Abstract
In α-chloralose-anesthetized rats, changes in the blood oxygenation level-dependent (BOLD) functional MRI (fMRI) signal (ΔS/S), and the relative spiking frequency of a neuronal ensemble (Δν/ν) were measured in the somatosensory cortex during forepaw stimulation from two different baselines. Changes in cerebral oxygen consumption (ΔCMRO2/CMRO2) were derived from the BOLD signal (at 7T) by independent determinations in cerebral blood flow (ΔCBF/CBF) and volume (ΔCBV/CBV). The spiking frequency was measured by extracellular recordings in layer 4. Changes in all three parameters (CMRO2, ν, and S) were greater from the lower baseline (i.e., deeper anesthesia). For both baselines, ΔCMRO2/CMRO2 and Δν/ν were approximately one order of magnitude larger than ΔS/S. The final values of CMRO2 and ν reached during stimulation were approximately the same from both baselines. If only increments were required to support functions then their magnitudes should be independent of the baseline. In contrast, if particular magnitudes of activity were required, then sizes of increments should inversely correlate with the baseline (being larger from a lower baseline). The results show that particular magnitudes of activity support neural function. The disregard of baseline activity in fMRI experiments by differencing removes a large and necessary component of the total activity. Implications of these results for understanding brain function and fMRI experiments are discussed.
Although much is known about the energetic costs of peripheral neurons (1, 2), only recently have quantitative relations between specific neuronal processes and their energetic costs been developed for the cerebral cortex (3, 4). A stoichiometric relation between the cerebral metabolic rate of oxygen consumption (CMRO2) and the glutamate–glutamine neurotransmitter flux (Vcyc) has been obtained by 13C magnetic resonance spectroscopy (MRS) measurements in vivo (5, 6). These 13C MRS experiments followed 13C label flow from [1-13C]glucose into neuronal glutamate and astrocytic glutamine pools to show that ΔCMRO2/CMRO2 ≈ ΔVcyc/Vcyc in rat brain under moderately anesthetized conditions. These experiments showed that ≈80% of brain energy consumption is dedicated to neuronal activity under resting awake conditions.
Recent MRI experiments in the anesthetized rat (7, 8) have compared (9) forepaw stimulation-induced changes in CMRO2 with extracellular recordings of relative spiking frequency (ν). These results showed that ΔCMRO2/CMRO2 ≈ Δν/ν during activation, similar to the established relation between Vcyc and CMRO2. Combining all these results we see that ΔCMRO2/CMRO2 ≈ ΔVcyc/Vcyc ≈ Δν/ν in the rat cortex over a wide range of activity, thereby providing an energetic basis for two parameters of neuronal activity in the brain. In this paper we explore the consequences of this relationship on the nature of functional activation and for the interpretation of neuroimaging experiments.
For technical reasons, functional MRI (fMRI) generally measures increments in regional brain activity. The ease of measuring incremental activity (ΔS/S) has been consistent with the widespread view in cognitive neuroscience that the increments represent the energy required for functional activation (10). However, the high level of resting brain activity observed in the 13C MRS experiments has raised questions about discussing cerebral function exclusively in terms of incremental activity (3, 11). The finding that stimulation-induced metabolic and neuroimaging changes in anesthetized animals were significantly larger, both in absolute and relative magnitude, than under awake conditions further questioned the reliance on incremental signals (11). A survey of literature values revealed that for a particular stimulus, the deeper the anesthesia the larger the increment, leading to the suggestion that the total energy rather than the increment was needed for function (11). In this paper we test this proposal under more dependable conditions.
We have recently measured increments in localized CMRO2 from calibrated fMRI and changes in ν of a neuronal ensemble by extracellular recordings (9) during sensory stimulation in α-chloralose anesthetized rats. Both increments were measured under the same experimental conditions in each rat, thereby providing more reliable comparisons than the previous literature data selected from different sources. Portions of the data are described in ref. 9, where we focused primarily on methodological concerns and derived the relationship between ΔCMRO2/CMRO2 and Δν/ν. Here we emphasize the increments of CMRO2 and ν observed during stimulation from two different levels of baseline neuronal activity to answer the following question: Does a cortical region require total or incremental neuronal activity for processing sensory information? If increments in CMRO2 and ν provide sufficient support for the neuronal activity processing sensory stimulation, then changes in CMRO2 and ν should be independent of the basal activity level. Alternatively, if the neuronal activity requires particular magnitudes of CMRO2 and ν, then their changes should be larger from deeper anesthesia. The answer to this question has consequences for understanding how the brain processes information and also influences the interpretation of fMRI data.
Materials and Methods
Animal Preparation.
