Abstract
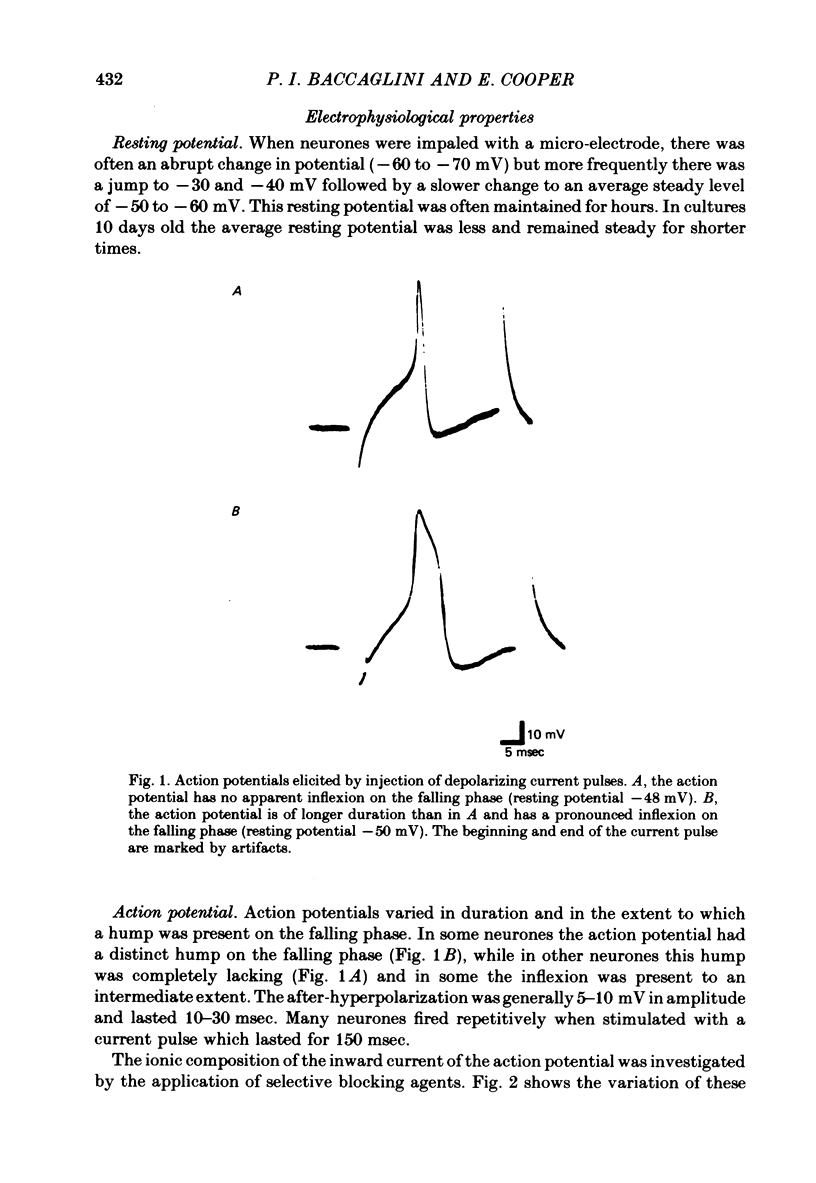
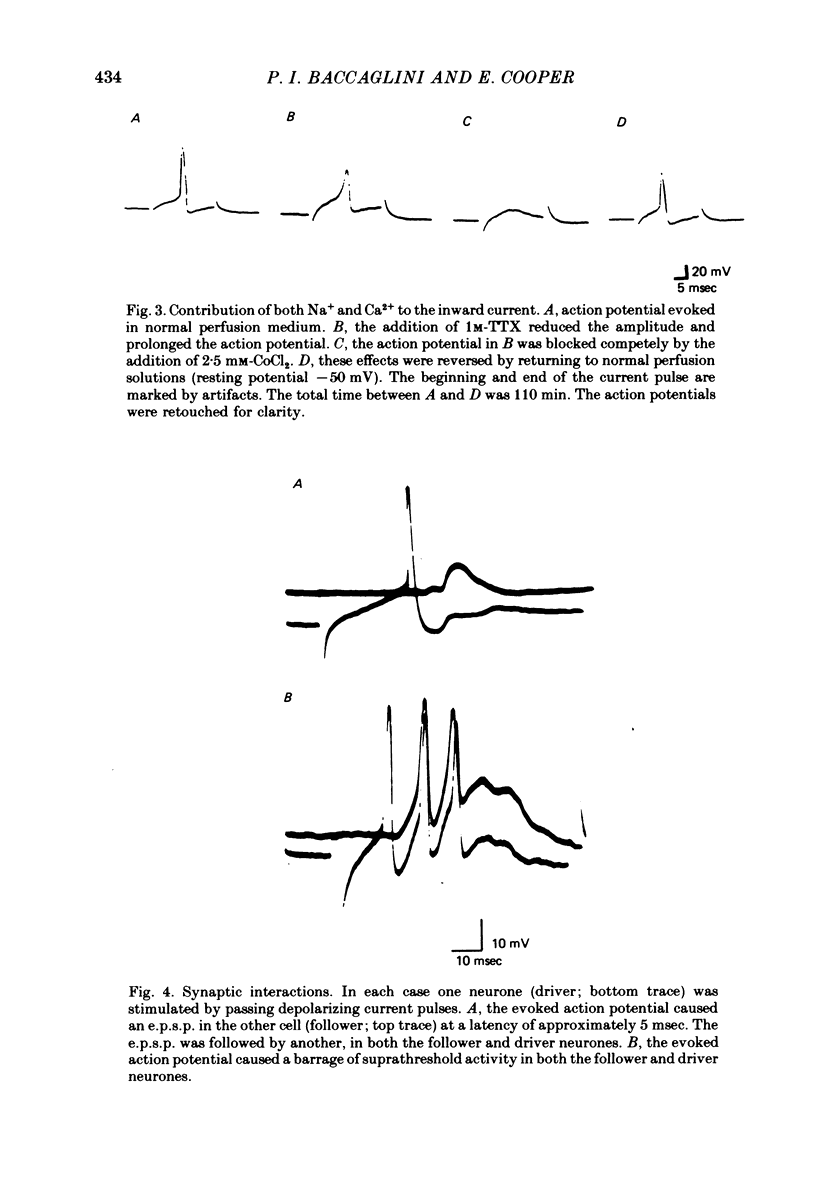
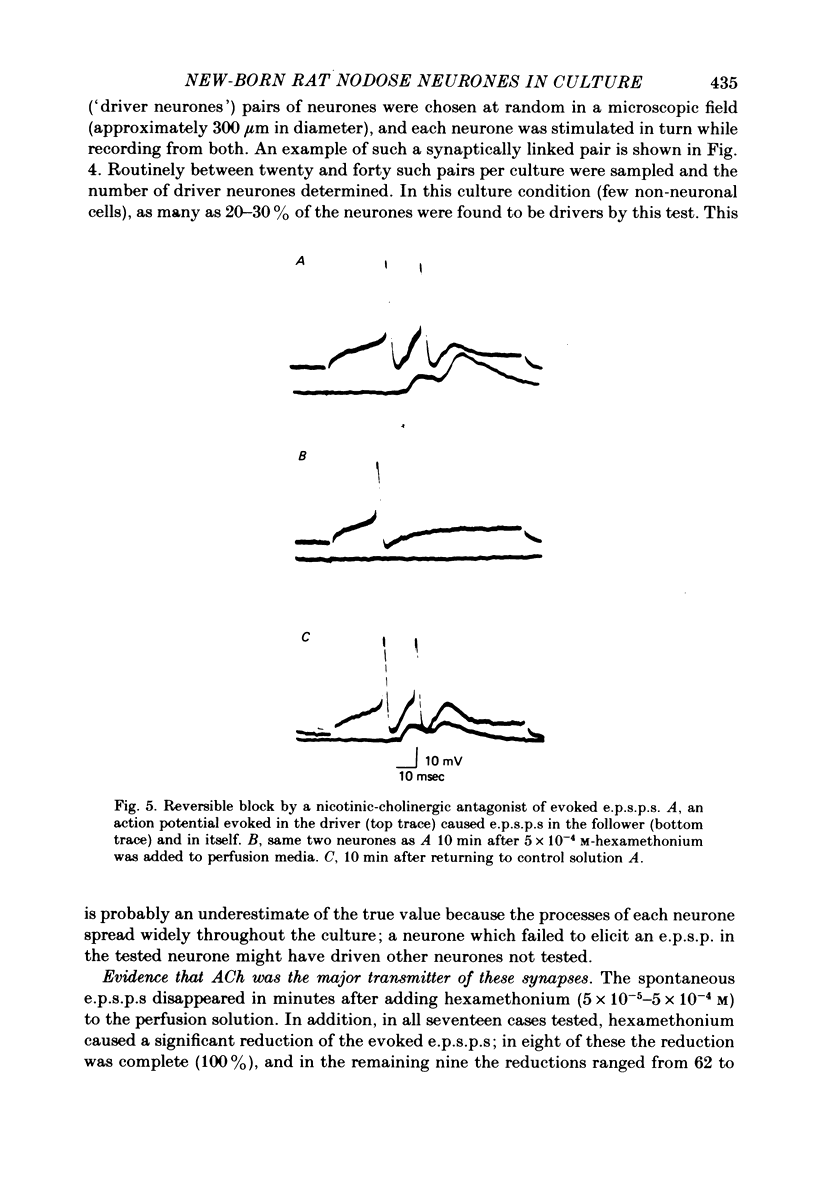
1. Neurones of the nodose ganglion of the vagus nerve were dissociated from new-born rats and grown in the virtual absence of non-neuronal cells and in the presence of nerve growth factor. 2. The resting potentials of the neurones ranged from -40 to -80 mV. Action potentials were of short duration, with no inflexion on the falling phase; others were of longer duration with a hump on the falling phase. 3. The inward current of the action potential was carried either predominantly by Na+ or by Na+ and Ca2+. 4. Tetrodotoxin (1 microM) blocked the Na+ channels of some neurones but in other neurones the Na+ channels were partially or completely resistant to tetrodotoxin (1-10 microM). 5. Many neurones formed excitatory synapses on neighbouring neurones which were blocked or greatly reduced by conventional ganglionic nicotinic antagonists. This indicates that these neurones secreted ACh and expressed ACh receptors at these synapses. 6. The accompanying paper (Baccaglini & Cooper, 1981) reports the effect of co-culturing nodose neurones with non-neuronal cells on the expression of functional nicotinic receptors.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AGOSTONI E., CHINNOCK J. E., DE DALY M. B., MURRAY J. G. Functional and histological studies of the vagus nerve and its branches to the heart, lungs and abdominal viscera in the cat. J Physiol. 1957 Jan 23;135(1):182–205. doi: 10.1113/jphysiol.1957.sp005703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARMETT C. J., RITCHIE J. M. The action of acetylcholine on conduction in mammalian non-myelinated fibres and its prevention by an anticholinesterase. J Physiol. 