Abstract
We have studied the subcellular localization of the acid S-like ribonuclease (RNase) LX in tomato (Lycopersicon esculentum Mill.) cells using a combination of biochemical and immunological methods. It was found that the enzyme, unexpectedly excluded from highly purified vacuoles, accumulates in the endoplasmic reticulum. The evidence that RNase LX is a resident of the endoplasmic reticulum (ER) is supported by an independent approach showing that the C-terminal peptide HDEF of RNase LX acts as an alternative ER retention signal in plants. For functional testing, the cellular distribution of chimeric protein constructs based on a marker protein, Brazil nut (Bertholletia excelsa) 2S albumin, was analyzed immunochemically in transgenic tobacco (Nicotiana tabacum) plants. Here, we report that the peptide motif is necessary and sufficient to accumulate 2S albumin constructs of both vacuolar and extracellular final destinations in the ER. We have shown immunochemically that RNase LX is specifically expressed during endosperm mobilization and leaf and flower senescence. Using immunofluorescence, RNase LX protein was detected in immature tracheary elements, suggesting a function in xylem differentiation. These results support a physiological function of RNase LX in selective cell death processes that are also thought to involve programmed cell death. It is assumed that RNase LX accumulates in an ER-derived compartment and is released by membrane disruption into the cytoplasma of those cells that are intended to undergo autolysis. These processes are accompanied by degradation of cellular components supporting a metabolic recycling function of the intracellular RNase LX.
Turnover and processing of macromolecules play a pivotal role in the metabolism of all organisms. Ribonucleases (RNases) usually terminate the life span of different RNA species by hydrolytic or phosphorolytic action producing oligo- or mononucleotides. In general, the catabolic products can be recycled by salvage pathways or are further catabolized by accessory enzymes. Nucleolytic enzymes with broad specificity are often sequestered and reside within organelles like lytic plant vacuoles, are secreted, or are inactivated by specific endogeneous inhibitors as known from fungal and mammalian enzymes (D'Alessio and Riordan, 1997). Nevertheless, depending on spatial and temporal expression patterns, such enzymes may exercise distinct and developmentally important functions, e.g. prevention of inbreeding in Solanaceae (McCubbin and Kao, 2000).
Cultivated tomato (Lycopersicon esculentum Mill.) cells synthesize different acid RNases when entering the late stationary phase. Alternatively, synthesis of RNases can also be initiated by transferring cells into phosphate-free cultivation medium (Löffler et al., 1992). Localization studies showed that RNase LX, the main intracellular enzyme activity, was unexpectedly excluded from isolated and highly purified vacuoles, whereas two minor posttranslational processing products of RNase LX designated RNase LV1 and LV2 were found in vacuoles (Löffler et al., 1992). RNase LX was purified and protein-sequenced, revealing high homology to the second, but secreted RNase, RNase LE (Löffler et al., 1993). RNases LE and LX, being single-copy genes in tomato, are members of the T2/S-RNase superfamily (Green, 1994). They were characterized as RNA-dependent, single strand-specific, endonucleolytic phosphotransferases releasing 2′,3′-cyclic nucleotides as primary reaction products (see review of Irie, 1999; Abel and Köck, 2001). Several studies revealed that RNase LX is involved in physiological processes such as inorganic phosphate (Pi) starvation and senescence (Köck et al., 1995; Bosse and Köck, 1998; Lers et al., 1998). However, the spatial and temporal expression pattern during plant development is still unknown.
The cDNA sequence of RNase LX contains an N-terminal secretory signal sequence of 24 amino acids conferring the co-translational transport into the endoplasmic reticulum (ER; Köck et al., 1995). By comparing the RNase LX protein sequence with those of homologous, but secreted RNases like RNase LE, it became obvious that RNase LX carries a unique sequence of nine amino acids, STNDDHDEF, at its C terminus (Löffler et al., 1993; Köck et al., 1995). Interestingly, the terminal tetrapeptide HDEF (His-Asp-Glu-Phe) shows a remarkable similarity to the ER retention signal HDEL, with exception of the last amino acid. Taking into account the localization of RNase LX in nonvacuolar fractions, it was supposed that the C-terminal peptide sequence might act as an ER retention signal in plants. A first study using baker's yeast (Saccharomyces cerevisiae) has shown that this motif is sufficient to retain plant RNase LX and a secreted form of chitinase in the ER of yeast cells (Kaletta et al., 1998). However, neither the actual intracellular localization of RNase LX in tomato cells nor the ER-targeting ability of the HDEF motif and the relevance of the other five amino acids (STNDD) in the plant system have been studied until now. Additionally, positive verification of RNase LX as a protein accumulating in the ER allows more detailed investigations of its spatial and temporal expression in tomato plants.
