Abstract
A core difficulty in developmental dyslexia is the accurate specification and neural representation of speech. We argue that a likely perceptual cause of this difficulty is a deficit in the perceptual experience of rhythmic timing. Speech rhythm is one of the earliest cues used by infants to discriminate syllables and is determined principally by the acoustic structure of amplitude modulation at relatively low rates in the signal. We show significant differences between dyslexic and normally reading children, and between young early readers and normal developers, in amplitude envelope onset detection. We further show that individual differences in sensitivity to the shape of amplitude modulation account for 25% of the variance in reading and spelling acquisition even after controlling for individual differences in age, nonverbal IQ, and vocabulary. A possible causal explanation dependent on perceptual-center detection and the onset-rime representation of syllables is discussed.
Children with developmental dyslexia have specific problems with reading and spelling that cannot be accounted for by low intelligence, poor educational opportunities, or obvious sensory/neurological damage. The accepted core problem across languages is a deficit in phonological representation (1, 2), although this can be accompanied by other deficits (3, 4). In developmental theories of language acquisition, phonological or speech-based representations in children no longer are thought to be organized around phonemic segments from the outset (5). The explicit phonemic representation of speech is thought to depend on being taught to read an alphabetic script (6). If it is accepted that phonemic representation is a product of literacy and constitutes a psychological process that is not logically necessary for speech perception and production (7), then the phonological deficit in dyslexia must arise at a developmentally earlier level of phonological representation than the phoneme. Obvious candidate levels are those of the syllable (pop-si-cle, gar-den) and onset rime (s-eat, sw-eet, str-eet). Phonological awareness of the syllables, onsets, and rimes in words develops before literacy across languages. For example, prereaders learning to speak English, Chinese, and German all perform well in “oddity” tasks in which they must select the odd word out that does not rhyme (e.g., pat, hat, man; refs. 8–10). Onset-rime awareness tasks (but not usually syllabic-awareness tasks) also are good predictors of literacy acquisition across languages (11–13). A perceptual deficit in the mechanisms used to extract the suprasegmental attributes of the speech stream thus may help to cause the phonological awareness and literacy problems characteristic of developmental dyslexia across orthographies.
Rhythm in speech is a property of the slow amplitude modulation (AM) of the waveform (14), corresponding roughly to the AM associated with syllables. Previous studies have shown that dyslexic children have difficulty in detection of AM and frequency modulation at rates (2–10 Hz) similar to those seen at the syllable level in speech (15, 16). To investigate the impact that these difficulties might have on the perception of rhythm in acoustic signals, we designed a perceptual task in which AM was varied to affect the perception of distinct, discrete “beats” in the auditory stream. Potential differences in the psychometric function for beat detection between dyslexic and matched control children, and between precocious readers and their matched controls, then were investigated.
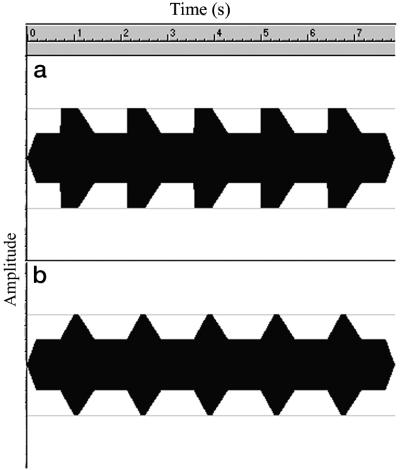
Our psychophysical task was based on a rate of AM change task, which takes advantage of the relationship between beat perception and the shape of AM change (17). The task was based on a sinusoid that was modulated in amplitude to a depth of 50%. Within this, the rate of amplitude change only was varied by varying the rise time of the modulation, while the overall rate of modulation was held constant at 0.7 Hz (see Fig. 1). Very slow rise times (>250 ms) give the percept of a continuous sound that varies in loudness. When the rise time is shortened sufficiently, however (e.g., to 120 ms), the percept changes to that of a continuous sound with a loud beat occurring rhythmically at the same rate as the modulation (18). Given that aspects of syllable processing (i.e., onset-rime awareness) are poorer in dyslexic children, we predicted that they would have poorer sensitivity to the perceptual consequences of AM than control children. As rise time is varied, dyslexic children should evidence less change in AM-related experiences of beat perception than their controls. Two studies of beat detection were carried out. In the first, dyslexic children were compared with reading and chronological age-matched control children in a cross-sectional design. In the second, young early readers participating in a longitudinal study were compared with their matched controls from the same study. We predicted that precocious readers should be significantly more sensitive to variations of rise time in the amplitude-modulated sequences than their controls, as indexed by their perception of beats.