Artificially ventilated (70% N2O/30% O2) Sprague–Dawley rats (adult, male, 150–210 g) were prepared and monitored as described (7–9). Femoral arterial and venous lines were used for physiological monitoring and infusion of an MRI contrast agent (AMI-227, Advanced Magnetics, Cambridge, MA) to measure blood volume (CBV) changes (12). Intraperitoneal lines were used for administration of α-chloralose (see below) and d-tubocurarine chloride (0.5 mg/kg/h). The anesthesia was first maintained at a low dose (36 ± 2 mg/kg/h; condition I) followed by a higher dose (46 ± 4 mg/kg/h; condition II) to achieve high and low basal activity levels, respectively, in the same rat (9). Electrical stimulation (2 mA, 0.3 ms, 3 Hz) was provided to the rat with two copper electrodes.
CMRO2 Measurements.
The fMRI data were collected on a modified 7T Bruker (Billerica, MA) horizontal-bore spectrometer (7–9). A multimodal fMRI method (13) with sequentially sampled echo-planar data (14) allowed interleaved measurements of changes in blood oxygenation level-dependent (BOLD) signal (ΔS/S) and blood flow (ΔCBF/CBF) under fully relaxed conditions. An MRI contrast agent was slowly infused after collection of ΔS/S and ΔCBF/CBF data in both conditions I and II, and ΔCBV/CBV was measured in condition II only (9). The multimodal data were then used to calculate ΔCMRO2/CMRO2 in conditions I and II (7, 8).
Extracellular Recordings.
The rat was placed on a stereotaxic holder (Kopf Instruments, Tujunga, CA) that rested on a vibration-free table inside a Faraday cage. Tungsten-iridium microelectrodes (FHC, Bowdoinham, ME) were placed in the contralateral and ipsilateral forepaw regions and extracellular signals were acquired as described in ref. 9. High signal-to-noise ratio spikes from the ensemble around the electrode tip (<1 μm) were identified by shape recognition with Spike-2 (Cambridge Electronic Design, Cambridge, U.K.). The spikes recognized in the first experimental run of condition I were used to process the data collected in all subsequent experimental runs in that condition, as well as condition II. The data were converted to reflect the ν of the neuronal ensemble by analyzing consecutive 10 s bins (9).
Results
Stimulation-Induced Changes in CMRO2 and ν at Different Levels of Baseline Activity.
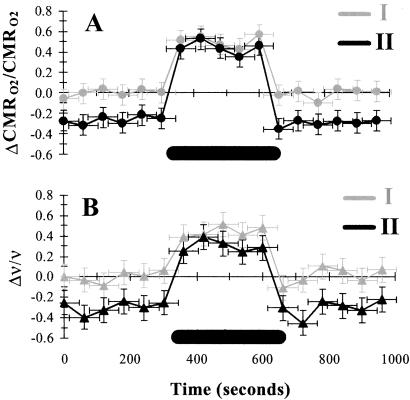
Fig. 1 summarizes the stimulation-induced localized changes in CMRO2 and ν in the contralateral cerebral cortex during high basal activity (condition I) and low basal activity (condition II). In the same rat, the same forepaw stimulation induced different increments in CMRO2 for conditions I and II (by ≈40% and ≈100%), and CMRO2 differed between the two baselines (by ≈30%) (Table 1). A similar dose-dependent decrease of glucose consumption (CMRglc) measured with [14C]2-deoxyglucose autoradiography has been observed in halothane (15), α-chloralose (16), and phenobarbital (17) anesthetized rats. The absolute values of ν of a neuronal ensemble were extracted from extracellular recordings (18, 19). No significant stimulation-induced changes in ν were observed ipsilaterally (data not shown). As in the CMRO2 measurements, the stimulation increased averaged contralateral firing frequency, ν, by ≈30% and ≈100% for conditions I and II, respectively, in the same rat. Baseline-dependent stimulation-induced increments in neuronal activity have been observed under anesthesia (11, 20, 21). The larger incremental change from condition II (in comparison to condition I) compensated for the lower baseline value. The incremental changes in both CMRO2 and ν were greater from condition II than from condition I where the baseline was lower by ≈30%. The final values for both CMRO2 and ν reached on stimulation from both conditions I and II were approximately equal.
Fig 1.
The changes in energy metabolism (CMRO2, n = 6) and relative spiking frequency (ν, n = 36) during forepaw stimulation in α-chloralose anesthetized rats. (A and B) Relative changes in CMRO2 (A) and ν (B) during stimulation in conditions I and II, which are shown in gray and black, respectively. The stimulus duration is shown with the black bar (see Table 1).
Table 1.