The ER, the most versatile and adaptable organelle of eucaryotic cells, is the first organelle in the secretory pathway. Its principal functions include the synthesis, processing, and sorting of proteins, glycoproteins, and lipidic molecules, the transport to their final destinations (e.g. cell surface, vacuoles), as well as the regulation of cytosolic calcium levels. It also became obvious that this three-dimensional network consists of a large number of discrete structural domains that serve different functions. Although considerable progress has been made in the biochemical, molecular, and functional analysis of distinct domains, e.g. oil and protein bodies, knowledge about other parts is still limited (Staehelin, 1997). Retention of ER-resident proteins is thought to be the result of a continuous recycling process (retrograde transport) that is mediated by intrinsic targeting sequences of transported proteins, receptors, regulatory proteins, and vesicles. Soluble ER-resident proteins are very likely trapped by interacting with specific receptors like ERD2, expressed in the cis-Golgi, and retrieved to the ER (for review, see Vitale and Denecke, 1999). Plant lumenal ER-resident proteins like binding protein (BiP), calreticulin, and protein-disulfide isomerase (referred to as reticuloplasmins), which function as chaperones and folding enzymes in the ER, carry a retention motif at their carboxyl terminus (Denecke et al., 1992, 1993). Although studies have demonstrated that HDEL and several variants of the KDEL sequence can act as ER retention signals in plant cells, HDEF has never been tested (Vitale et al., 1993; Gomord and Faye, 1996).
In this report, the subcellular localization of RNase LX is studied by biochemical and immunocytochemical techniques. Since the low expression level of RNase LX does not allow localization studies in tomato plants, a tomato cell culture system in which the RNase LX protein amount can be increased was chosen as experimental system. For functional testing of the ER-targeting ability of the C-terminal peptide HDEF in plant cells, the cellular distribution of chimeric protein constructs based on a marker protein, Brazil nut (Bertholletia excelsa) 2S albumin, was analyzed in transgenic tobacco (Nicotiana tabacum) plants. Here, we provide compelling evidence that RNase LX accumulates in the ER of tomato cells by virtue of this alternative retention motif HDEF.
In addition, to investigate functional aspects of RNase LX during plant development, we screened tomato plants for RNase LX expression. Detailed immunochemical and microscopic analysis of RNase LX expression in tomato plants suggests its involvement in physiological cell death processes in plants. The tissue- and organ-specific expression pattern will be discussed with respect to the intracellular location of RNase LX. To our knowledge, this study represents the first report describing the relationship between subcellular localization and physiological role of an S-like RNase.
RESULTS
Biochemical Evidence for the Accumulation of RNase LX in the ER of Tomato Cells
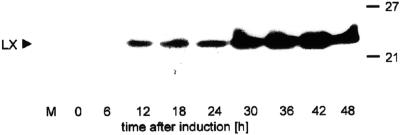
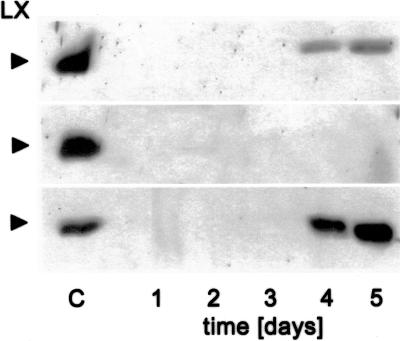
The precise cellular localization of the RNase LX has not yet been determined, although the presence of a hydrophobic signal peptide indicates transit through the secretory pathway (Köck et al., 1995). The analysis of isolated and highly purified vacuoles revealed RNase LX is absent from this organelle as well as from the medium of cultivated tomato cells (Löffler et al., 1992). To identify the microsomal compartment in which RNase LX is located, we conducted localization studies with biochemical and immunological methods. The preferred experimental system is the well-characterized tomato cell culture, which has already served as source for RNase LX protein isolation and purification (Löffler et al., 1993). Under normal growth conditions, neither RNase LX transcript nor enzyme activity are detectable, but expression is induced to high levels after depletion of inorganic phosphate in the cultivation medium (Köck et al., 1995). To determine synthesis and intracellular accumulation of RNase LX protein, a specific polyclonal rabbit antiserum directed against the C-terminal part of the RNase LX protein was generated. No cross-reactivity with related RNase LE protein secreted into the cultivation medium could be detected (Fig. 1, lane M). RNase LX is the dominant intracellularly localized RNase of Pi-starved tomato cells. The amount of RNase LX protein that accumulates in cells during phosphate starvation allowed localization studies to be performed (Fig. 1).
