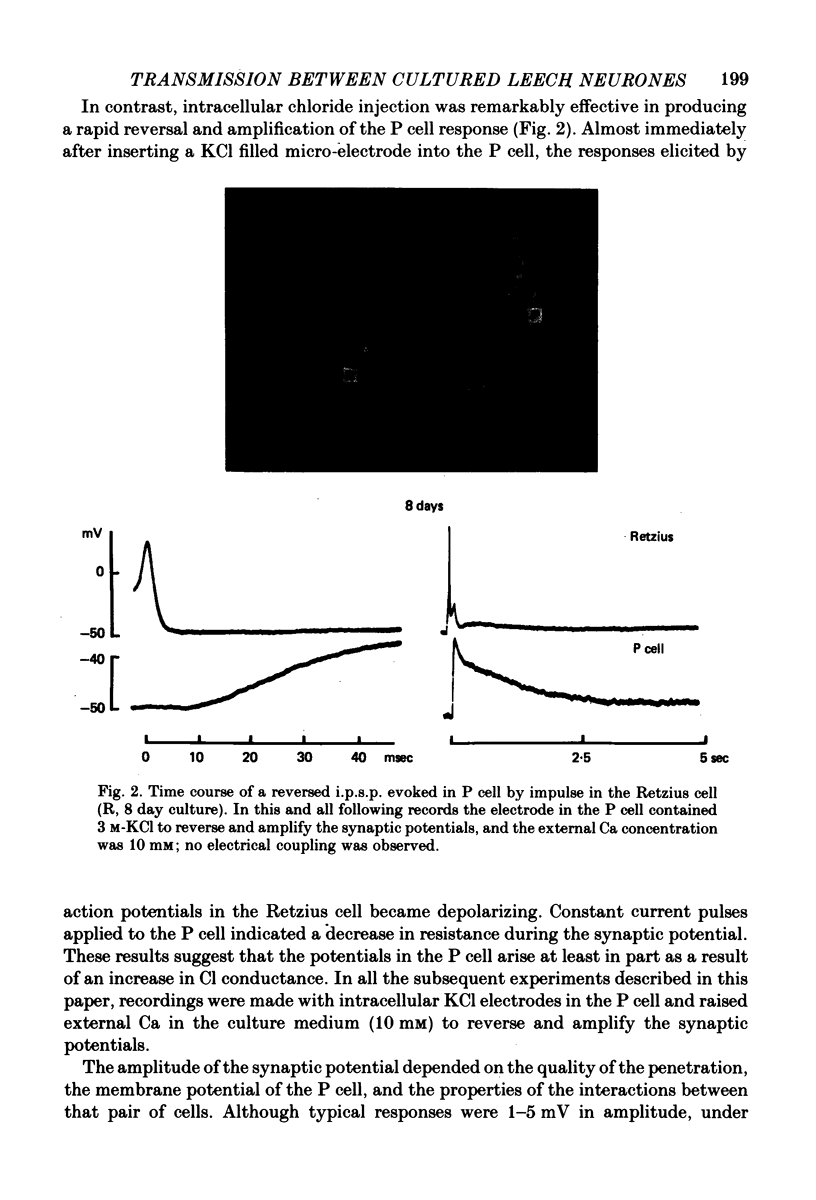
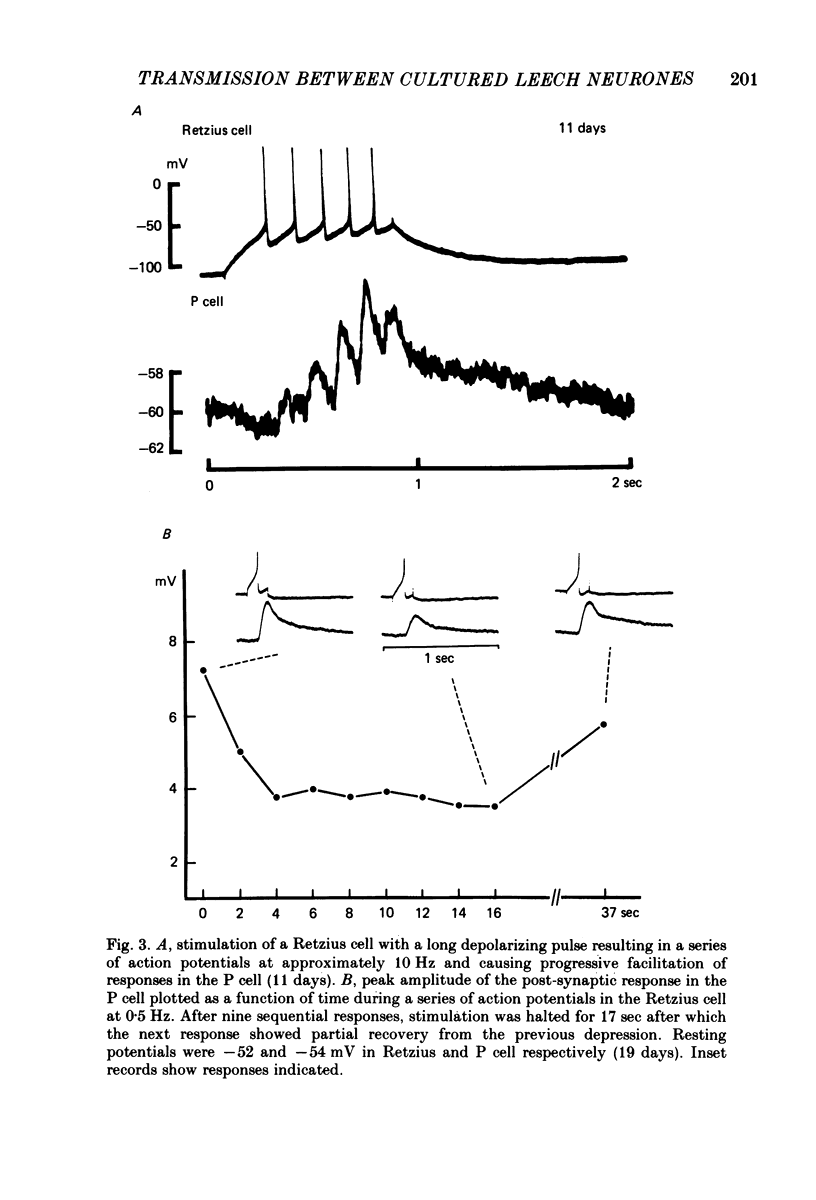
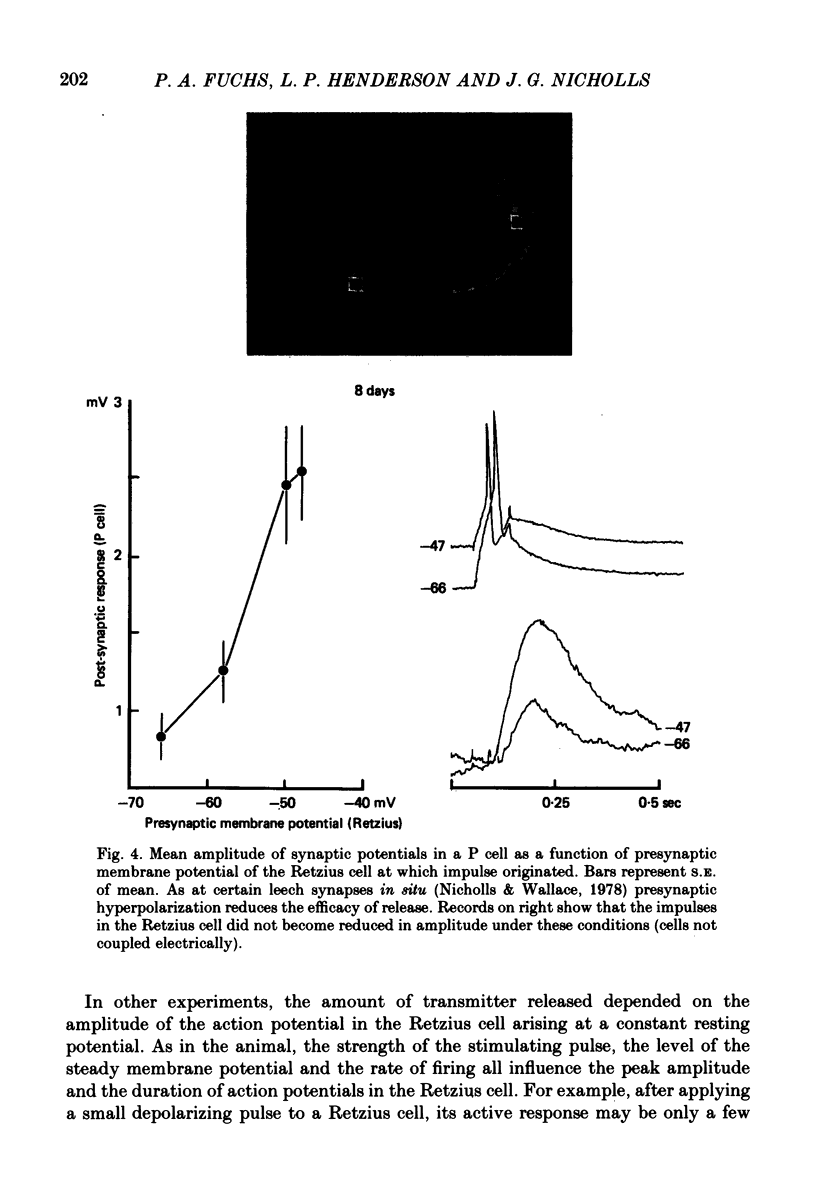
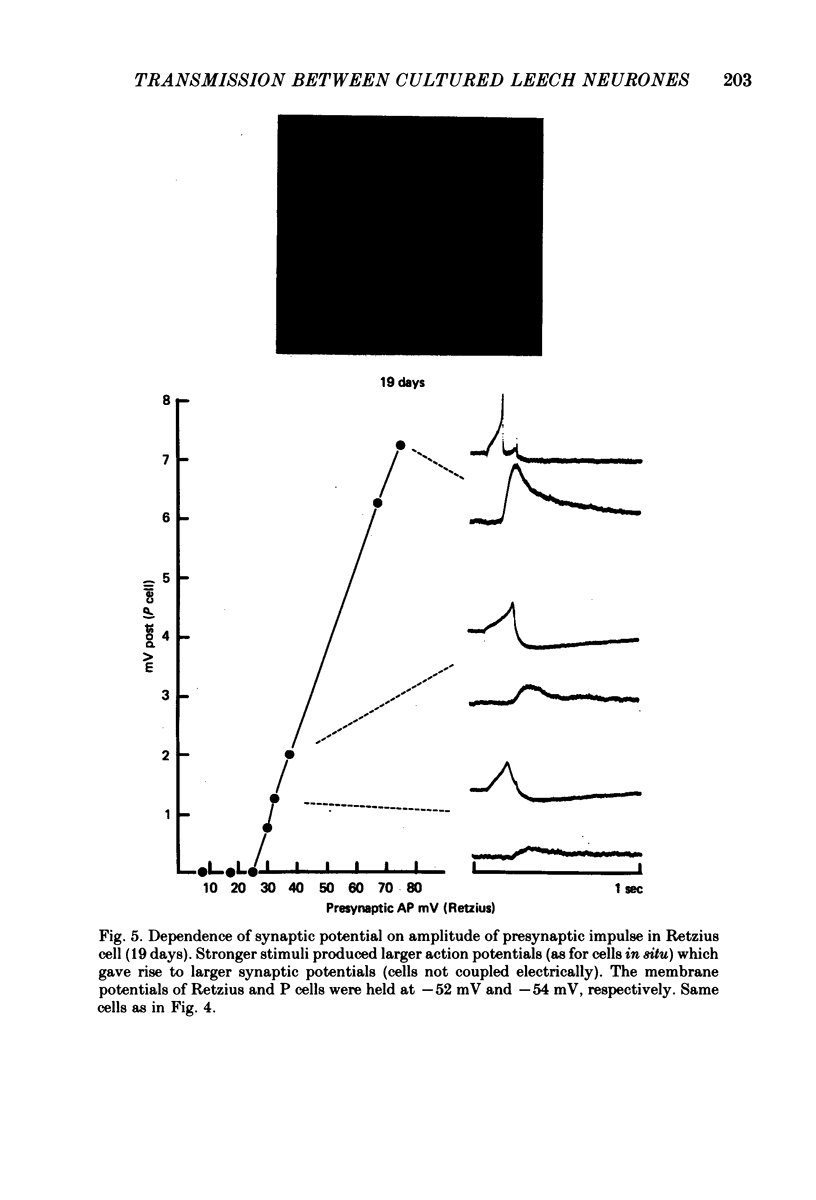
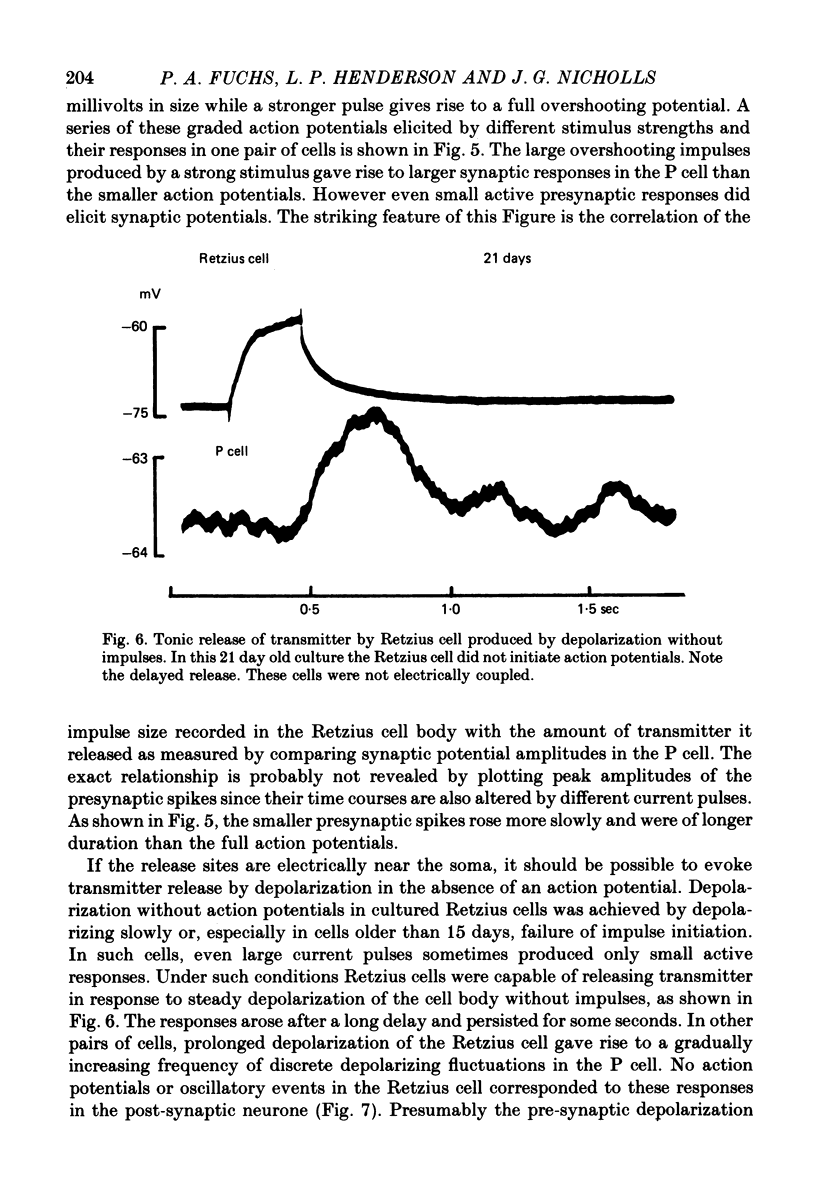
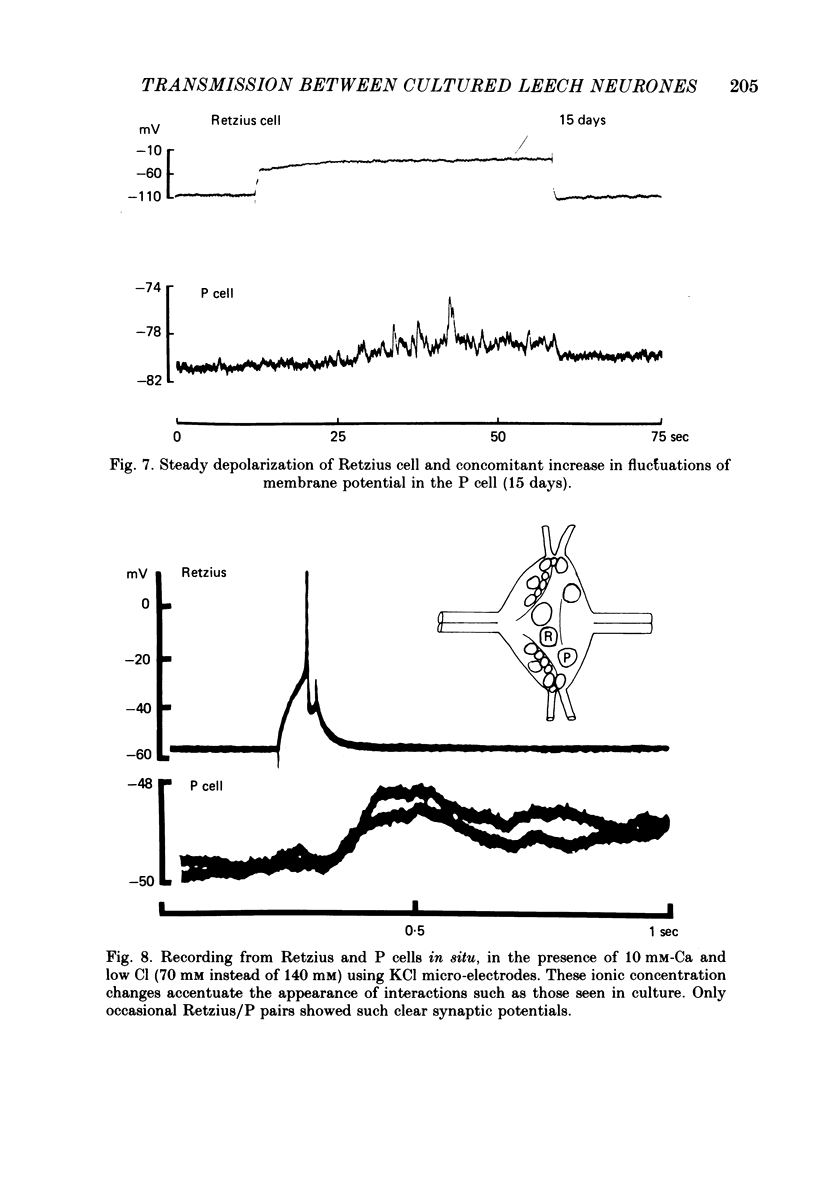
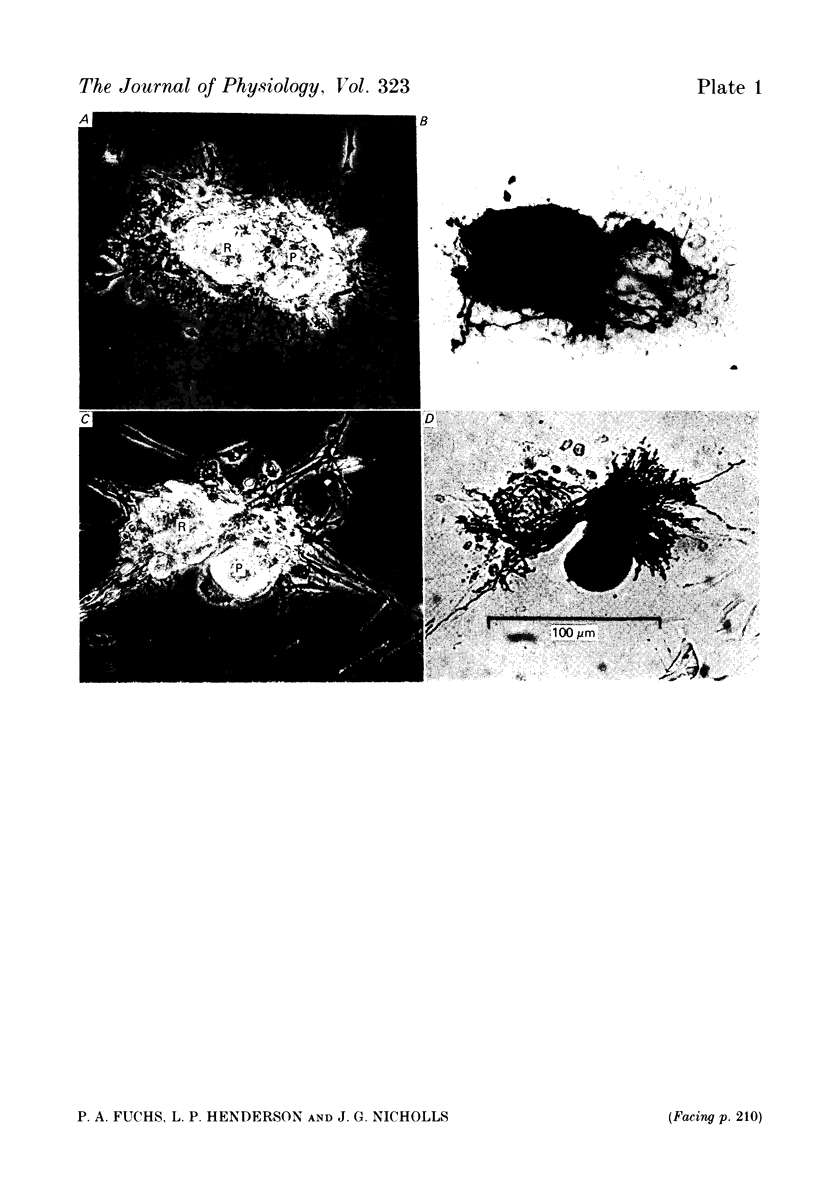
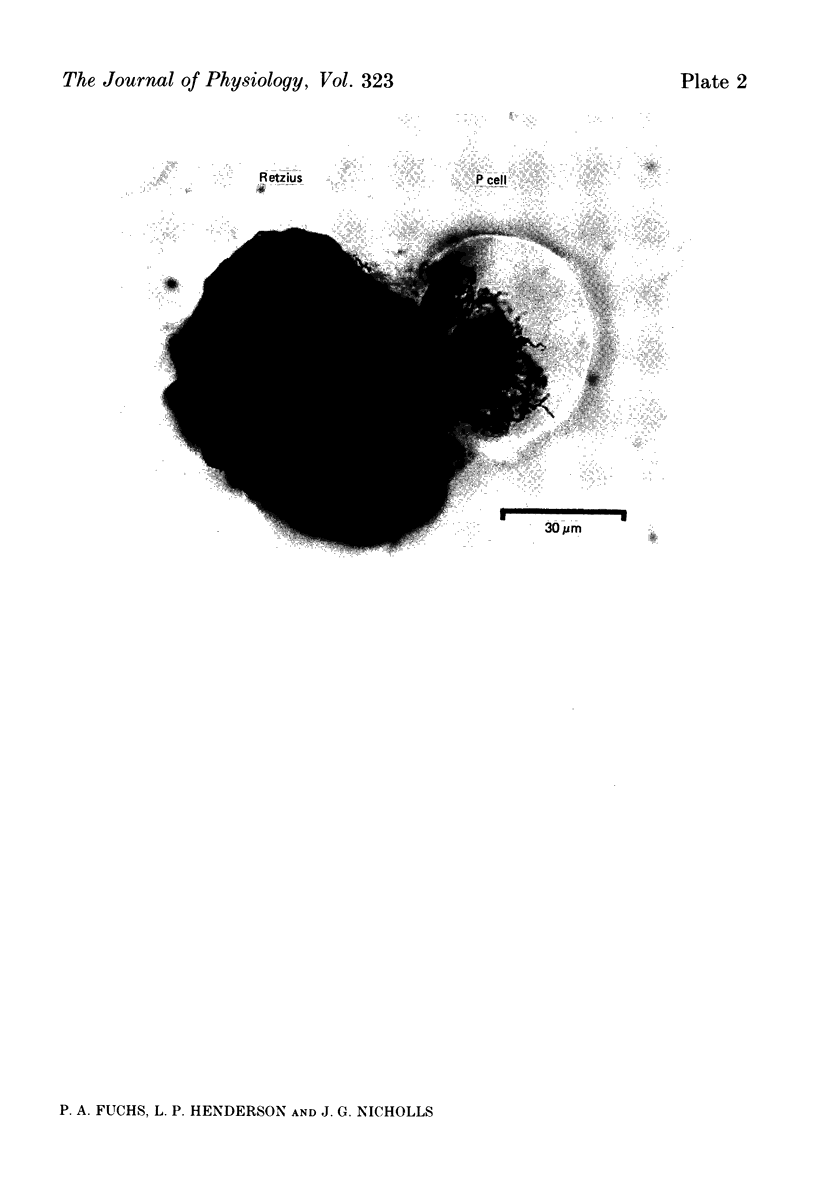
1. Chemical synaptic transmission develops between individual identified neurones dissected from leech ganglia and maintained in culture. Impulses in Retzius cells give rise to hyperpolarizing synaptic potentials in pressure (P) sensory cells. In suitable medium the potentials develop by 3 days and can be observed for more than 3 weeks. 