Fig 1.
Examples of the stimulus wave form for rise times of 15 (a) and 300 (b) ms.
The “phonological deficit” in developmental dyslexia is indexed typically by behavioral difficulties in three related areas of phonological processing, all of which we expected to be related to beat detection. We therefore measured phonological awareness (using the rhyme oddity task), rapid “automatized” naming (or RAN) of letters and pictures, and phonological short-term memory (PSTM, repetition of triples of nonwords) in our dyslexic children and their controls. We also measured spelling as well as reading. In languages other than English, developmental dyslexia is diagnosed on the basis of a severe spelling deficit accompanied by extremely slow performance in phonological processing tasks (because orthographic transparency makes decoding very accurate). To contribute to developmental dyslexia across languages, therefore, beat detection should be related to spelling as well as to reading.
On the basis of the prior basic auditory processing literature with dyslexic children, we also included two rapid temporal-processing tasks in our study. One was a version of the rapid-frequency discrimination (RFD) task pioneered by Tallal and coworkers (19). The other was a temporal order-judgement (TOJ) task based on easily labeled environmental sounds (dog/car horn). We expected dyslexic children to show deficits in both tasks, because both require rapid spectrotemporal integration (suggested to be the basis of the phonological deficit in dyslexia by Tallal et al. in refs. 20 and 21). However, deficits in rapid spectrotemporal integration (and processing related acoustic cues such as place of articulation and voice-onset time) have not always been found in dyslexic children (22).
Methods
Subjects.
One hundred and one children were tested, of whom 24 had a statement of dyslexia from their local education authority. In the United Kingdom, “statements of dyslexia” depend on extensive testing by educational psychologists and are the basis for service provision. Twenty of the dyslexic children were at special schools with curricula focused on remediating the phonological deficit. Because of this remediation, single-word decoding for this group was in the normal range. Subject characteristics are shown in Table 1. None of the dyslexic children had additional difficulties (e.g., dyspraxia, attention deficit/hyperactivity disorder, autistic spectrum disorder, or specific language impairment) according to their specialist assessments. The control children for the dyslexics (n = 49) were drawn from local schools and comprised those in the right age/reading age range whose parents returned consent forms. Twenty-eight children who were participating in a longitudinal study of precocious readers were tested also with the beat-detection task. All of these participants had been followed from 4 years of age and were aged 11 at the time of testing. Of these children, 14 were young early readers, and 14 were young early controls who had been individually matched to the young early readers on the basis of socioeconomic status and vocabulary ability at age 4 (23).
Table 1.
Participant characteristics
| Group
|
Standardized tests | ||||
|---|---|---|---|---|---|
| Dyslexic | CA match | RL match | Young early readers | Non-early readers | |
| N | 24 | 25 | 24 | 14 | 14 |
| Age in years and months | 9, 0 (11) | 9, 0 (8) | 7, 11 (4) | 11, 4 (4) | 11, 4 (4) |
| Reading standard score | 101.1 (11.7) | 142.5 (14.7) | 108.3 (13.0) | 117.4 (3.6) | 110.9 (5.5) |
| Spelling standard score | 69.0 (12.1) | 107.8 (16.2) | 85.5 (12.1) | 124.4 (8.3) | 109.7 (6.7) |
| Nonword reading/20 | 7.4 (5.5) | 15.7 (4.0) | 11.3 (5.1) | — | — |
| IQ | 109.1 (11.4) | 111.9 (11.0) | 105.7 (10.6) | 50.4 (3.3) | 47.8 (5.4) |
Standard deviations are shown in parentheses.
