Abstract
1. The steady-state and kinetic characteristics of the processes of activation and inactivation of the Na+ permeability, PNa, were measured in cut skeletal muscle fibres from Rana temporaria under voltage-clamp conditions.
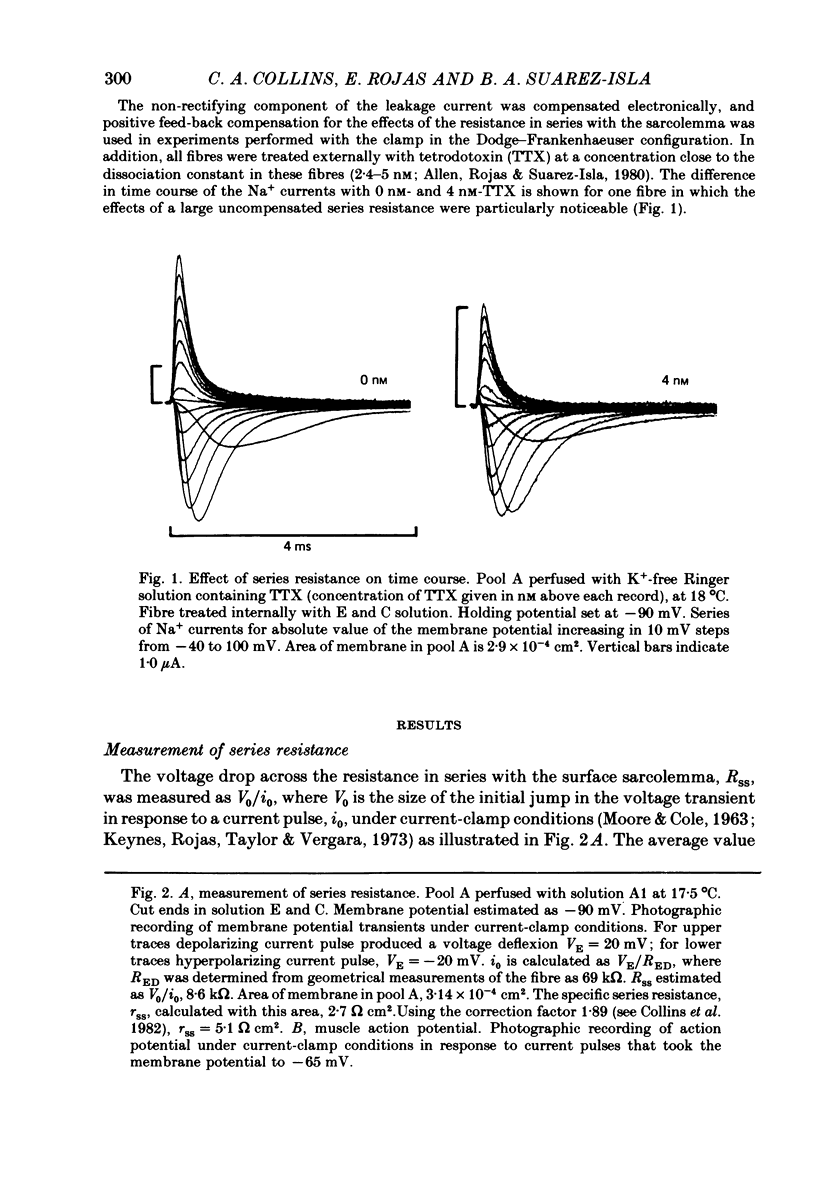
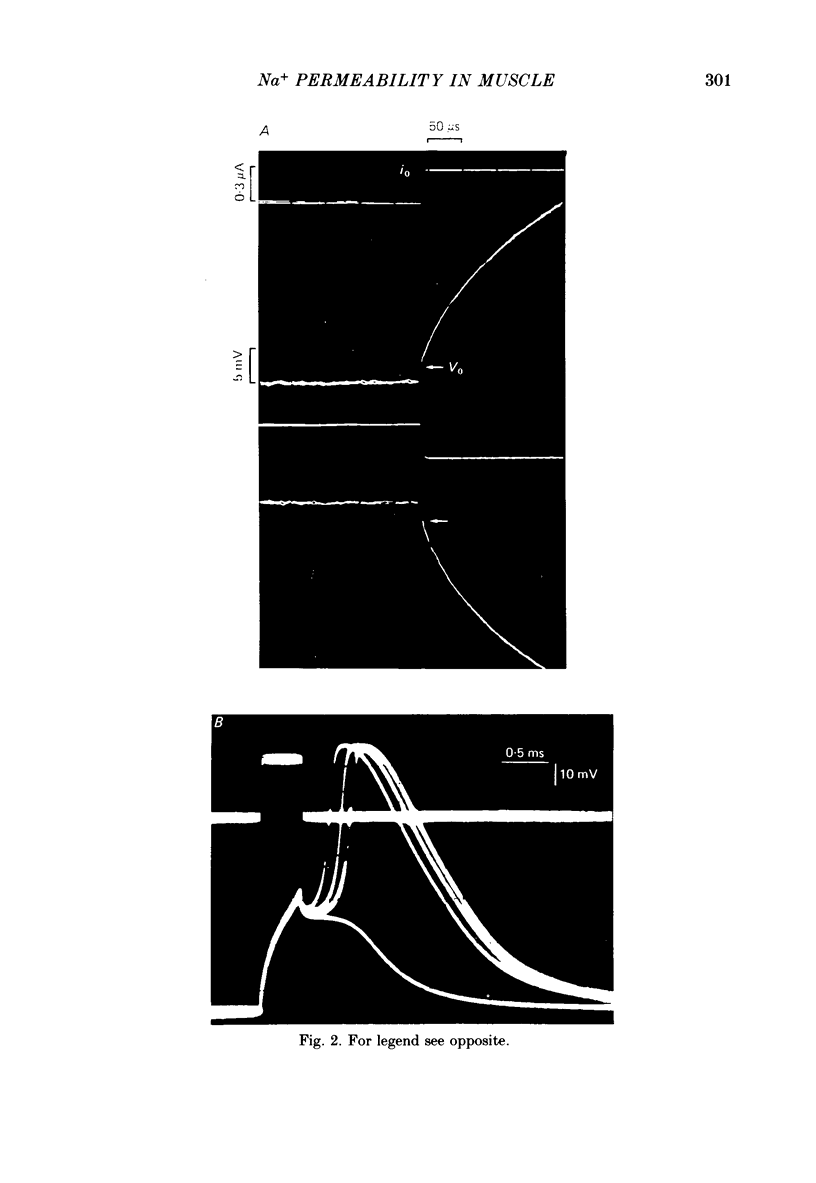
2. The specific resistance, rss, in series with the surface sarcolemma, was estimated as 6 Ω cm2 by measuring the initial value of the membrane potential transient in response to current pulses under current-clamp conditions. To reduce the error in the potential across the sarcolemma introduced by rss, Na+ currents were recorded using positive feed-back compensation, in the presence of tetrodotoxin (2·4-5 nm).
