Abstract
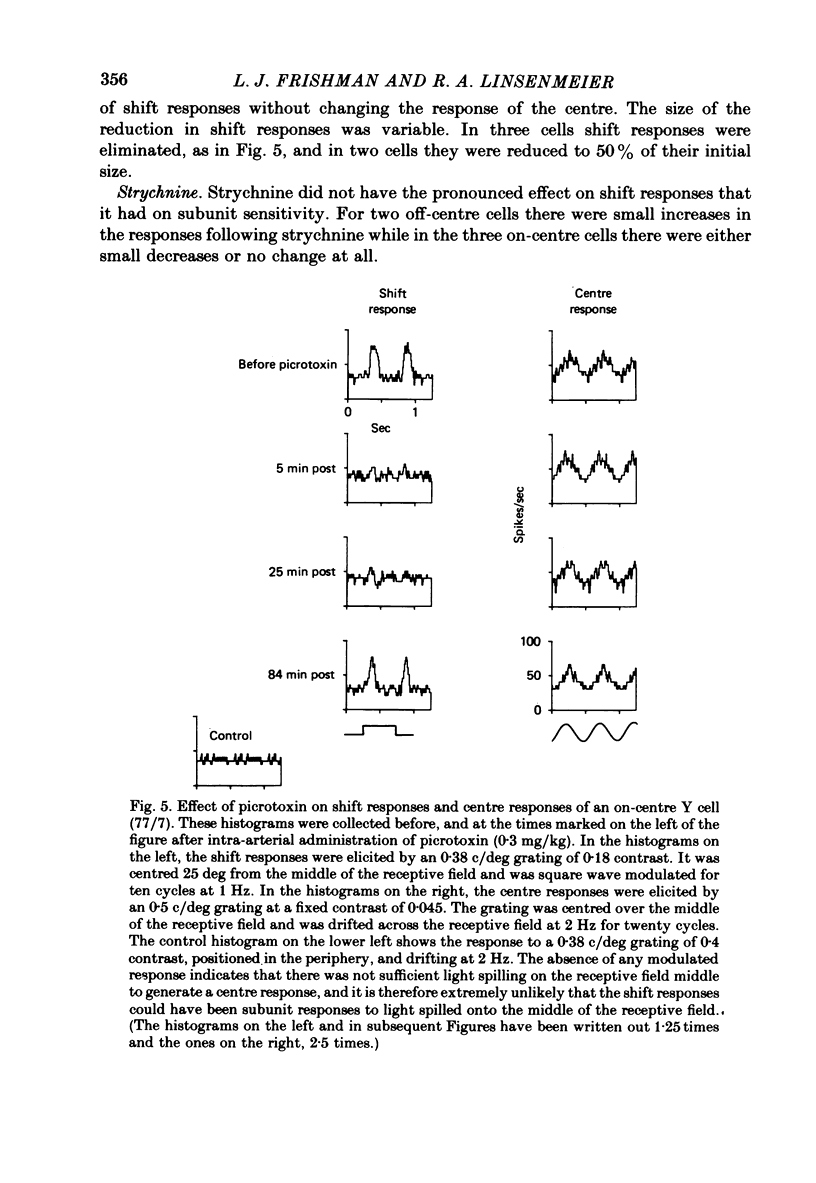
1. The effects of neurotransmitter antagonists on spatially linear and non-linear responses of Y cat retinal ganglion cells were studied. 2. The contrast sensitivity of the spatially linear receptive field centre and surround at mesopic and photopic levels of illumination was affected very little by picrotoxin, but the sensitivity of the non-linear subunits was reduced. 3. Picrotoxin also reduced two other non-linear effects: 'shift responses' and the suppression of the response to a centred test flash caused by movement of a peripheral pattern. 4. In contrast to picrotoxin, strychnine decreased the contrast sensitivity of the receptive field centre, and increased the sensitivity of the subunits. 5. The results support the idea that the non-linear responses may all be generated by similar pathways, which are distinct from those which generate linear responses. Because both picrotoxin and strychnine affect subunit responses, cells using gamma-aminobutyric acid (GABA) and glycine as transmitters are probably involved in subunit pathways.