2. The synaptic potentials occur after a synaptic delay, exhibit facilitation and depression and are reversed by hyperpolarization. The blocking effects of reduced calcium and raised magnesium concentrations in the bathing fluid provide additional evidence for the chemical nature of transmission. 3. An increase in chloride conductance is involved in the generation of the synaptic potential in the P cell. With high intracellular Cl in the post-synaptic cell, the synaptic potentials become reversed and amplified. The amplitudes of these reversed responses range from 1 to 20 mV with a falling phase lasting for seconds. 4. Changes in the membrane potential of the presynaptic cell that modify the amplitude and duration of the action potential influence the efficacy of transmission. In addition, impulses in Retzius cells initiated from hyperpolarized values of membrane potential evoke smaller synaptic potentials in the P cells than impulses arising from a depolarized level. 5. With neurones placed directly next to one another in the dish, maintained depolarization of the presynaptic Retzius cell in the absence of conducted action potentials gives rise to slow synaptic potentials in the P cells. In some pairs, the response in the P cell consists of a marked increase in 'noise'. 6. Injection of horseradish peroxidase into the Retzius cell reveals neurites with distinctive varicosities growing over the P cell.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burrows M. Graded synaptic interactions between local premotor interneurons of the locust. J Neurophysiol. 1979 Jul;42(4):1108–1123. doi: 10.1152/jn.1979.42.4.1108. [DOI] [PubMed] [Google Scholar]