Dyslexics and CA and RL controls: British Ability Scales. Young early readers, non-early readers, WORD.
Dyslexics and CA and RL controls: WISC. Young early readers, non-early readers, Ravens raw score.
Auditory Processing Tasks.
AM/beat-perception task.
The children were presented with 7.857-s sound sequences, all of which were sinusoidal carriers at 500 Hz, were amplitude-modulated at a rate of 0.7 Hz, and had a depth of 50%. The underlying modulation envelope was based on a square wave, but the fall time was fixed at 350 ms, and the rise time could be varied from 15 to 300 ms (logarithmically spaced over a continuum of 40 stimuli). Before testing, the children were trained by using the two extremes of the continuum. The 15-ms stimulus (which yielded a clear beat) was presented as the sound of two toys (Tigger and Eeyore) swinging on a double-toy swing. The back-and-forth rhythm of their swing coincided with the beat in the signal. The 300-ms stimulus was presented as the sound of Winnie the Pooh sliding down a solid plastic straw in the form of a spiral (he got nearer to the child or further away as the training sound got louder and quieter, respectively). The children then were asked to decide whether subsequent stimuli (given by computer through headphones) belonged to Winnie the Pooh or to Tigger and Eeyore. Performance on this task was measured by using Levitt's adaptive procedure (24) with modifications to increase efficiency (25). Two independent adaptive tracks were used to estimate the points on the rise-time continuum at which the stimuli were labeled as Winnie the Pooh 29 and 71% of the time, with a maximum of 40 trials. Tracks started at the endpoints of the continuum, with rise times of 15 and 300 ms. The categorization function was derived from all trials in a particular test, and summary statistics for slope and category boundary estimated by Probit analysis (26). Shallower slopes indicate less sensitivity to variations in the acoustic feature varied across the continuum (here, rise time).
RFD task.
The nonspeech same/different task was similar to that described in ref. 19. The basic stimuli were two vowel-like 50-ms complex periodic tones (rise and fall times of 5 ms) with fundamental frequencies of 100 and 305 Hz. Every trial consisted of two stimuli presented sequentially with an interstimulus interval (ISI) of 0, 10, 50, 100, or 400 ms. All four possible stimulus orders were presented (low-low, low-high, high-low, and high-high), and listeners responded by indicating “same” or “different.” Trials were presented in a random order, with one occurrence of each ISI and stimulus order, making 20 trials (5 ISIs × 4 orders).
Dog/car-horn TOJ task.
This task used two stimuli that were readily identifiable without prior training as a dog bark and a car horn. The dog bark was aperiodic, whereas the car horn was periodic with a fundamental frequency of ≈400 Hz. Starting from sounds accompanying a children's computer game, various manipulations of amplitude envelope and duration were used to create stimuli with a total duration of 115 ms each, with rise and fall times of 5 ms. The two stimuli then were normalized to have the same rms level. The continuum of sounds consisted of 204 stimuli in which the stimulus onset asynchrony varied from +405 ms (horn leading dog) to −405 ms (dog leading horn) in 4-ms steps. Stimuli were allowed to overlap to the degree necessary to create the specified stimulus onset asynchronys. For testing, the same adaptive procedure was used as for the beat-detection task, but the children indicated simply which sound they heard first. If dyslexic children are poorer at TOJs, then their psychometric functions in this task also should be flatter than those of controls.
Phonological Processing Tasks.
Oddity task.
The child listened to sets of three words and had to select the nonrhyme (e.g., gap, nap, Jack).
PSTM.
The child listened to sets of three nonwords and had to repeat them (e.g., loff, bup, heg). Both tasks were presented via headphones by using digitized speech.
RAN task.
The child had to name familiar pictures and letters under timed conditions.
Standardized Psychometric Tests.
The children received four subsets of the Wechsler Intelligence Scale for Children (WISC): blocks, picture arrangement, similarities, and vocabulary. The British Ability Scales reading, spelling, and mathematics subscales also were administered, along with the Graded Test of Nonword Reading (27).