1960 Jun;152:141–158. doi: 10.1113/jphysiol.1960.sp006476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccaglini P. I., Cooper E. Influences on the expression of acetylcholine receptors on rat nodose neurones in cell culture. J Physiol. 1982 Mar;324:441–451. doi: 10.1113/jphysiol.1982.sp014123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchini V., Angeletti P. U. The nerve growth factor: purification as a 30,000-molecular-weight protein. Proc Natl Acad Sci U S A. 1969 Oct;64(2):787–794. doi: 10.1073/pnas.64.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara M., Kurahashi K., Mizuno N., Nakamura Y. Involvement of nicotinic and muscarinic receptors in synaptic transmission in cat superior cervical ganglions reinnervated by vagal primary afferent axons. J Pharmacol Exp Ther. 1978 Apr;205(1):77–90. [PubMed] [Google Scholar]
- GRAY J. A., DIAMOND J. Pharmacological properties of sensory receptors and their relation to those of the autonomic nervous system. Br Med Bull. 1957 Sep;13(3):185–188. doi: 10.1093/oxfordjournals.bmb.a069611. [DOI] [PubMed] [Google Scholar]
- Gallego R., Eyzaguirre C. Membrane and action potential characteristics of A and C nodose ganglion cells studied in whole ganglia and in tissue slices. J Neurophysiol. 1978 Sep;41(5):1217–1232. doi: 10.1152/jn.1978.41.5.1217. [DOI] [PubMed] [Google Scholar]
- Hawrot E., Patterson P. H. Long-term culture of dissociated sympathetic neurons. Methods Enzymol. 1979;58:574–584. doi: 10.1016/s0076-6879(79)58174-9. [DOI] [PubMed] [Google Scholar]
- Hedlund K. O., Ebendal T. The chick embryo nodose ganglion: effects of nerve growth factor in culture. J Neurocytol. 1980 Oct;9(5):665–682. doi: 10.1007/BF01205032. [DOI] [PubMed] [Google Scholar]