- Dennis M. J., Ziskind-Conhaim L., Harris A. J. Development of neuromuscular junctions in rat embryos. Dev Biol. 1981 Jan 30;81(2):266–279. doi: 10.1016/0012-1606(81)90290-6. [DOI] [PubMed] [Google Scholar]
- Ehinger B., Falck B., Myhrberg H. E. Biogenic monoamines in Hirudo medicinalis. Histochemie. 1968;15(2):140–149. doi: 10.1007/BF00306364. [DOI] [PubMed] [Google Scholar]
- Fuchs P. A., Nicholls J. G., Ready D. F. Membrane properties and selective connexions of identified leech neurones in culture. J Physiol. 1981 Jul;316:203–223. doi: 10.1113/jphysiol.1981.sp013783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb D. I., Glaser L. Cellular recognition during neural development. Annu Rev Neurosci. 1980;3:303–318. doi: 10.1146/annurev.ne.03.030180.001511. [DOI] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE EFFECT OF CALCIUM ON ACETYLCHOLINE RELEASE FROM MOTOR NERVE TERMINALS. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:496–503. doi: 10.1098/rspb.1965.0017. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. A study of synaptic transmission in the absence of nerve impulses. J Physiol. 1967 Sep;192(2):407–436. doi: 10.1113/jphysiol.1967.sp008307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe J. S., Marty A. Certain slow synaptic responses: their properties and possible underlying mechanisms. Annu Rev Biophys Bioeng. 1980;9:437–465. doi: 10.1146/annurev.bb.09.060180.002253. [DOI] [PubMed] [Google Scholar]