Summary of ΔCMRO2/CMRO2, Δν/ν, and ΔS/S in the contralateral cerebral cortex
| ΔCMRO2/CMRO2 | Δν/ν | ΔS/S | |
|---|---|---|---|
| Stimulation increment (from baseline I) | 0.40 ± 0.13 | 0.29 ± 0.13 | 0.02 ± 0.01 |
| Baseline decrement (from baseline I to baseline II) | −0.32 ± 0.08 | −0.29 ± 0.11 | −0.03 ± 0.01 |
| Stimulation increment (from baseline II) | 1.02 ± 0.22 | 1.04 ± 0.32 | 0.05 ± 0.02 |
ΔCMRO2/CMRO2 was averaged across all rats (n = 6) from 2 × 2 voxels in contralateral forepaw region (9). ΔCMRO2/CMRO2 was calculated from ΔCBF/CBF − {(ΔS/S)/Á + ΔCBV/CBV}(1 + ΔCBF/CBF), where Á has been determined (8). Although absolute values of CMRO2 were not measured directly, the use of the data obtained in the baseline of condition I allowed all increments and decrements in activity to be normalized to that baseline value (Fig. 2, Table 2).
Δν/ν was averaged across all rats (n = 36) from all extracellular recordings in the contralateral side (9). Δν/ν was measured at a depth of layer 4 where ≈60% of the data showed an increase, ≈10% of the data showed a decrease, and ≈30% of the data showed no change (9). The absolute values of ν for the two baseline conditions were significantly different (9.5 ± 2.4 and 6.7 ± 1.8 Hz; P < 0.01), whereas the two stimulation conditions were approximately similar (12.3 ± 4.0 and 13.7 ± 3.2 Hz; P > 0.25). Because absolute values of ν were measured in all conditions, the relative changes were directly obtained from comparison of ν values obtained in the different conditions (Fig. 2, Table 2).
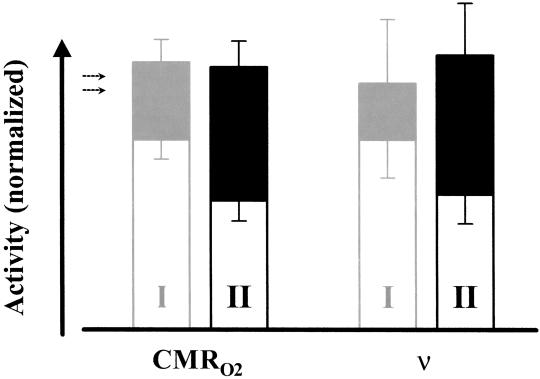
Fig. 2 shows the values and changes in CMRO2 and ν normalized relative to the baseline in condition I (Table 2). We first discuss changes in CMRO2. Because absolute values of CMRO2 were not measured directly, all fractional changes in activity were normalized relative to the baseline of condition I. The stimulation induced an increase of 40% ± 13% in CMRO2 from the baseline of condition I. The baseline of condition II was lowered by 32% ± 8% from the baseline of condition I. In condition II the stimulation increased CMRO2 by 102% ± 22% from the baseline. Stimulation increased ν by 29% ± 13% and by 104% ± 32% in condition I and II, respectively, very similar to those in CMRO2. From the baseline of condition I to the baseline of condition II there was a decrease in ν of 29% ± 11%. In both conditions, stimulation produced approximately the same total magnitude of activity in both CMRO2 and ν, whereas the increments varied accordingly.
Fig 2.
The changes in CMRO2 and ν relative to the baseline in condition I. Although the increments (filled) of CMRO2 and ν were different in conditions I (gray) and II (black), the same activity level was reached on stimulation where the size of the increment depended on the baseline level (open). The dotted arrows represent the projected baseline value of CMRO2 in the resting awake condition (ref. 8; see Table 2).
Table 2.
Summary of incremental changes in CMRO2 and ν relative to baseline I
| CMRO2 | ν | |
|---|---|---|
| Baseline I | 1.00 ± 0.19 | 1.00 ± 0.25 |
| Stimulation I | 1.40 ± 0.46 | 1.29 ± 0.58 |
| Baseline II | 0.68 ± 0.17 | 0.71 ± 0.27 |
| Stimulation II | 1.38 ± 0.30 | 1.44 ± 0.44 |
The stimulation induced similar averaged increases in CMRO2 (relative) and ν (relative) for conditions I (≈40% and ≈30%, respectively) and II (≈100% and ≈100%, respectively), where the larger incremental change from condition II (in comparison to condition I) was observed because of the lowered starting baseline value (by ≈30%). Because absolute values of CMRO2 were not measured directly by the calibrated BOLD method (8), the changes in CMRO2 (as shown here) reflect relative changes when the values obtained in the baseline period of condition I is used for normalization. Although absolute values of ν were measured in all conditions, the changes in ν (as shown here) are normalized to the baseline period of condition I.
The final CMRO2 values were insignificantly different (P > 0.36) on stimulation from baseline conditions I and II (see Fig. 2).