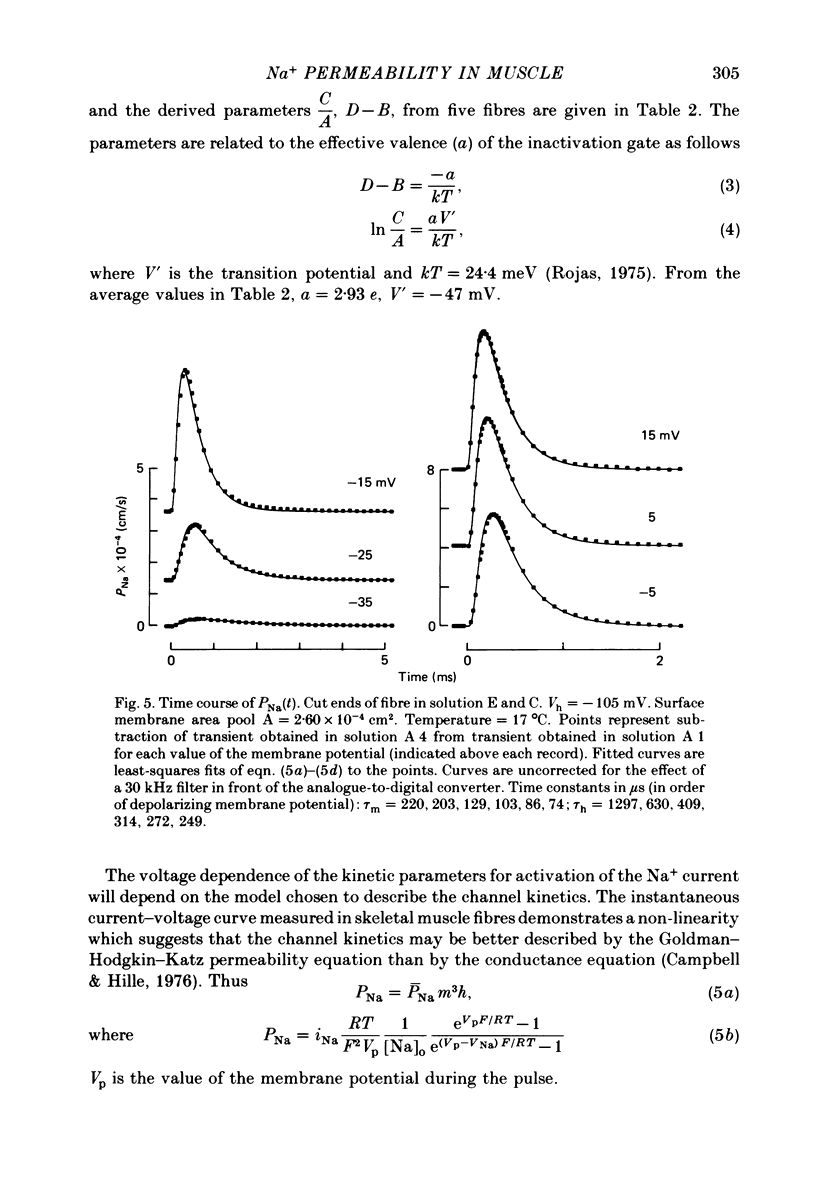
3. PNa(t) was fitted with m3h kinetics assuming a voltage-dependent delay, δt, to the start of the activation process.
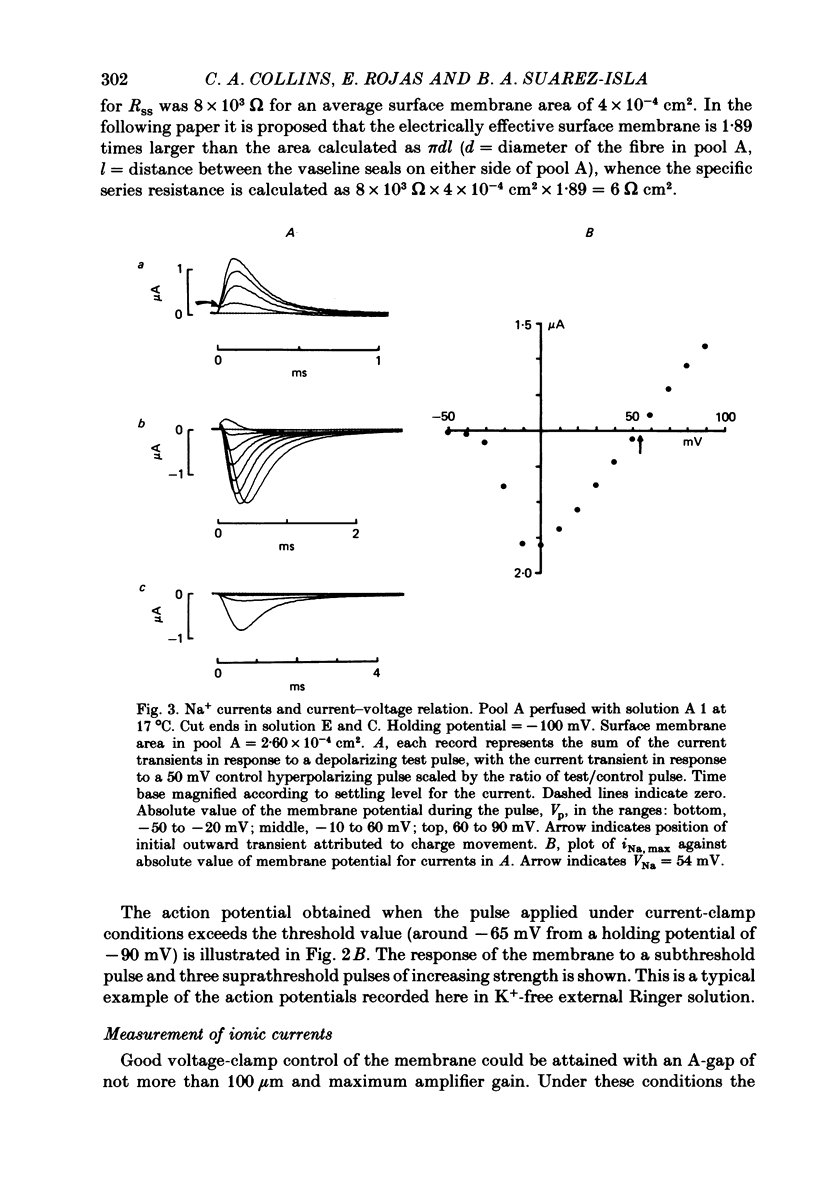
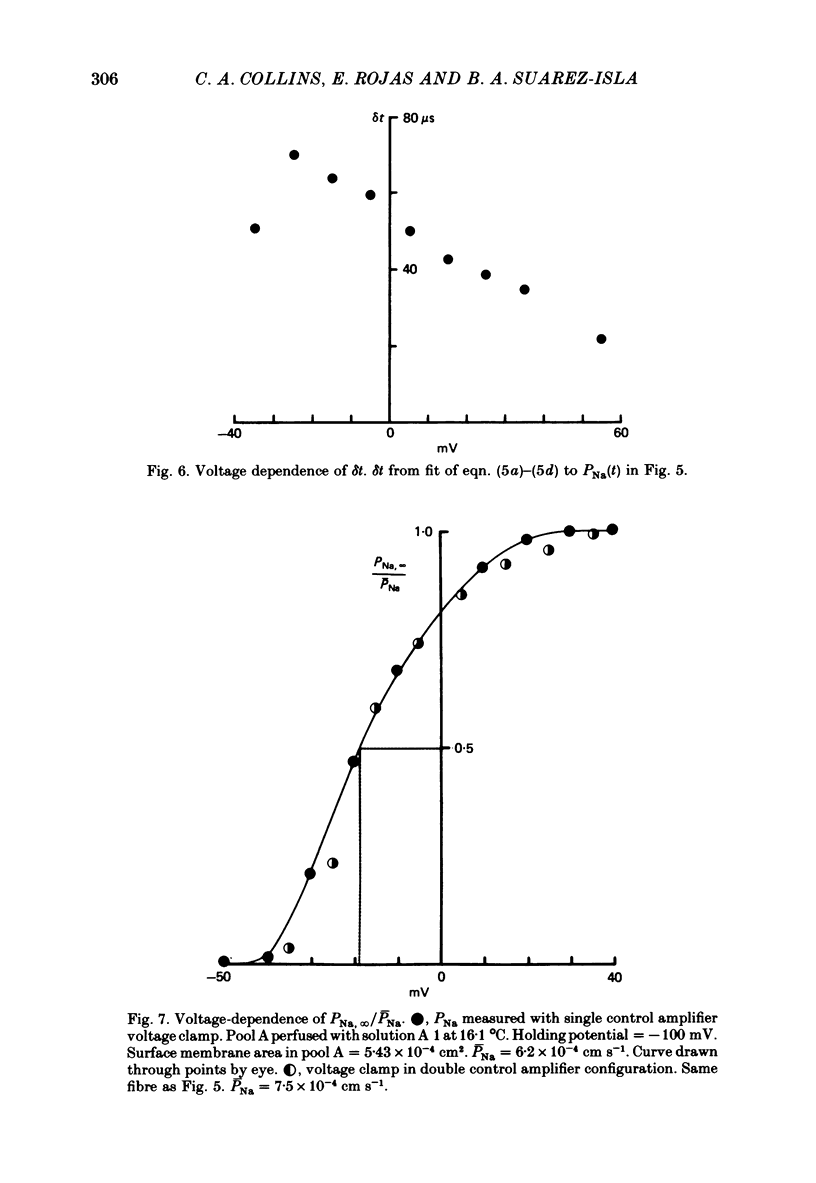