Figure 1.
Accumulation of RNase LX protein in cultivated tomato cells. Time course of RNase LX induction under phosphate-deficient growth conditions in cultivated tomato cells. Tomato cells cultivated without inorganic phosphate were harvested at indicated time points. Aliquots of protein extracts were loaded (100 μg). Lane M represents protein from 2 mL of cultivation medium containing extracellular RNase LE. Proteins were electrophoretically separated, blotted, and probed with the anti-RNase LX (LX) antiserum.
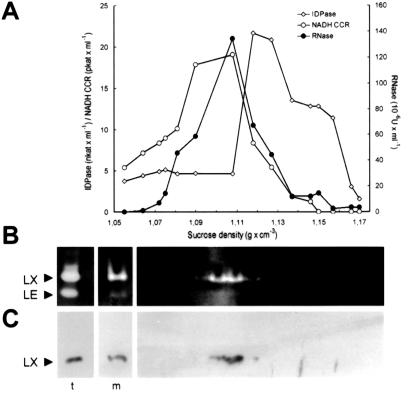
To demonstrate the actual intracellular localization of RNase LX, in the first step, an enriched microsomal fraction from tomato protoplasts was isolated on a discontinuous Suc density gradient (Table I). The comparison of marker enzyme concentrations in the protoplast homogenate and the microsomal fraction revealed the enrichment of the microsomal markers, whereas the cytosolic and mitochondrial markers (1.6% and 10.2%, respectively) drastically decreased. The percentage of RNase activity in the “crude” microsomal fraction was lower than the activity of microsomal marker enzymes since total RNase activity of cell extracts is shared between activities of vacuolar forms and RNase LX. To prove that the increase of RNase activity of the microsomal fraction is due to the accumulation of RNase LX, the RNase pattern has been inspected by in-gel-activity staining and RNase LX immunodetection (Fig. 2B). Both methods confirmed the almost exclusive enrichment of RNase LX in the total microsomal fraction. In the second step, this microsomal fraction was diluted, loaded onto a continuous gradient of 13% to 50% Suc, and fractionated by isopycnic density gradient centrifugation. The positions of the ER and Golgi apparatus membranes in the gradient were determined by analysis of 1-mL fractions. Antimycin-insensitive cytochrome C reductase, the marker enzyme for the ER, peaked at a Suc density of 1.09 to 1.11 g mL−1 and the activity of the Golgi marker enzyme, inosine diphosphatase, peaked at a density of 1.12 to 1.13 g mL−1 (Fig. 2A). RNase LX activity, which was calculated by activity assay as well as by activity staining of polyacrylamide gels, is found in ER fractions (Fig. 2B). Immunoblot analysis of samples containing equal volumes of fractions were performed with the anti-RNase LX antibody. It also clearly detects RNase LX in fractions that have been shown to contain ER membranes (Fig. 2C).
Table I.