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker J. L., Nicoll R. A., Padjen A. Studies on convulsants in the isolated frog spinal cord. I. Antagonism of amino acid responses. J Physiol. 1975 Mar;245(3):521–536. doi: 10.1113/jphysiol.1975.sp010859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Derrington A. M., Harris L. R., Lennie P. The effects of remote retinal stimulation on the responses of cat retinal ganglion cells. J Physiol. 1977 Jul;269(1):177–194. doi: 10.1113/jphysiol.1977.sp011898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaventure N., Wioland N., Mandel P. Antagonists of the putative inhibitory transmitter effects of taurine and GABA in the retina. Brain Res. 1974 Nov 15;80(2):281–289. doi: 10.1016/0006-8993(74)90691-x. [DOI] [PubMed] [Google Scholar]
- Bowery N. G., Brown D. A. Depolarizing actions of gamma-aminobutyric acid and related compounds on rat superior cervical ganglia in vitro. Br J Pharmacol. 1974 Feb;50(2):205–218. doi: 10.1111/j.1476-5381.1974.tb08563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell J. H., Daw N. W. Effects of picrotoxin and strychnine on rabbit retinal ganglion cells: changes in centre surround receptive fields. J Physiol. 1978 Mar;276:299–310. doi: 10.1113/jphysiol.1978.sp012234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell J. H., Daw N. W., Wyatt H. J. Effects of picrotoxin and strychnine on rabbit retinal ganglion cells: lateral interactions for cells with more complex receptive fields. J Physiol. 1978 Mar;276:277–298. doi: 10.1113/jphysiol.1978.sp012233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan R. Y., Naka K. The amacrine cell. Vision Res. 1976;16(10):1119–1129. doi: 10.1016/0042-6989(76)90252-2. [DOI] [PubMed] [Google Scholar]
- Cunningham R., Miller R. F. Electrophysiological analysis of taurine and glycine action on neurons of the midpuppy retina. I. Intracellular recording. Brain Res. 1980 Sep 15;197(1):123–138. doi: 10.1016/0006-8993(80)90439-4. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Duggan A. W., Johnston G. A. The specificity of strychnine as a glycine antagonist in the mammalian spinal cord. Exp Brain Res. 1971 Jun 29;12(5):547–565. doi: 10.1007/BF00234248. [DOI] [PubMed] [Google Scholar]
- Derrington A. M., Lennie P., Wright M. J. The mechanism of peripherally evoked responses in retinal ganglion cells. J Physiol. 1979 Apr;289:299–310. doi: 10.1113/jphysiol.1979.sp012738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Goldstick T. K., Linsenmeier R. A. The contrast sensitivity of cat retinal ganglion cells at reduced oxygen tensions. J Physiol. 1980 Jul;304:59–81. doi: 10.1113/jphysiol.1980.sp013310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Hertz G., Lennie P. Cone signals in the cat's retina. J Physiol. 1977 Jul;269(2):273–296. doi: 10.1113/jphysiol.1977.sp011902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Jakiela H. G. Suppression of cat retinal ganglion cell responses by moving patterns. J Physiol. 1980 May;302:49–72. doi: 10.1113/jphysiol.1980.sp013229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer B., Krüger J., Droll W. Quantitative aspects of the shift-effect in cat retinal ganglion cells. Brain Res. 1975 Jan 17;83(3):391–403. doi: 10.1016/0006-8993(75)90832-x. [DOI] [PubMed] [Google Scholar]
- Hochstein S., Shapley R. M. Linear and nonlinear spatial subunits in Y cat retinal ganglion cells. J Physiol. 1976 Nov;262(2):265–284. doi: 10.1113/jphysiol.1976.sp011595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstein S., Shapley R. M. Quantitative analysis of retinal ganglion cell classifications. J Physiol. 1976 Nov;262(2):237–264. doi: 10.1113/jphysiol.1976.sp011594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. S., Roberts M. H., Straughan D. W. Amino-acid induced depression of cortical neurones. Br J Pharmacol. 1970 Apr;38(4):659–666. doi: 10.1111/j.1476-5381.1970.tb09875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby A. W., Enroth-Cugell C. The involvement of gamma-aminobutyric acid in the organization of cat retinal ganglion cell receptive fields. A study with picrotoxin and bicuculline. J Gen Physiol. 1976 Oct;68(4):465–484. doi: 10.1085/jgp.68.4.