Results
Beat Detection in Dyslexic and Normally Reading Children.
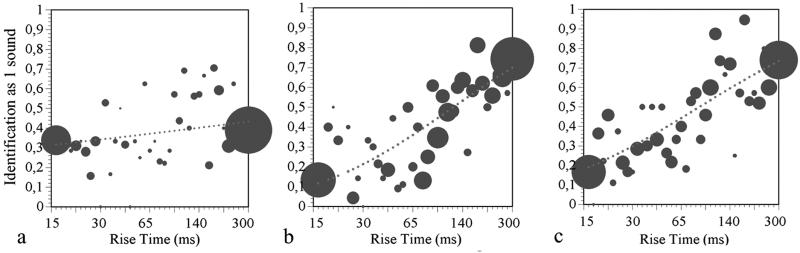
A significant difference was found between the group of dyslexic children and their chronological age controls in the slope of the categorization function (see Fig. 2), with the dyslexics showing flatter slopes as predicted [mean slope = −0.03 for dyslexics (SD = 0.04) and −0.12 for controls (SD = 0.08), P < 0.000]. The reading age match controls showed intermediate slopes (mean slope = −0.06, SD = 0.05). Detection of beats in AM signals thus was poorer in the dyslexic children than in their peers and seemed to vary with reading level.
Fig 2.
Bubble plots of the psychometric functions from the dyslexic (a), chronological age (CA) control (b), and reading level (RL) control (c) groups for the beat-detection task. The size of the bubbles represents the number of trials.
To explore the relationship between beat detection and phonological processing, reading and spelling, partial correlations controlling for age, and WISC IQ (WISC short form) were calculated. Group performance in the behavioral tasks is shown in Table 2, and the partial correlations are shown in Table 3. As predicted, there were highly significant relationships between beat detection and RAN, phonological memory, phonological awareness, reading, spelling, and nonword reading. On the rapid spectrotemporal integration hypothesis, significant relationships with phonological processing and literacy would be expected also for the RFD and the dog/car-horn TOJ tasks (group performance shown in Figs. 3 and 4); this was the case, but the relationships found were not as strong as those for the beat-detection task (see Table 3). Both the RFD task and the beat-detection task showed a significant relationship with mathematical ability, which was not predicted. This result could reflect the short-term memory demands of the mental arithmetic tasks in the standardized mathematical assessment used here.
Table 2.
Mean performance for the dyslexics and CA and RL controls on the behavioral tasks
| Dyslexics | CA match | RL match | |
|---|---|---|---|
| Oddity, % correct | 46.9 (16.7) | 74.2 (12.2) | 53.5 (14.3) |
| PSTM, % phonemes correct | 79.0 (8.8) | 86.5 (5.1) | 79.9 (10.0) |
| RAN mean speed, sec | 36.7 (7.5) | 29.1 (3.7) | 34.6 (5.6) |
| Mathematics, standard score | 92.7 (20.3) | 114.6 (16.0) | 94.4 (14.0) |
| Beat detection: slope | −0.03 (−0.04) | −0.12 (−0.08) | −0.06 (−0.05) |
| RFD task, % correct | 75.7 (13.3) | 88.6 (10.7) | 72.3 (18.2) |
| Dog/car-horn TOJ: slope | −0.03 (−0.02) | −0.04 (−0.03) | −0.03 (−0.02) |
Standard deviations are shown in parentheses.
Dyslexics < CA at P < 0.05.
RL < CA at P < 0.05.
Table 3.
Partial correlations between the basic auditory-processing measures and the experimental variables controlling for age and WISC IQ
| P-center slope | Dog/car TOJ | RFD | |
|---|---|---|---|
| Reading | −0.59 | 0.27 | 0.40 |
| Spelling | −0.56 | 0.25 | 0.29 |
| Nonword reading | −0.43 | 0.20 | 0.42 |
| Mathematics | −0.34 | 0.06 | 0.24 |
| Oddity | −0.43 | 0.28 | 0.40 |
| RAN | 0.36 | −0.12 | −0.23 |
| PSTM | −0.36 | 0.25 | 0.38 |
| P center | −0.25 | −0.32 | |
| Dog/car-horn TOJ | −0.25 | 0.45 | |
| RFD | −0.32 | 0.45 |
P < 0.0001.