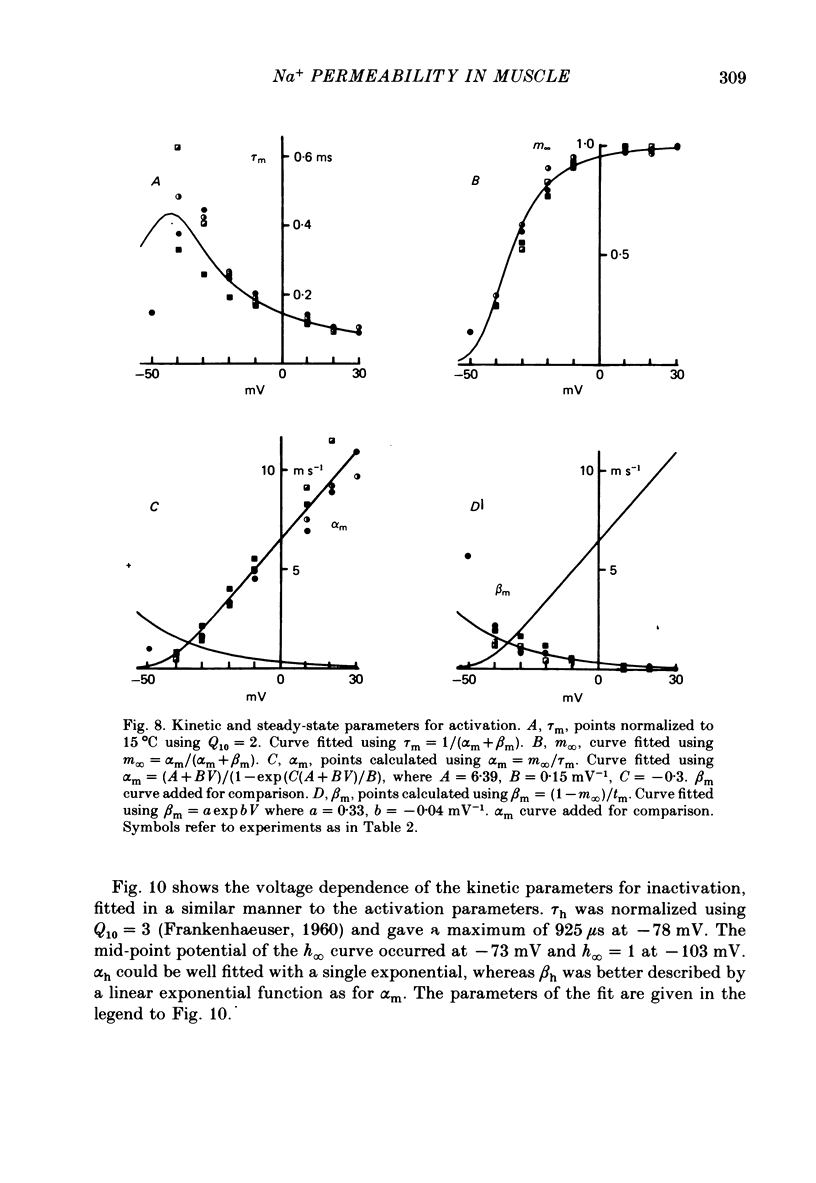
4. The PNa—Vp curve exhibited saturation at potentials more positive than 30 mV. m∞, calculated as (PNa, ∞/¯PNa)⅓ as a function of Vp, was a sigmoid curve with a mid point at -35 mV. The