The final ν values were insignificantly different (P > 0.25) on stimulation from baseline conditions I and II (see Fig. 2).
Effect of Stimulation and Baseline Activity on ν Distribution.
The distributions of ν for all measurements (data not shown; see Fig. 2 of ref. 9) for the two baseline and stimulation conditions showed that although the ensemble spiking frequency were different at baseline, on stimulation the ensemble spiking frequency became similar (see caption of Table 1). The increment in ν on stimulation was significantly larger from the lower baseline level of condition II (i.e., deeper anesthesia level), and the final distributions of ν reached on stimulation from both baseline levels were not statistically different. The same stimulation applied at the two α-chloralose conditions induced different increments, resulting in the same total response of the neuronal ensemble (see Fig. 1). The distributions of ν shifted from low frequencies at baseline to higher frequencies during stimulation. At the deeper level of α-chloralose anesthesia (condition II) the difference between baseline and stimulation frequencies became more significant (Tables 1 and 2).
Although the fMRI spatial resolution (≈0.2 μl per voxel) was coarse in comparison to the electrical recordings (≈0.05 μl per electrode), energy utilization (CMRO2) is proportional to ensemble spiking frequency (ν) as shown by the approximately equal magnitudes of ΔCMRO2/CMRO2 and Δν/ν observed during all changes (Figs. 1 and 2). This leads to a ≈1:1 relationship between ΔCMRO2/CMRO2 and Δν/ν observed over the conditions examined (9). The present results are consistent with the results from studies of peripheral neurons (2), where CMRglc increased proportionately with the stimulation frequency.
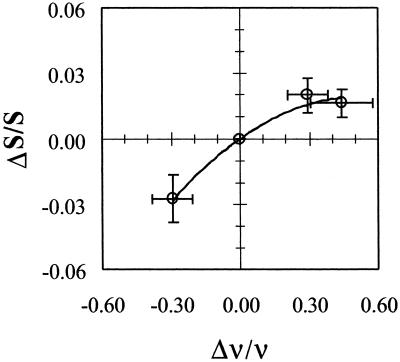
The relation between changes in the measured BOLD signal (ΔS/S) and in the spiking frequency (Δν/ν) of the neuronal ensemble illustrate the extent to which the BOLD signal itself can be used to represent neuronal firing frequency. Fig. 3 shows a monotonic but nonlinear relationship between ΔS/S and Δν/ν observed over a large range of neuronal activity. It should be noted that compared with changes in ΔCMRO2/CMRO2 and Δν/ν, the changes in ΔS/S are lower by an order of magnitude (Table 1).
Fig 3.
Changes in relative spiking frequency (Δν/ν) in relation to changes in the BOLD signal (ΔS/S). The origin is with respect to the baseline in condition I. The final levels reached on stimulation from both baseline conditions are approximately similar, but as shown in Table 1 that while Δν/ν ≈ ΔCMRO2/CMRO2 (within experimental errors) and Δν/ν ≫ ΔS/S over the wide range of activities measured.
This difference in the sensitivity of the fMRI signal and CMRO2 toward changes in neuronal activity is due to the mechanism of the BOLD phenomenon. The BOLD signal, which is an indirect measure of red blood cell hemoglobin oxygenation (22–24), depends on the balance between oxygen delivery by perfusion (i.e., CBF and CBV) and the rate of oxygen use (i.e., CMRO2) at steady-state (22, 25). Ogawa et al. (22) showed that the BOLD signal is a difference between the larger changes in CBF and CMRO2,
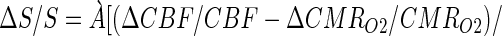 |
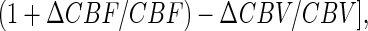 |
where Á is a magnetic field dependent physiologic constant (22, 25) that can be measured (7, 8). By calibrating the BOLD signal so as to obtain ΔCMRO2/CMRO2 from Eq. 1 (7, 8), it is possible to infer the local spiking frequency of the neuronal ensemble (9). To interpret ΔS/S directly without deriving a value of ΔCMRO2/CMRO2, we note that although ΔS/S is not linear with Δν/ν over the large range studied, it is a monotonic relationship, and over a small range should approach linearity. These relative results are in accord with other reports of calibrated BOLD experiments in human brain showing near linearity between ΔS/S and ΔCMRO2/CMRO2 (26, 27). However, direct interpretation of ΔS/S in terms of neuronal activity required that ΔCMRO2/CMRO2 be derived from calibrated BOLD experiments (9).