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby A. W., Schweitzer-Tong D. E. GABA-antagonists and spatial summation in Y-type cat retinal ganglion cells. J Physiol. 1981 Mar;312:335–344. doi: 10.1113/jphysiol.1981.sp013631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby A. W., Schweitzer-Tong D. E. Gaba-antagonists alter spatial summation in receptive field centres of rod- but not cone-drive cat retinal ganglion Y-cells. J Physiol. 1981 Nov;320:303–308. doi: 10.1113/jphysiol.1981.sp013950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby A. W. The effect of strychnine, bicuculline, and picrotoxin on X and Y cells in the cat retina. J Gen Physiol. 1979 Jul;74(1):71–84. doi: 10.1085/jgp.74.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb H., Famiglietti E. V. Rod and cone pathways in the inner plexiform layer of cat retina. Science. 1974 Oct 4;186(4158):47–49. doi: 10.1126/science.186.4158.47. [DOI] [PubMed] [Google Scholar]
- Krüger J., Fischer B. Strong periphery effect in cat retinal ganglion cells. Excitatory responses in ON- and OFF- center neurones to single grid displacements. Exp Brain Res. 1973 Oct 26;18(3):316–318. doi: 10.1007/BF00234601. [DOI] [PubMed] [Google Scholar]
- Landau E. M. The effect of strychnine on the neuro-muscular junction of the rat. Life Sci. 1967 Dec 1;6(23):2515–2517. doi: 10.1016/0024-3205(67)90315-3. [DOI] [PubMed] [Google Scholar]
- Levick W. R. Another tungsten microelectrode. Med Biol Eng. 1972 Jul;10(4):510–515. doi: 10.1007/BF02474199. [DOI] [PubMed] [Google Scholar]
- Linsenmeier R. A., Hertz B. G. Eye movements in paralyzed cats induced by drugs and sympathetic stimulation. Vision Res. 1979;19(11):1249–1252. doi: 10.1016/0042-6989(79)90191-3. [DOI] [PubMed] [Google Scholar]
- Linsenmeier R. A., Jakiela H. G. Non-linear spatial summation in cat retinal ganglion cells at different background levels. Exp Brain Res. 1979 Jul 2;36(2):301–309. doi: 10.1007/BF00238913. [DOI] [PubMed] [Google Scholar]
- Marshall J., Voaden M. Autoradiographic identification of the cells accumulating 3H gamma-aminobutyric acid in mammalian retinae: a species comparison. Vision Res. 1975 Mar;15(3):459–461. doi: 10.1016/0042-6989(75)90102-9. [DOI] [PubMed] [Google Scholar]
- Masland R. H., Mills J. W. Autoradiographic identification of acetylcholine in the rabbit retina. J Cell Biol. 1979 Oct;83(1):159–178. doi: 10.1083/jcb.83.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., McGuire B. A., Sterling P. Interplexiform cell in cat retina: identification by uptake of gamma-[3H]aminobutyric acid and serial reconstruction. Proc Natl Acad Sci U S A. 1980 Jan;77(1):658–661. doi: 10.1073/pnas.77.1.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R., Famiglietti E. V., Jr, Kolb H. Intracellular staining reveals different levels of stratification for on- and off-center ganglion cells in cat retina. J Neurophysiol. 1978 Mar;41(2):472–483. doi: 10.1152/jn.1978.41.2.472. [DOI] [PubMed] [Google Scholar]
- Pourcho R. G. Autoradiographic localization of [3H]muscimol in the cat retina. Brain Res. 1981 Jun 29;215(1-2):187–199. doi: 10.1016/0006-8993(81)90501-1. [DOI] [PubMed] [Google Scholar]
- Shapley R. M., Victor J. D. The effect of contrast on the non-linear response of the Y cell. J Physiol. 1980 May;302:535–547. doi: 10.1113/jphysiol.1980.sp013259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapley R. M., Victor J. D. The effect of contrast on the transfer properties of cat retinal ganglion cells. J Physiol. 1978 Dec;285:275–298. doi: 10.1113/jphysiol.1978.sp012571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So Y. T., Shapley R. Spatial properties of X and Y cells in the lateral geniculate nucleus of the cat and conduction veolcities of their inputs. Exp Brain Res. 1979 Aug 1;36(3):533–550. doi: 10.1007/BF00238521. [DOI] [PubMed] [Google Scholar]
- Toyoda J. Frequency characteristics of retinal neurons in the carp. J Gen Physiol. 1974 Feb;63(2):214–234. doi: 10.1085/jgp.63.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin F. S. Lateral interactions at inner plexiform layer of vertebrate retina: antagonistic responses to change. Science. 1972 Mar 3;175(4025):1008–1010. doi: 10.1126/science.175.4025.1008. [DOI] [PubMed] [Google Scholar]