slope, dm∞/dVp, at this point was 0·032 mV-1.
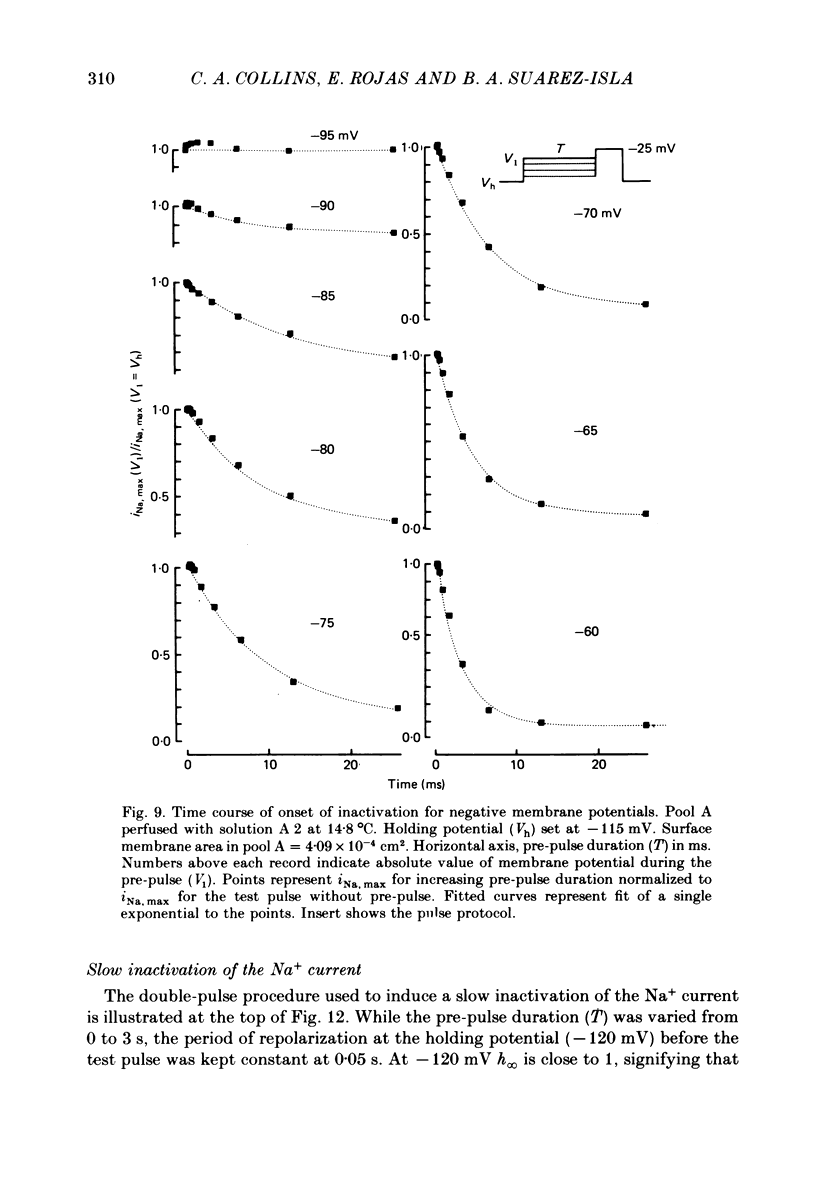
5. Using a double-pulse protocol a non-exponential time course for the development of fast inactivation at small depolarizations was observed.