Enrichment of microsomes by discontinuous Suc density gradient centrifugation
| Enzyme Activity | Protoplast Homogenate | Total Microsomal Fraction | Percent in Microsomal Fraction |
|---|---|---|---|
| Ethanol dehydrogenase | 0.93 | 0.015 | 1.6 |
| Malate dehydrogenase | 2.94 | 0.3 | 10.2 |
| α-Mannosidase | 1.80 | 0.63 | 35.0 |
| Inosine diphosphatase | 282 | 225 | 79.8 |
| Antimycin-insensitivecytochrome C reductase | 87.0 | 81.0 | 93.1 |
| RNase | 384 | 264 | 68.8 |
Protoplasts of cultivated tomato cells were homogenized and centrifuged. The supernatant was loaded on a discontinuous Suc gradient. After centrifugation, the turbid fraction at the interphase was collected (total microsomal fraction). Different marker enzymes were assayed. Activities of enzymes are given in pkat at 30°C, except IDPase in nkat and RNase in units, as described in Abel and Köck (2001).
Figure 2.
Determination of the microsomal compartment containing RNase LX. A, Suc density gradient fractionation of the enriched microsomal fraction of Pi-induced tomato cells and distribution of RNase LX in relation to organelle enzymatic marker activities. The total microsomal fraction of phosphate-starved tomato cells was diluted and loaded onto a continuous gradient of 13% to 50% (w/v) Suc. After centrifugation, 16 1-mL fractions were collected and Suc concentrations were measured refractometrically. Antimycin A-insensitive NADH cytochrome C reductase (ER) and inosine diphosphatase (Golgi apparatus) as well as EDTA-insensitive RNase activity were determined. Note that total RNase activity colocalizes with the ER marker enzyme. B, In-gel assay for determination of the RNase activity pattern in gradient fractions corresponding to A by native PAGE and negative activity staining. Two additional lanes on the left show the pattern in total cellular extract (t) and in an aliquot of the enriched microsomal fraction (m), which was loaded on the continuous Suc gradient. Positions of RNase LX and RNase LE in gels are marked on the left. C, Protein gel-blot analysis in gradient fractions corresponding to A by using anti-RNase LX antiserum (LX). On the left, the presence of RNase LX is shown in total cellular extract (t) and in the enriched microsomal fraction (m) before loading onto continuous Suc gradients. RNase LX protein is present in the ER-containing fractions.
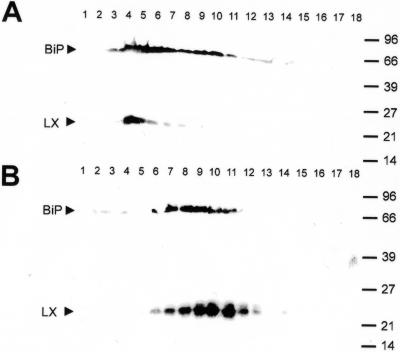
To further substantiate the characterization of the RNase LX containing fractions as those containing ER membranes, homogenization and fractionation studies were performed in the presence of either magnesium ions or in the presence of EDTA. Treatment with EDTA releases ribosomes from the ER and therefore lowers its density, but not that of the other organelles. This behavior results in a shift of ER fractions to lighter density in the Suc gradient. The detection of the protein of interest in fractions of lighter density would represent a strong indication for its location in the ER. Gradient fractions were analyzed by SDS-PAGE followed by immunodetection of RNase LX and BiP, which was used as a lumenal marker of the ER (Denecke et al., 1991). As shown in Figure 3, RNase LX was found exclusively in Suc gradient fractions that also contained BiP, and both BiP and RNase LX were found in fractions of lighter density when gradients were performed in the presence of EDTA. Although there is a slight difference in the shape and distribution of BiP and RNase LX signals in gradients, e.g. total numbers and fractions of highest protein content, it is more important that both proteins are detected significantly in fractions of lighter density due to the shift of ER membranes after treatment with EDTA. The results indicate that the ER marker BiP and the RNase LX are located in the same compartment.
Figure 3.
Subcellular colocalization of the RNase LX with the ER marker protein BiP using different procedures of ER preparation. A, Protein gel-blot analysis of fractions collected from isopycnic linear Suc gradients (10%–50%, w/v), which were prepared from cultured cells in the presence of MgCl2-containing buffer by using both anti-BiP (BiP) and anti-RNase LX (LX) antibodies. B, Protein gel-blot analysis of fractions collected from isopycnic linear Suc gradients (10%–50%, w/v), which were prepared from cultured cells in the presence of EDTA-containing buffer by using both anti-BiP (BiP) and anti-RNase LX (LX) antibodies. Fractions are numbered consecutively from the bottom to the top and represent identical Suc densities. Compared with preparations in the presence of magnesium ions, EDTA treatment results in a shift of ER fractions to lighter density in Suc gradients. Both BiP and RNase LX were shifted into other fractions, indicating the same localization of BiP and RNase LX.