Discussion
In the present α-chloralose anesthetized rat study, both CMRO2 and ν were measured (Fig. 1) under high (I) and low (II) basal activity levels to determine the fractional increases of each parameter on stimulation (Fig. 2). The changes in CMRO2 and ν on forepaw stimulation from the two conditions I and II were localized to the contralateral somatosensory cortex, as established for the forepaw stimulation model (28, 29). The values of ΔCMRO2/CMRO2 approximately equaled Δν/ν under all conditions examined (Table 1) and both were significantly larger for condition II (Table 2). The present results extend our previous in vivo 13C MRS studies of rat brain (5, 6), which showed that ΔCMRO2/CMRO2 is proportional to ΔVcyc/Vcyc under moderate anesthetized levels (as in the present study). These combined results show that ΔCMRO2/CMRO2 ≈ ΔVcyc/Vcyc ≈ Δν/ν in the moderately anesthetized rat cortex (9).
The experimentally derived values of ΔCMRO2/CMRO2 and Δν/ν were quite large, sometimes higher than 100% in the most intense voxels or electrode placements, which were often observed in condition II. In contrast, the measured values of ΔS/S under the same conditions were smaller by at least an order of magnitude in comparison to ΔCMRO2/CMRO2 and Δν/ν (Table 1). Although the relationship between ΔS/S and Δν/ν is highly nonlinear over a wide range of activity (Fig. 3), the increments in S for conditions I and II followed approximately the increments detected for CMRO2 and ν (Fig. 2). The same stimulation induced different increments in CMRO2, ν, and S from each condition so that approximately the same final values were reached. This finding agrees with a previous hypothesis based on a survey of changes in CMRglc and single-unit spiking activity on stimulation in anesthetized animals (11). The current results do not suggest that there is a ceiling level of activity being reached with stimulation of anesthetized animals, because the cortex still has the capacity to increase CMRO2 and CBF from the stimulated/awake values (8) by at least 80% (e.g., seizure induced by bicuculine).
We carried out the current experiments with two baseline levels (in the same rat) during which the same forepaw stimulation was applied (Figs. 1 and 2) to determine whether a cortical region uses total or incremental neuronal activity for encoding sensory information. The determination of whether total or incremental activity is used for function has consequences for realizing how brain uses energy to process and transfer information. If only the increments in activity support the neuronal activity, then the sizes of increments should be independent of the basal activity level. Alternatively, if the neuronal activity required particular magnitudes of activity, then the sizes of the increments should be inversely correlated with the basal activity level, being larger from a lower baseline activity level. In the former case the total disregard of the baseline data in neuroimaging would be justified because the baseline activity does not contribute to the encoding of information, whereas in the latter case, the disregard of the baseline activity would not be justified. Our results from the rat brain show that the total energy is required to support neuronal activity (Figs. 1 and 2).
Comparison with Previous Results.
The finding that the same forepaw stimulation induced different increments from each baseline condition so that approximately the same final values were reached (Figs. 1 and 2) is quite reproducible under the present anesthetized conditions with α-chloralose and pentobarbital (9, 11, 20, 21). However, some previous results do not agree. Nakao et al. (30) reported that whisker stimulation of rats anesthetized with α-chloralose do not induce significant increases in total CMRglc (and CBF), which were measured by autoradiographic methods. This interpretation contrasts with previous [14C]2-deoxyglucose autoradiography (28, 29, 31) and with 13C MRS (32–34) measurements of significant increases in CMRglc and CMRO2 in the cortex with functional activation of α-chloralose anesthetized rats. The localized values of CMRglc reported by Nakao et al. (30) probably underestimate the metabolic rate in activated tissue by including large nonactivated regions. Our results show that the activated regions are only a fraction of the total cortex (8, 9, 34).
Human positron emission tomography (PET) studies measuring changes in CBF at different levels of propofol anesthesia did not show increasing increments to sensory stimulation at deeper levels of anesthesia (35), as would be expected from our results. However, the authors acknowledged that localized changes in CBF were confounded by elevated blood pCO2 (that increased with depth of anesthesia), which in turn raised CBF globally. Furthermore, the somatosensory stimulation used did not show PET activations under any conditions.
Another possible limitation of our results is that the observations are specific to α-chloralose and would not generally apply to stimulation under other anesthetics or in the nonanesthetized state. The [14C]2-deoxyglucose autoradiography experiments of Ueki et al. (29) showed that α-chloralose resulted in stimulation-induced metabolic responses, whereas other anesthetics (e.g., halothane and nitrous oxide) did not. Although little is known about the molecular mechanisms of the action of α-chloralose (36), it seems to show a major slowing of axonal conduction and/or synaptic transmission without a loss of synaptic efficacy (37). In the current study, ΔCMRO2/CMRO2 and Δν/ν were measured from two baseline levels in the same rat, where a larger magnitude was observed from a deeper level of anesthesia (Figs. 1 and 2). Because different doses of α-chloralose were used to alter the baseline activity levels (in the same rats), presumably the same molecular mechanisms were involved (to different extents) in both baseline conditions. By use of the same stimulation in both baseline conditions (in the same rats), we tried to minimize any preferential action of the anesthetic to investigate the stimulation-induced changes in CMRO2 and ν (Figs. 1 and 2). Future studies with other anesthetics, as well as the awake condition, would provide more accurate comparisons for the current experimental findings.