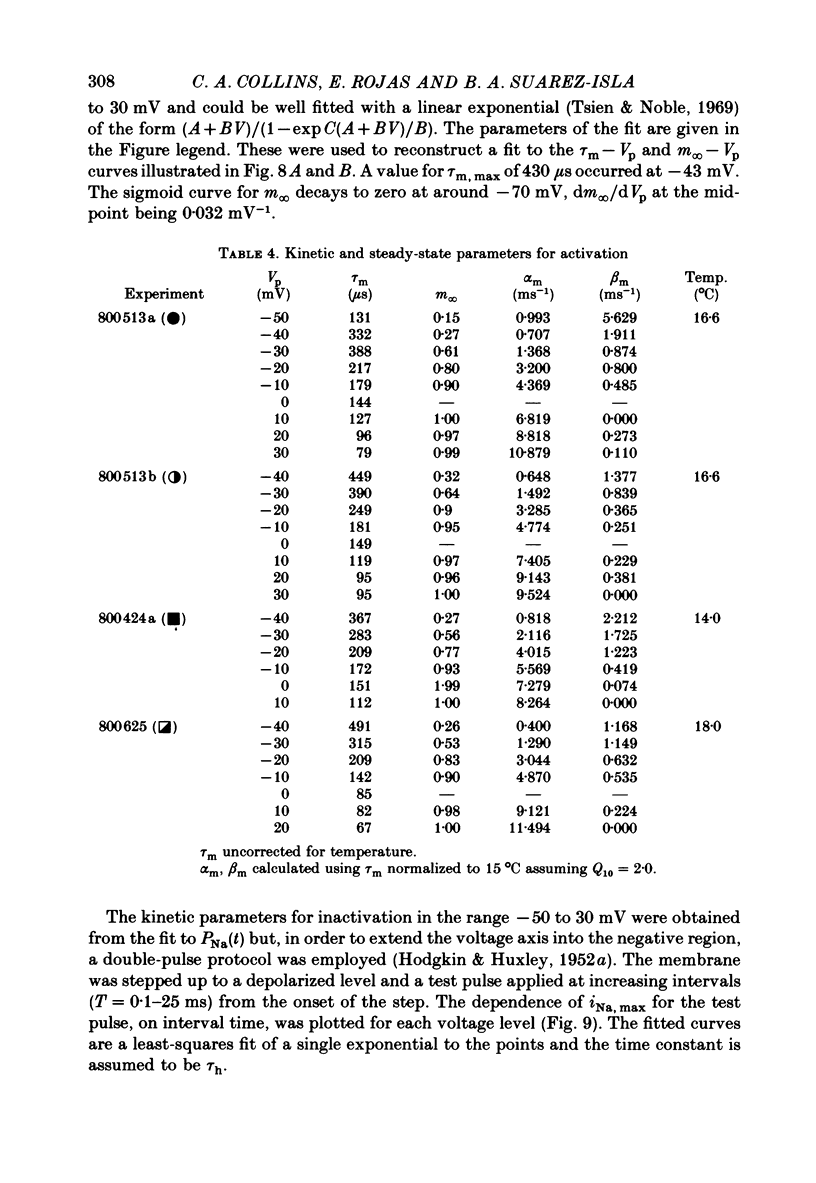
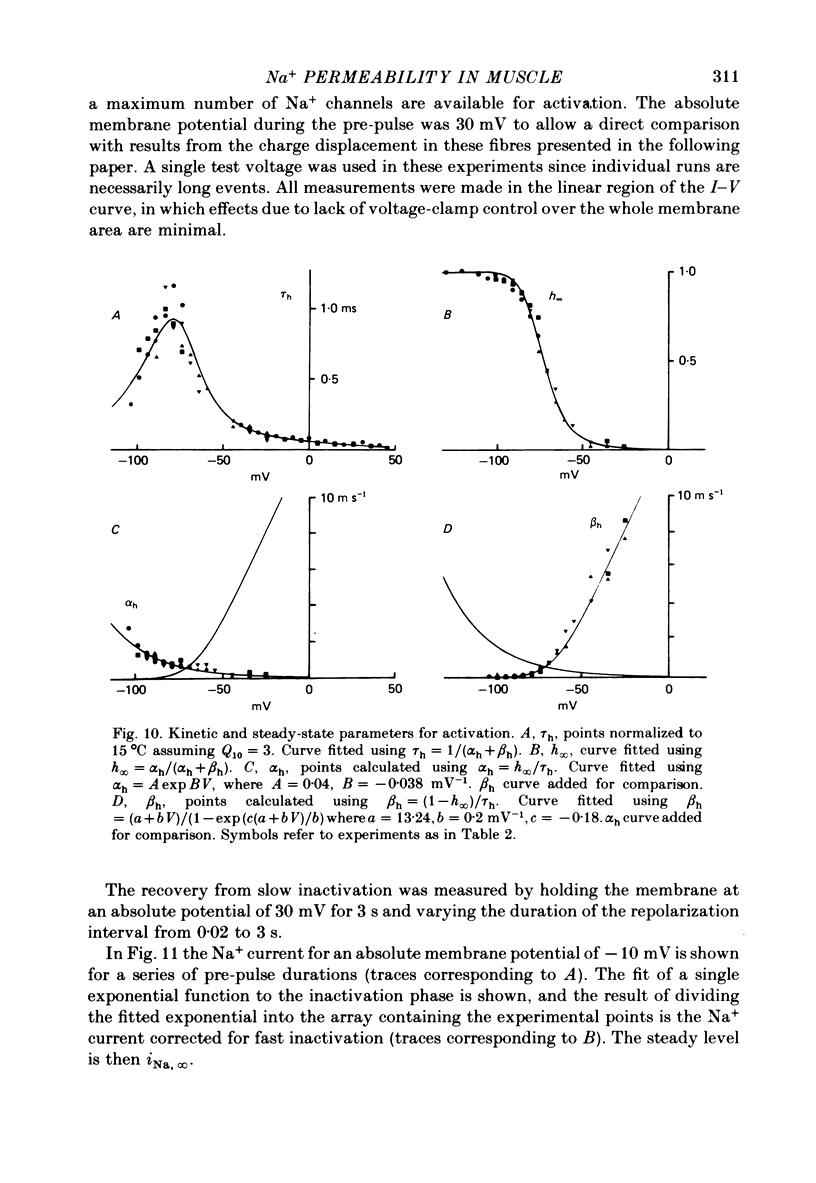
6. The time constant for activation, τm, as a function of Vp, and τh as a function of Vp, could be fitted with an approximately bell-shaped function, maximum of 430 μs at -43 mV and 925 μs at -78 mV respectively, at 15 °C.