P < 0.05.
P < 0.01.
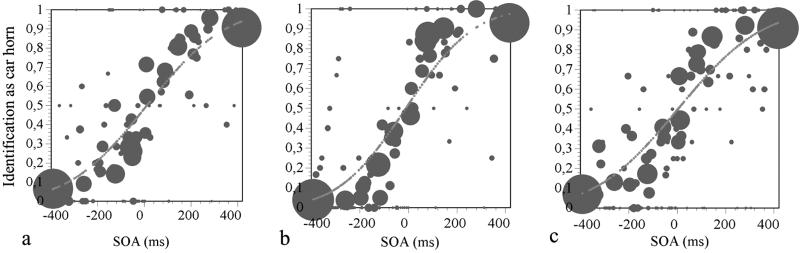
Fig 3.
Bubble plots of the psychometric functions from the dyslexic (a), CA control (b), and RL control (c) groups for the dog/car-horn TOJ task. The stimulus onset asynchrony (SOA) values refer to the stimulus onset asynchrony of the dog in relation to the car horn (e.g., −400 ms means the dog barked 400 ms before the horn sounded).
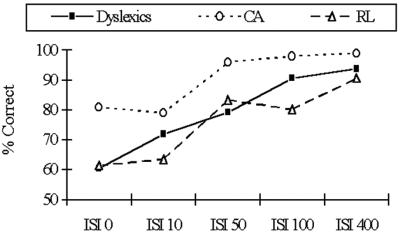
Fig 4.
Performance on the RFD task by group. ISI, interstimulus interval.
If basic auditory processing is important in causing the phonological deficit that characterizes developmental dyslexia, then measures of basic auditory processing should predict reading, spelling, and phonological ability even when age, nonverbal IQ, and vocabulary are controlled. To determine predictive relationships, a series of four-step fixed-entry multiple regression equations were computed on the data set (73 children). The dependent variables were reading ability, spelling ability, nonword reading, rime oddity, RAN, and PSTM. The independent variables were (in a fixed order) (i) age, (ii) nonverbal IQ, (iii) vocabulary, and (iv) an auditory-processing measure (beat detection, RFD, or dog/car-horn TOJ). The beat-detection measure accounted for an additional 25% of the variance in reading and spelling in these stringent analyses (see Table 4). The RFD measure did not predict spelling, but it did predict reading and nonword reading, accounting for an additional 10 and 12% of the variance, respectively. The TOJ measure was less sensitive, predicting a significant proportion of the variance in reading (6%) only. All three measures predicted phonological awareness and PSTM, but only the beat-detection measure predicted RAN performance (see Table 4).
Table 4.
Percentage of variance in reading, spelling, nonword reading, phonological awareness (oddity), PSTM, and RAN explained by the different independent variables in separate fixed-entry multiple-regression equations
|
|
Dependent variable (columns show separate equations), R2 | |||||
|---|---|---|---|---|---|---|
| Reading | Spelling | Nonword R | Oddity | PSTM | RAN | |
| Step 1: age | 0.09 | 0.03 | 0.01 | 0.00 | 0.00 | 0.11 |
| Step 2: blocks | 0.05 | 0.04 | 0.04 | 0.13 | 0.00 | 0.05 |
| Step 3: vocabulary | 0.11 | 0.07 | 0.02 | 0.04 | 0.05 | 0.00 |
| Step 4: P center | 0.25 | 0.25 | 0.14 | 0.13 | 0.12 | 0.08 |
| Step 4: RFD | 0.10 | 0.04 | 0.12 | 0.09 | 0.13 | 0.01 |
| Step 4: Dog/car horn | 0.06 | 0.05 | 0.03 | 0.06 | 0.06 | 0.00 |
Steps 1–3 were always the same (age, nonverbal IQ, and vocabulary). Step 4 was a basic auditory-processing variable (P centers, RFD, or dog/car TOJ).