Biophysics of Brain Activity.
In steady-state conditions, the almost exclusive energy source is glucose oxidation in the adult mammalian brain (38). The finding that ΔCMRO2/CMRO2 ≈ ΔVcyc/Vcyc ≈ Δν/ν establishes a quantitative relation between three important biophysical parameters, associating two specific parameters of neuronal activity to energy metabolism: the flux of the glutamate neurotransmitter release and its cycling back to neurons via astrocytic glutamine (Vcyc), and the relative spiking frequency of an ensemble of glutamatergic neurons (ν) are both stoichiometrically coupled to glucose oxidation (CMRO2). This approach differs from previous proposals of phenomenological relationships observed between measures of electrical activity and the BOLD signal (39, 40). We believe the disagreements between these studies could be reconciled if the BOLD measurements were quantified in terms of neuroenergetics (9).
The energetics of neuronal activity derived from these experiments has found strong support in a recent theoretical energy budget for rat brain by Attwell and Laughlin (4). From literature values of individual processes for glutamatergic neurons during firing and nonfiring periods (at spiking frequency of 4 Hz), they calculated that ≈15% of the energy is used to maintain resting membrane potentials in nonfiring epochs while the remaining ≈85% is used to restabilize membrane potentials in firing epochs. This estimate agreed extremely well with our 13C MRS experiments showing that the high resting brain activity consumes ≈80% of the energy (5). They further calculated the absolute amounts of energy expended in signaling by glutamatergic neurons, and once again their estimation agreed with our finding that ≈80% at rest of the energy is dedicated to processes of neuronal firing (3). Of direct relevance to the present results they also showed that the functional neuroenergetic costs of glutamatergic neurons are proportional to glutamate release. The present results are in agreement with this prediction.
Implications for the Interpretation of fMRI Studies.
The present results support our previous conclusion based on 13C MRS results of high brain activity at rest (3, 41). Earlier we examined the consequences of the large baseline activity (quantitated from 13C MRS studies) for interpreting neuroimaging data. Our interpretation explained the negative neuroimaging signals, sometimes observed with fMRI, which at that time had been considered an anomaly. Raichle (42) had noted that negative signals were not expected on the prevailing view of brain function as inferred from the differencing approach used in functional imaging. We explained that negative signals in a localized region could be interpreted as a reduction in total activity (in the stimulation period) given the large resting activity observed (in the baseline period). More recently, the existence and the importance of significant neuronal activity at rest as a special property of certain brain regions (based on a meta-analysis of neuroimaging data) has been recognized by Gusnard and Raichle (43). Implications for the interpretation of neuroimaging data, many of which have been suggested previously (3, 11, 41), can now be extended based on our current findings.
Using energy as the measure of neuronal activity, we find that incremental stimulation-induced energy is similar to energy consumed at rest (albeit to different extents). Generally the sizes of stimulation-induced incremental activity in awake human experiments are smaller than the baseline activity, whereas in anesthetized animals the sizes of incremental and baseline activities depend on depth of anesthesia (11). Therefore, the focus on only the magnitude of incremental activity neglects contributions of the neuronal activity (10) and can be misleading (3). For example, if ΔCMRO2/CMRO2 in a localized region for one task is 1% and for another task it is 2%, it would be misleading to conclude that twice the neuronal activity is required in the second task. Assuming that approximately 75% of CMRO2 is associated with neuronal activity in humans (44, 45), a more accurate conclusion would be an increase in neuronal activity of 76% to 77%.
The current results are in good agreement with an earlier report (11), which showed that total amount of activity is required for a stimulus to be processed under anesthesia, which in turn explained the large increments reported in neuroimaging studies of anesthetized animals (32, 46). To respond to the stimulus, the energy is raised from the anesthetized levels to total energy above or near the resting awake level (11, 29, 33). The high levels of activity required for function have been supported by the present results, under the more reliable conditions of same animal, same stimulation, and same anesthetic at different doses.
Measuring the high baseline activity, which is subtracted away in processing neuroimaging data, increases concerns about the “injection” hypothesis, which only measures incremental signals (41). One such reinterpretation suggests that the disappearance of an incremental signal (e.g., in cases of repetition or practice) does not mean that the regional neuronal activity is zero, but only that on repetition of the task no more (or less) energy is needed than was available in the resting state. Another implication is that the incremental signals depend on, among other things, the starting resting brain activity itself. The importance of total energetics for neural function suggests that the planning and interpretation of neuroimaging experiments be reevaluated so as to include the functional requirement for high levels of localized brain activity.