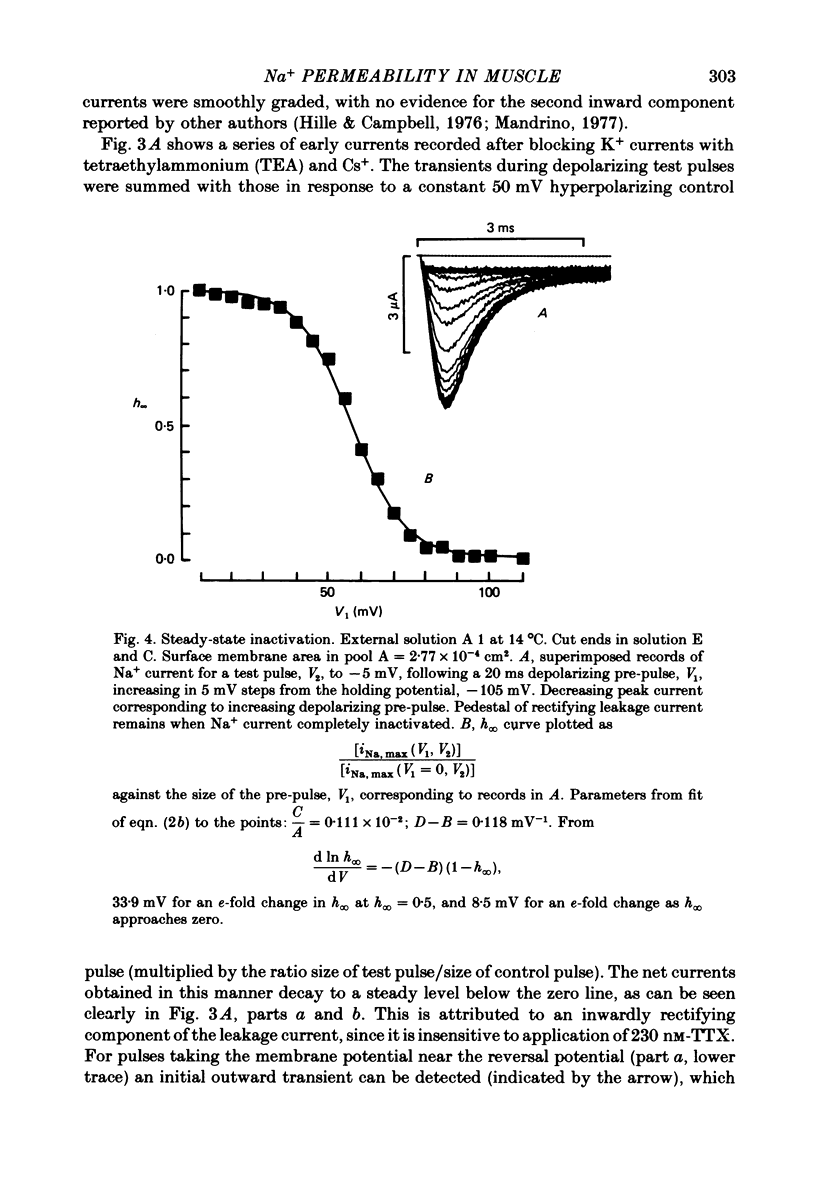
7. The mid-point potential of the h∞—Vl curve occurred at -58 mV, and h∞ approached 1 for V1 values more negative than -103 mV.