P < 0.01.
P < 0.05.
P < 0.001.
Whereas the RFD and TOJ tasks are thought to tap the ability to detect rapid acoustic change (at a time scale of <40 ms), the rise times that yield the perceptual experience of beats are considerably longer in duration (up to 150 ms or more). To determine whether there was overlap in the variance in reading accounted for by the beat-detection and RFD tasks, a pair of five-step multiple regression equations were computed, both entering (i) age, (ii) nonverbal IQ, and (iii) vocabulary followed by the two auditory measures in either order. When entered last, the beat-detection measure accounted for an additional 19% of the variance in reading (P < 0.001). The RFD measure entered last accounted for an additional 4% (P < 0.02). A large proportion of the variance in reading predicted by the RFD task was clearly shared with the beat-detection task but not vice versa. To determine whether individual differences in these basic processing abilities still would be predictive of reading even when phonological awareness was controlled, a second pair of five-step multiple regression equations were computed, entering (i) age, (ii) nonverbal IQ, (iii) vocabulary, (iv) oddity, and (v) beat detection or RFD. Here only beat detection remained a significant predictor of reading, accounting for an additional 9% of the variance (P < 0.001, the oddity measure at step four accounted for 31% of the variance in reading).
Beat Detection in Young Early Readers and Normally Developing Children.
As a further test of the hypothesis that AM-driven beat detection is associated with the phonological determinants of reading ability, we also assessed beat detection in a group of young early readers who had learned to read without parental instruction before entering school (see ref. 23). These children, now aged 11, had taught themselves to read on the basis of their superior phonological skills at age 4. Theoretically, these superior phonological skills may have developed at least partly because of excellent rhythm perception (i.e., enhanced ability to perceive beats in amplitude-modulated sequences). Compared with control children from the same longitudinal study, the young early readers showed greater sensitivity to beats, with significantly sharper psychometric functions [young early readers, mean slope = −0.14 (SD 0.06), matched controls = −0.10 (SD 0.04), P < 0.04]. Sensitivity to AM was also significantly related to reading progress in this cohort, both in terms of reading comprehension (r = −0.42) and development of the orthographic lexicon (word chains test, r = −0.43).
Discussion
Our hypothesis was that the potential deficits in AM and frequency-modulation detection in dyslexic individuals reported by other groups (see refs. 15 and 16) might relate to deficits in the processing of acoustic structure at the level of the syllable. This processing is best described as rhythm detection. If this version of a syllabic hypothesis is correct, then children with phonological developmental dyslexia should be characterized by poorer AM beat detection. This hypothesis was supported. Dyslexic children showed significantly inferior detection of AM beats compared with controls, and children with superior literacy acquisition showed significantly superior detection of AM beats. This report demonstrates a developmental continuum in a basic auditory-processing ability (beat detection) from dyslexic to exceptional child readers.
Theoretically, the detection of beats in AM sequences such as those used here corresponds to the detection of “perceptual centers” (P centers) in acoustic signals. P centers are the perceptual moments of occurrence in speech (28) and musical (29) sounds. Determined by the onsets of signals (30), P centers are associated in speech with rapid increases of midband spectral energy, typically occurring around the onset of a vowel (31). From a speech-development perspective therefore, they constitute a nonspeech-specific mechanism for segregating syllable onsets and rhymes. Their accurate detection should be important for the quality of phonological representation. In line with this hypothesis, beat detection was shown to be related to individual differences in phonological processing, although the strongest relationships found were for reading and spelling progress. Beat detection was a significant predictor of literacy even when phonological processing was controlled, which could reflect developmental factors. Stronger relationships between beat detection and phonological processing might be found in younger children who are just beginning to read. Note that because beats/P centers are a consequence of the processing of complex sound, both speech and nonspeech, it is difficult to argue that differences in such sensitivity are a product of reading acquisition. Nevertheless, this possibility cannot be ruled out on the basis of the current data.