Acknowledgments
We thank engineers T. Nixon, P. Brown, and S. McIntyre for maintenance of the spectrometer and the radio-frequency probe designs, and B. Wang for technical support. This work was supported by National Institutes of Health Grants NS-37203 (to F.H.), DC-003710 (to F.H.), NS-32126 (to D.L.R.), and DK-27121 (to R.G.S.); National Science Foundation Grants DBI-9730892 (to F.H.) and DBI-0095173 (to F.H.); and the James S. McDonnell Foundation (F.H.).
Abbreviations
fMRI, functional MRI
BOLD, blood oxygenation level-dependent
MRS, magnetic resonance spectroscopy
CBF, cerebral blood flow
CBV, cerebral blood volume
CMRO2, cerebral metabolic rate of oxygen consumption
ν, relative spiking frequency
See commentary on page 10237.
References
- 1.Ritchie J. M. (1973) Prog. Biophys. Mol. Biol. 26, 147-187. [DOI] [PubMed] [Google Scholar]
- 2.Sokoloff L. (1993) Dev. Neurosci. 15, 194-206. [DOI] [PubMed] [Google Scholar]
- 3.Shulman R. G. & Rothman, D. L. (1998) Proc. Natl. Acad. Sci. USA 95, 11993-11998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Attwell D. & Laughlin, S. B. (2001) J. Cereb. Blood Flow Metab. 21, 1133-1145. [DOI] [PubMed] [Google Scholar]
- 5.Sibson N. R., Dhankhar, A., Mason, G. F., Rothman, D. L., Behar, K. L. & Shulman, R. G. (1998) Proc. Natl. Acad. Sci. USA 95, 316-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rothman D. L., Sibson, N. R., Hyder, F., Shen, J., Behar, K. L. & Shulman, R. G. (1999) Philos. Trans. R. Soc. London B 354, 1165-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kida I., Kennan, R. P., Rothman, D. L., Behar, K. L. & Hyder, F. (2000) J. Cereb. Blood Flow Metab. 20, 847-860. [DOI] [PubMed] [Google Scholar]
- 8.Hyder F., Kida, I., Behar, K. L., Kennan, R. P., Maciejewski, P. K. & Rothman, D. L. (2001) NMR Biomed. 14, 413-431. [DOI] [PubMed] [Google Scholar]
- 9.Smith A. J., Blumenfeld, H., Behar, K. L., Rothman, D. L., Shulman, R. G. & Hyder, F. (2002) Proc. Natl. Acad. Sci. USA 99, 10765-10770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Posner M. I. & Raichle, M. E. (1998) Proc. Natl. Acad. Sci. USA 95, 763-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shulman R. G., Rothman, D. L. & Hyder, F. (1999) Proc. Natl. Acad. Sci. USA 96, 3245-3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kennan R. P., Scanley, B. E. & Gore, J. C. (1997) Magn. Reson. Med. 37, 953-956. [DOI] [PubMed] [Google Scholar]
- 13.Hyder F., Renken, R., Kennan, R. P. & Rothman, D. L. (2000) Magn. Reson. Imaging 18, 227-235. [DOI] [PubMed] [Google Scholar]
- 14.Hyder F., Rothman, D. L. & Blamire, A. M. (1995) Magn. Reson. Imaging 13, 97-103. [DOI] [PubMed] [Google Scholar]
- 15.Savaki H. E., Desban, M., Glowinski, J. & Besson, M. J. (1981) J. Comp. Neurol. 213, 36-45. [DOI] [PubMed] [Google Scholar]
- 16.Dudley R. E., Nelson, S. R. & Samson, F. (1982) Brain Res. 233, 173-180. [DOI] [PubMed] [Google Scholar]
- 17.Hodes J. E., Soncrant, T. T., Larson, D. M., Carlson, S. G. & Rapoport, S. I. (1985) Anesthesiology 63, 633-639. [DOI] [PubMed] [Google Scholar]
- 18.Chapin J. K. & Nicolelis, M. A. (1999) J. Neurosci. Methods 94, 121-140. [DOI] [PubMed] [Google Scholar]
- 19.Zouridakis G. & Tam, D. C. (2000) Comput. Methods Programs Biomed. 61, 91-98. [DOI] [PubMed] [Google Scholar]
- 20.Harding G. W., Stogsdill, R. M. & Towe, A. L. (1979) Neuroscience 4, 369-378. [DOI] [PubMed] [Google Scholar]
- 21.Bonvento G., Charbonne, R., Correze, J. L., Borredon, J., Seylaz, J. & Lacombe, P. (1994) Brain Res. 665, 213-221. [DOI] [PubMed] [Google Scholar]