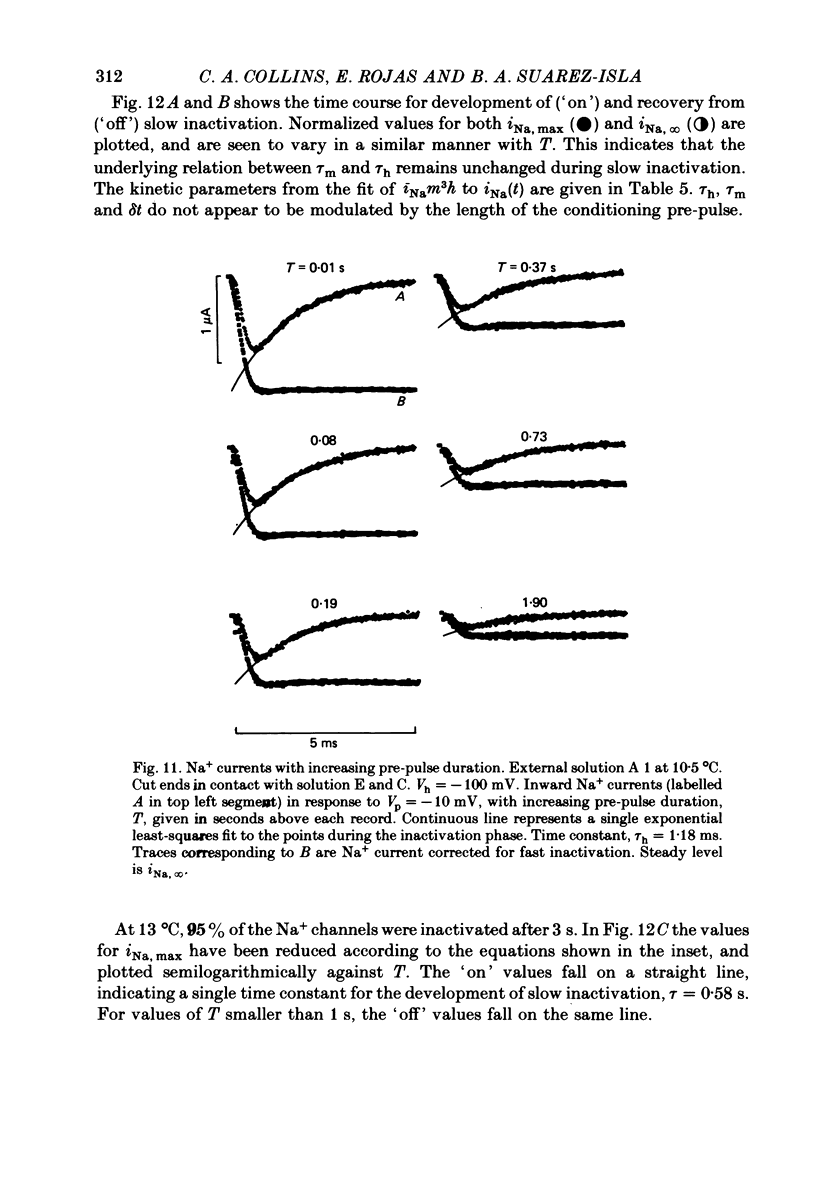
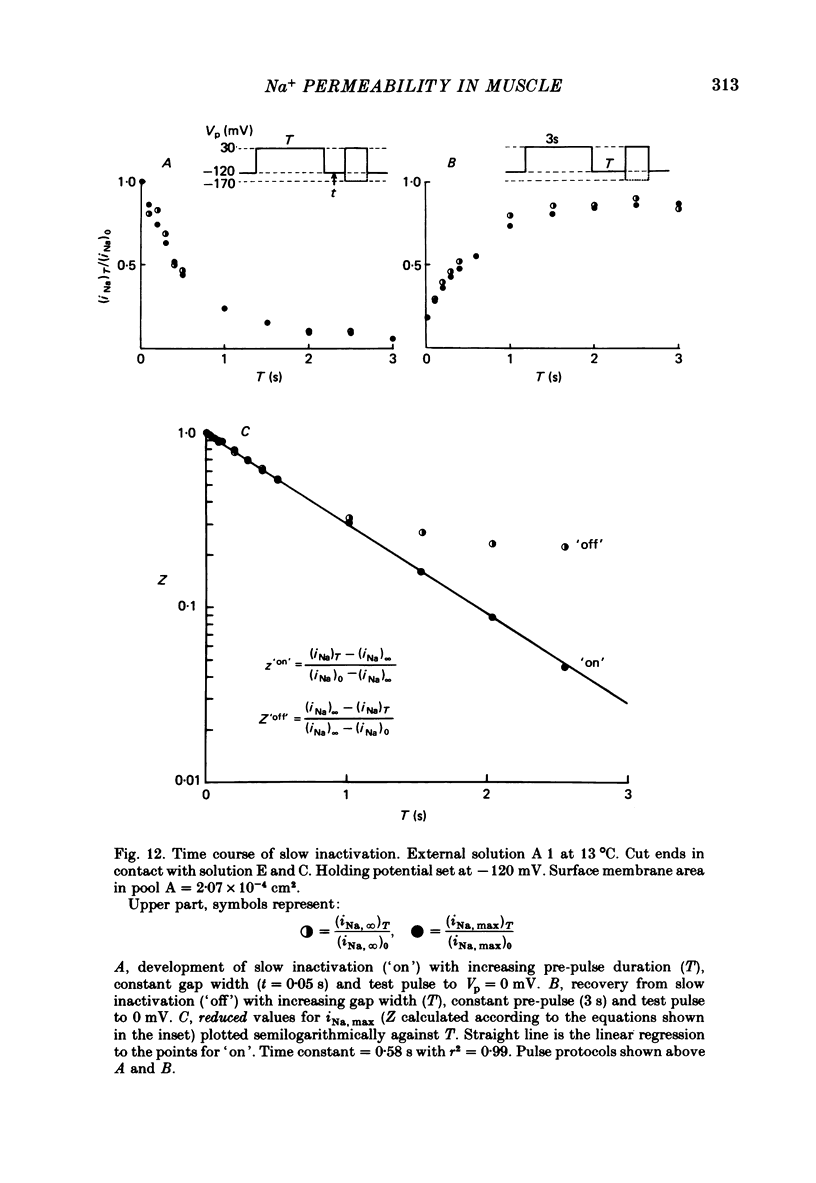
8. Using a double-pulse procedure the development of a slow inactivation of the Na+ current was demonstrated. Its time course could be described in terms of a single exponential function, time constant equal to 0·58 s. The recovery from slow inactivation could be described by a similar exponential for recovery times smaller than 1 s.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman W. J., Jr, Palti Y. The effects of external potassium and long duration voltage conditioning on the amplitude of sodium currents in the giant axon of the squid, Loligo pealei. J Gen Physiol. 1969 Nov;54(5):589–606. doi: 10.1085/jgp.54.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Chandler W. K., Hodgkin A. L. Voltage clamp experiments in striated muscle fibres. J Physiol. 1970 Jul;208(3):607–644. doi: 10.1113/jphysiol.1970.sp009139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Peachey L. D. Reconstruction of the action potential of frog sartorius muscle. J Physiol. 1973 Nov;235(1):103–131. doi: 10.1113/jphysiol.1973.sp010380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M., Bezanilla F. Charge movement associated with the opening and closing of the activation gates of the Na channels. J Gen Physiol. 1974 May;63(5):533–552. doi: 10.1085/jgp.63.5.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brismar T. Slow mechanism for sodium permeability inactivation in myelinated nerve fibre of Xenopus laevis. J Physiol. 1977 Sep;270(2):283–297. doi: 10.1113/jphysiol.1977.sp011952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell D. T., Hille B. Kinetic and pharmacological properties of the sodium channel of frog skeletal muscle. J Gen Physiol. 1976 Mar;67(3):309–323. doi: 10.1085/jgp.67.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins C. A., Rojas E., Suarez-Isla B. A. Fast charge movements in skeletal muscle fibres from Rana temporaria. J Physiol. 1982 Mar;324:319–345. doi: 10.1113/jphysiol.1982.sp014115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGE F. A., FRANKENHAEUSER B. Membrane currents in isolated frog nerve fibre under voltage clamp conditions. J Physiol. 1958 Aug 29;143(1):76–90. doi: 10.1113/jphysiol.1958.sp006045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B. Quantitative description of sodium currents in myelinated nerve fibres of Xenopus laevis. J Physiol. 1960 Jun;151:491–501. doi: 10.1113/jphysiol.1960.sp006455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D. E. POTENTIAL, IMPEDANCE, AND RECTIFICATION IN MEMBRANES. J Gen Physiol. 1943 Sep 20;27(1):37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):497–506. doi: 10.1113/jphysiol.1952.sp004719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B., Campbell D. T. An improved vaseline gap voltage clamp for skeletal muscle fibers. J Gen Physiol. 