Working from the association of beats in a perceptual sequence and P centers, our hypothesis is that the primary auditory-processing deficit in dyslexia is related to P-center processing of speech and nonspeech sounds. AM rise time contributes to this perceptual primitive, and thus other observed auditory deficits (e.g., auditory-stream segregation and backward masking; refs. 32–34) may arise in part because the stimuli used in these judgement tasks of necessity have P centers. A P-center hypothesis also can explain dyslexic children's difficulties in producing speech in time with a metronome and finger-tapping in time with a metronome or an internally generated rhythm (35, 36). It further explains why a focus on rhyme and rhythm in preschool (e.g., clapping out nursery rhymes, which in effect gives children practice in coordinating a manual rhythm with the P centers of certain syllables) is important for later literacy development across languages (37, 38). Note, however, that the potential P-center deficit in dyslexia is a subtle one. The deficit is not sufficient to interfere markedly with the acquisition of spoken language, although spoken-language processing in metalinguistic tasks remains effortful and slow. More serious deficits in P-center perception theoretically should cause spoken- as well as written-language impairments of the kind found in specific language impairment.
In the current study, two measures of rapid spectrotemporal integration (RSI) were also administered to the dyslexic children and their controls. These two tasks were highly correlated and were both performed poorly by dyslexic children. These tasks measure the importance of rapid changes in the signal, which should affect the child's ability to detect changes in speech at the segmental level (e.g., “p” vs. “b”). The beat-detection task measures the importance of the syllabic information given by amplitude envelope onsets, which in speech affect suprasegmental attributes of the vowel onsets. Both aspects of auditory processing seem to be poor in dyslexic children, but most of the variance in reading accounted for by the RSI tasks is shared with beat detection (although not vice versa). As children become aware of onsets and rimes without being taught to read, we argue that the ability to process amplitude envelope onsets accurately may constitute the primary deficit in developmental dyslexia. Detailed experiments, ideally across languages, are required to test this hypothesis further.
Acknowledgments
We thank the children who participated in this research and their schools. Research at the Institute of Child Health and Great Ormond Street Hospital benefits from research and development funding received from the National Health Service Executive.
Abbreviations
AM, amplitude modulation
RAN, rapid automatized naming
PSTM, phonological short-term memory
RFD, rapid-frequency discrimination
TOJ, temporal order judgement
CA, chronological age
RL, reading level
WISC, Wechsler Intelligence Scale for Children
P center, perceptual center
References
- 1.Goswami U. (2000) Dyslexia 6, 133-151. [DOI] [PubMed] [Google Scholar]
- 2.Snowling M. J., (2000) Dyslexia (Blackwell, Oxford).
- 3.Stein J. & Walsh, V. (1997) Trends Neurosci. 20, 147-152. [DOI] [PubMed] [Google Scholar]
- 4.Nicholson R., Fawcett, A. & Dean, P. (1996) Ann. Dyslexia 46, 259-283. [DOI] [PubMed] [Google Scholar]
- 5.Metsala J. L. & Walley, A. C. (1998) in Word Recognition in Beginning Literacy, eds. Metsala, J. L. & Ehri, L. C. (Erlbaum, Hillsdale, NJ), pp. 89–120.