- Helfand S. L., Riopelle R. J., Wessells N. K. Non-equivalence of conditioned medium and nerve growth factor for sympathetic, parasympathetic, and sensory neurons. Exp Cell Res. 1978 Apr;113(1):39–45. doi: 10.1016/0014-4827(78)90085-x. [DOI] [PubMed] [Google Scholar]
- Jaffe R. A., Sampson S. R. Analysis of passive and active electrophysiologic properties of neurons in mammalian nodose ganglia maintained in vitro. J Neurophysiol. 1976 Jul;39(4):802–815. doi: 10.1152/jn.1976.39.4.802. [DOI] [PubMed] [Google Scholar]
- Johnson E. M., Jr, Gorin P. D., Brandeis L. D., Pearson J. Dorsal root ganglion neurons are destroyed by exposure in utero to maternal antibody to nerve growth factor. Science. 1980 Nov 21;210(4472):916–918. doi: 10.1126/science.7192014. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R., Caramia F., Luse S. A., Angeletti P. U. In vitro effects of the nerve growth factor on the fine structure of the sensory nerve cells. Brain Res. 1968 May;8(2):347–362. doi: 10.1016/0006-8993(68)90054-1. [DOI] [PubMed] [Google Scholar]
- Lindsay R. M. Adult rat brain astrocytes support survival of both NGF-dependent and NGF-insensitive neurones. Nature. 1979 Nov 1;282(5734):80–82. doi: 10.1038/282080a0. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Hökfelt T., Nilsson G., Terenius L., Rehfeld J., Elde R., Said S. Peptide neurons in the vagus, splanchnic and sciatic nerves. Acta Physiol Scand. 1978 Dec;104(4):499–501. doi: 10.1111/j.1748-1716.1978.tb06307.x. [DOI] [PubMed] [Google Scholar]
- MATSUMURA M., KOELLE G. B. The nature of synaptic transmission in the superior cervical ganglion following reinnervation by the afferent vagus. J Pharmacol Exp Ther. 1961 Oct;134:28–46. [PubMed] [Google Scholar]