1976 Mar;67(3):265–293. doi: 10.1085/jgp.67.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ildefonse M., Rougier O. Voltage-clamp analysis of the early current in frog skeletal muscle fibre using the double sucrose-gap method. J Physiol. 1972 Apr;222(2):373–395. doi: 10.1113/jphysiol.1972.sp009803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ildefonse M., Roy G. Kinetic properties of the sodium current in striated muscle fibres on the basis of the Hodgkin-Huxley theory. J Physiol. 1972 Dec;227(2):419–431. doi: 10.1113/jphysiol.1972.sp010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes R. D., Rojas E., Taylor R. E., Vergara J. Calcium and potassium systems of a giant barnacle muscle fibre under membrane potential control. J Physiol. 1973 Mar;229(2):409–455. doi: 10.1113/jphysiol.1973.sp010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes R. D., Rojas E. The temporal and steady-state relationships between activation of the sodium conductance and movement of the gating particles in the squid giant axon. J Physiol. 1976 Feb;255(1):157–189. doi: 10.1113/jphysiol.1976.sp011274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrino M. Voltage-clamp experiments on frog single skeletal muscle fibres: evidence for a tubular sodium current. J Physiol. 1977 Aug;269(3):605–625. doi: 10.1113/jphysiol.1977.sp011918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore L. E. Effect of temperature and calcium ions on rate constants of myelinated nerve. Am J Physiol. 1971 Jul;221(1):131–137. doi: 10.1152/ajplegacy.1971.221.1.131. [DOI] [PubMed] [Google Scholar]
- Nakajima S., Bastian J. Double sucrose-gap method applied to single muscle fiber of Xenopus laevis. J Gen Physiol. 1974 Feb;63(2):235–256. doi: 10.1085/jgp.63.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumcke B., Fox J. M., Drouin H., Schwarz W. Kinetics of the slow variation of peak sodium current in the membrane of myelinated nerve following changes of holding potential or extracellular pH. Biochim Biophys Acta. 1976 Mar 5;426(2):245–257. doi: 10.1016/0005-2736(76)90335-7. [DOI] [PubMed] [Google Scholar]
- Nonner W. A new voltage clamp method for Ranvier nodes. Pflugers Arch. 1969;309(2):176–192. doi: 10.1007/BF00586967. [DOI] [PubMed] [Google Scholar]
- Pynsent P. B., Rojas E. Voltage clamp and data acquisition method for single myelinated nerve fibre work [proceedings]. J Physiol. 1979 Jun;291:14P–15P. [PubMed] [Google Scholar]
- Rojas E. Gating mechanism for the activation of the sodium conductance in nerve membranes. Cold Spring Harb Symp Quant Biol. 1976;40:305–320. doi: 10.1101/sqb.1976.040.01.031. [DOI] [PubMed] [Google Scholar]
- Schauf C. L., Pencek T. L., Davis F. A. Slow sodium inactivation in Myxicola axons. Evidence for a second inactive state. Biophys J. 1976 Jul;16(7):771–778. doi: 10.1016/S0006-3495(76)85727-X. [DOI] [PMC free article] [PubMed] [Google Scholar]