- 6.Boucher V. J. (1994) J. Phon. 22, 1-18. [Google Scholar]
- 7.Warren R. M., Bashford, J. A. & Gardner, D. A. (1990) Percept. Psychophys. 47, 423-432. [DOI] [PubMed] [Google Scholar]
- 8.Bradley L. & Bryant, P. E. (1978) Nature (London) 271, 746-747. [DOI] [PubMed] [Google Scholar]
- 9.Siok W. T. & Fletcher, P. (2001) Dev. Psychol. 37, 886-899. [PubMed] [Google Scholar]
- 10.Wimmer H., Landerl, K. & Schneider, W. (1994) Br. J. Dev. Psychol. 12, 469-484. [Google Scholar]
- 11.Hoien T., Lundberg, L., Stanovich, K. E. & Bjaalid, I. K. (1995) Read Writing 7, 171-188. [Google Scholar]
- 12.Bradley L. & Bryant, P. E. (1983) Nature (London) 310, 419-421. [Google Scholar]
- 13.Lundberg I., Olofsson, A. & Wall, S. (1980) Scand. J. Psychol. 21, 159-173. [Google Scholar]
- 14.Rosen S. (1992) Philos. Trans R. Soc. London B 336, 367-373. [DOI] [PubMed] [Google Scholar]
- 15.Talcott J. B., Witton, C., McLean, M. F., Hansen, P. C., Rees, A., Green, G. G. R. & Stein, J. F. (2000) Proc. Natl. Acad. Sci. USA 97, 2952-2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Witton C., Talcott, J. B., Hansen, P. C., Richardson, A. J., Griffiths, T. D., Rees, A., Stein, J. F. & Green, G. G. R. (1998) Curr. Biol. 8, 791-797. [DOI] [PubMed] [Google Scholar]
- 17.Scott S. K. (1998) Psychol. Res. 61, 4-11. [Google Scholar]
- 18.Bregman A. S. (1993) in Thinking in Sound: The Cognitive Psychology of Human Audition, eds. McAdams, S. & Bigand, E. (Oxford Univ. Press, Oxford), pp. 10–36.
- 19.Tallal P. & Piercy, M. (1973) Nature (London) 241, 468-469. [DOI] [PubMed] [Google Scholar]
- 20.Tallal P. (1980) Brain Lang. 9, 182-198. [DOI] [PubMed] [Google Scholar]
- 21.Tallal P., Merzenich, M. M., Miller, S. & Jenkins, W. (1998) Exp. Brain Res. 123, 210-219. [DOI] [PubMed] [Google Scholar]
- 22.McArthur G. M. & Bishop, D. V. M. (2001) Dyslexia 7, 150-170. [DOI] [PubMed] [Google Scholar]
- 23.Stainthorp R. & Hughes, D. (1998) J. Res. Read 21, 53-68. [Google Scholar]
- 24.Levitt H. (1971) J. Acoust. Soc. Am. 49, 467-477. [PubMed] [Google Scholar]
- 25.Baker R. J. & Rosen, S. (2001) Br. J. Audiol. 35, 43-52. [DOI] [PubMed] [Google Scholar]
- 26.Finney D. J., (1971) Probit Analysis (Cambridge Univ. Press, Cambridge, U.K.).
- 27.Snowling M. J., Stothard, S. E. & McLean, J., (1996) The Graded Nonword Reading Test (Thames Valley Test Co., Reading, U.K.).
- 28.Morton J., Marcus, S. M. & Frankish, C. (1976) Psychol. Rev. 83, 405-408. [Google Scholar]
- 29.Gordon J. W. (1987) J. Acoust. Soc. Am. 82, 88-105. [DOI] [PubMed] [Google Scholar]
- 30.Vos J. & Rasch, R. (1981) Percept. Psychophys. 29, 323-335. [DOI] [PubMed] [Google Scholar]
- 31.Marcus S. M. (1981) Percept. Psychophys. 30, 247-256. [DOI] [PubMed] [Google Scholar]
- 32.Helenius P., Uutela, K. & Hari, R. (1999) Brain 122, 907-913. [DOI] [PubMed] [Google Scholar]
- 33.Wright B. A., Bowen, R. W. & Zecker, S. G. (2000) Curr. Opin. Neurobiol. 10, 482-486. [DOI] [PubMed] [Google Scholar]
- 34.Rosen S. & Manganari, E. (2001) J. Speech Lang Hear. Res. 44, 720-736. [DOI] [PubMed] [Google Scholar]
- 35.Wolff P. H., Michel, G. F. & Ovrut, M. (1990) J. Speech Hear. Res. 33, 281-289. [DOI] [PubMed] [Google Scholar]
- 36.Wolff P. H. (1993) Ann. N.Y. Acad. Sci. 682, 87-103. [DOI] [PubMed] [Google Scholar]
- 37.Lundberg I., Frost, J. & Petersen, O. P. (1988) Read. Res. Q. 23, 263-284. [Google Scholar]
- 38.Schneider W., Roth, E. & Ennemoser, M. (2000) J. Educ. Psychol. 92, 284-295. [Google Scholar]