- Kleinhaus A. L., Prichard J. W. Calcium dependent action potentials produced in leech Retzius cells by tetraethylammonium chloride. J Physiol. 1975 Mar;246(2):351–369. doi: 10.1113/jphysiol.1975.sp010894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lent C. M. Retzius cells: neuroeffectors controlling mucus release by the leech. Science. 1973 Feb 16;179(4074):693–696. doi: 10.1126/science.179.4074.693. [DOI] [PubMed] [Google Scholar]
- Muller K. J. Synapses between neurones in the central nervous system of the leech. Biol Rev Camb Philos Soc. 1979 May;54(2):99–134. doi: 10.1111/j.1469-185x.1979.tb00869.x. [DOI] [PubMed] [Google Scholar]
- Nicholls J. G., Baylor D. A. Specific modalities and receptive fields of sensory neurons in CNS of the leech. J Neurophysiol. 1968 Sep;31(5):740–756. doi: 10.1152/jn.1968.31.5.740. [DOI] [PubMed] [Google Scholar]
- Nicholls J., Wallace B. G. Modulation of transmission at an inhibitory synapse in the central nervous system of the leech. J Physiol. 1978 Aug;281:157–170. doi: 10.1113/jphysiol.1978.sp012414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ready D. F., Nicholls J. Identified neurones isolated from leech CNS make selective connections in culture. Nature. 1979 Sep 6;281(5726):67–69. doi: 10.1038/281067a0. [DOI] [PubMed] [Google Scholar]
- Rude S., Coggeshall E., Van Orden L. S., 3rd Chemical and ultrastructural identification of 5-hydroxytryptamine in an identified neuron. J Cell Biol. 1969 Jun;41(3):832–854. doi: 10.1083/jcb.41.3.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent P. B. Synthesis of acetylcholine by excitatory motoneurons in central nervous system of the leech. J Neurophysiol. 1977 Mar;40(2):453–460. doi: 10.1152/jn.1977.40.2.453. [DOI] [PubMed] [Google Scholar]
- Stuart A. E., Hudspeth A. J., Hall Z. W. Vital staining of specific monoamine-containing cells in the leech nervous system. Cell Tissue Res. 1974;153(1):55–61. doi: 10.1007/BF00225445. [DOI] [PubMed] [Google Scholar]
- Waziri R. Presynaptic electrical coupling in Aplysia: effects on postsynaptic chemical transmission. Science. 1977 Feb 25;195(4280):790–792. doi: 10.1126/science.189390. [DOI] [PubMed] [Google Scholar]