- 22.Ogawa S., Menon, R. S., Tank, D. W., Kim, S. G., Merkle, H., Ellermann, J. M. & Ugurbil, K. (1993) Biophys. J. 64, 803-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kennan R. P., Zhong, J. & Gore, J. C. (1994) Magn. Reson. Med. 31, 9-21. [DOI] [PubMed] [Google Scholar]
- 24.Weisskoff R. M., Zuo, C. S., Boxerman, J. L. & Rosen, B. R. (1994) Magn. Reson. Med. 31, 601-610. [DOI] [PubMed] [Google Scholar]
- 25.Hyder F., Shulman, R. G. & Rothman, D. L. (1998) J. Appl. Physiol. 85, 554-564. [DOI] [PubMed] [Google Scholar]
- 26.Davis T. L., Kwong, K. K., Weisskoff, R. M. & Rosen, B. R. (1998) Proc. Natl. Acad. Sci. USA 95, 1834-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoge R. D., Atkinson, J., Gill, B., Crelier, G. R., Marrett, S. & Pike, G. B. (1999) Proc. Natl. Acad. Sci. USA 96, 9403-9408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santori E. M., Der, T. & Collins, R. C. (1986) J. Neurosci. 6, 463-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ueki M., Mies, G. & Hossmann, K. A. (1992) Acta Anaesthesiol. Scand. 36, 318-322. [DOI] [PubMed] [Google Scholar]
- 30.Nakao Y., Itoh, Y., Kuang, T. Y., Cook, M., Jehle, J. & Sokoloff, L. (2001) Proc. Natl. Acad. Sci. USA 98, 7593-7598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kossut M., Hand, P. J., Greenberg, J. & Hand, C. L. (1988) J. Neurophysiol. 60, 829-852. [DOI] [PubMed] [Google Scholar]
- 32.Hyder F., Chase, J. R., Behar, K. L., Mason, G. F., Siddeek, M., Rothman, D. L. & Shulman, R. G. (1996) Proc. Natl. Acad. Sci. USA 93, 7612-7617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hyder F., Rothman, D. L., Mason, G. F., Rangarajan, A., Behar, K. L. & Shulman, R. G. (1997) J. Cereb. Blood Flow Metab. 17, 1040-1047. [DOI] [PubMed] [Google Scholar]
- 34.Hyder F., Kennan, R. P., Kida, I., Mason, G. F., Behar, K. L. & Rothman, D. L. (2000) J. Cereb. Blood Flow Metab. 20, 485-498. [DOI] [PubMed] [Google Scholar]
- 35.Bonhomme V., Fiset, P., Meuret, P., Backman, S., Plourde, G., Paus, T., Bushnell, M. C. & Evans, A. C. (2001) J. Neurophysiol. 85, 1299-1308. [DOI] [PubMed] [Google Scholar]
- 36.Lees P. (1972) Vet. Rec. 91, 330-333. [DOI] [PubMed] [Google Scholar]
- 37.Richard C. D. (1998) Toxicol. Lett. 100-101, 41-50. [DOI] [PubMed] [Google Scholar]
- 38.Siesjo B. K., (1978) Brain Energy Metabolism (Wiley, New York).
- 39.Rees G., Friston, K. & Koch, C. (2000) Nat. Neurosci. 3, 716-723. [DOI] [PubMed] [Google Scholar]
- 40.Logothetis N. K., Pauls, J., Augath, M., Trinath, T. & Oeltermann, A. (2001) Nature (London) 412, 150-157. [DOI] [PubMed] [Google Scholar]
- 41.Shulman R. G. (2001) Am. J. Psychiatry 158, 11-20. [DOI] [PubMed] [Google Scholar]
- 42.Raichle M. E. (1998) Proc. Natl. Acad. Sci. USA 95, 765-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gusnard D. A. & Raichle, M. E. (2001) Nat. Rev. Neurosci. 2, 685-694. [DOI] [PubMed] [Google Scholar]
- 44.Lebon V., Petersen, K. F., Cline, G. W., Shen, J., Mason, G. F., Dufour, S., Behar, K. L., Shulman, G. I. & Rothman, D. L. (2002) J. Neurosci. 22, 1523-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shen J., Petersen, K. F., Behar, K. L., Brown, P., Nixon, T. W., Mason, G. F., Petroff, O. A., Shulman, G. I., Shulman, R. G. & Rothman, D. L. (1999) Proc. Natl. Acad. Sci. USA 96, 8235-8240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ueki M., Linn, F. & Hossman, K. A. (1988) J. Cereb. Blood Flow Metab. 8, 486-494. [DOI] [PubMed] [Google Scholar]