- Mains R. E., Patterson P. H. Primary cultures of dissociated sympathetic neurons. I. Establishment of long-term growth in culture and studies of differentiated properties. J Cell Biol. 1973 Nov;59(2 Pt 1):329–345. doi: 10.1083/jcb.59.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Lague P. H., MacLeish P. R., Nurse C. A., Claude P., Furshpan E. J., Potter D. D. Physiological and morphological studies on developing sympathetic neurons in dissociated cell culture. Cold Spring Harb Symp Quant Biol. 1976;40:399–407. doi: 10.1101/sqb.1976.040.01.038. [DOI] [PubMed] [Google Scholar]
- O'Lague P. H., Potter D. D., Furshpan E. J. Studies on rat sympathetic neurons developing in cell culture. I. Growth characteristics and electrophysiological properties. Dev Biol. 1978 Dec;67(2):384–403. doi: 10.1016/0012-1606(78)90208-7. [DOI] [PubMed] [Google Scholar]
- Paintal A. S. Vagal sensory receptors and their reflex effects. Physiol Rev. 1973 Jan;53(1):159–227. doi: 10.1152/physrev.1973.53.1.159. [DOI] [PubMed] [Google Scholar]
- Talman W. T., Perrone M. H., Reis D. J. Evidence for L-glutamate as the neurotransmitter of baroreceptor afferent nerve fibers. Science. 1980 Aug 15;209(4458):813–815. doi: 10.1126/science.6105709. [DOI] [PubMed] [Google Scholar]
- Thoenen H., Barde Y. A. Physiology of nerve growth factor. Physiol Rev. 1980 Oct;60(4):1284–1335. doi: 10.1152/physrev.1980.60.4.1284. [DOI] [PubMed] [Google Scholar]
- Vera C. L., Luco J. V. Reinnervation of smooth and striated muscle by sensory nerve fibers. J Neurophysiol. 1967 May;30(3):620–627. doi: 10.1152/jn.1967.30.3.620. [DOI] [PubMed] [Google Scholar]
- Yoshida S., Matsuda Y., Samejima A. Tetrodotoxin-resistant sodium and calcium components of action potentials in dorsal root ganglion cells of the adult mouse. J Neurophysiol. 1978 Sep;41(5):1096–1106. doi: 10.1152/jn.1978.41.5.1096. [DOI] [PubMed] [Google Scholar]