Immunocytochemical Evidence for the Localization of RNase LX in the ER
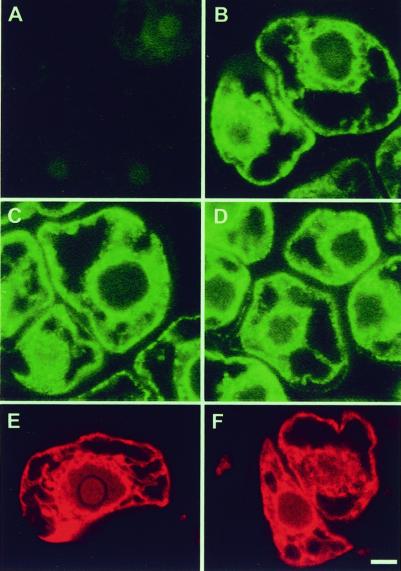
We have used immunofluorescence microscopy to further verify the compartment in which the RNase LX is located in tomato cells. Cell sections were prepared and stained individually with antisera directed against RNase LX and the ER-intrinsic protein BiP. Results are presented in Figure 4. It can be seen that both anti-RNase LX (Fig. 4B) and anti-BiP (Fig. 4D) gave a strong staining pattern in cells, which is characteristic for the ER. BiP is detectable as green dots in the cells and marks the ER around the nuclei and the cortical ER. Cell samples were stained in parallel after cultivation in the presence or the absence of phosphate in the medium for 24 h. Whereas the concentration of BiP is unchanged in both conditions and is recognized by the specific antiserum (Fig. 4, C and D), RNase LX protein is not detectable in phosphate-supplied cells due to the absence of RNase LX in uninduced tomato cells (Fig. 4A). These data are consistent with the results of the biochemical localization studies described above and illustrate the specificity of the used anti-RNase LX antiserum. In addition, we applied the fluorescent dye rhodamine B hexyl ester, which has been shown to accumulate in the ER of plant cells (Grabski et al., 1993). Rhodamine B clearly shows a similar staining pattern of the ER of tomato cells as labeling with BiP or RNase LX antibodies, respectively. This staining pattern is independent from the Pi-dependent induction status of cells (Fig. 4, E and F).
Figure 4.
Immunolocalization of RNase LX in cultured tomato cells. A through F, Immunofluorescence detection of ER-localized RNase LX (A and B) in comparison with ER marker protein BiP (C and D) and rhodamine B hexyl ester (E and F) using tomato cells grown in suspension with phosphate supply (A, C, and E) or grown for 24 h without phosphate source (B, D, and F), which causes RNase LX protein synthesis (B). Location of RNase LX and BiP were visualized by immunofluorescence with anti-RNase LX and anti-BiP antibodies, respectively, followed by application of BODIPY-conjugated secondary antibodies. Bars = 5 μm.
The Peptide HDEF Is Able to Retain Chimeric Reporter Gene Constructs in the ER of Tobacco Plants
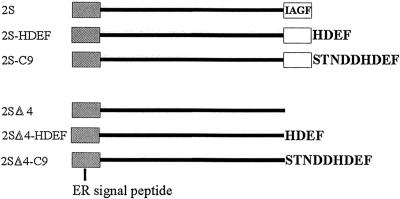
Generally soluble proteins that accumulate in the ER of plant cells carry C-terminal retention signals in their sequences. Taking into account the localization of RNase LX in ER fractions, it was supposed that the C-terminal peptide sequence might act as an ER retention signal in plants. To examine whether the peptide HDEF can retain vacuolar or secreted proteins in the ER of plant cells, we have analyzed the fate of chimeric protein constructs in which C-terminal segments of RNase LX were fused to the C terminus of a plant reporter protein. For this purpose, the seed-storage protein 2S albumin, which normally accumulates in storage vacuoles of Brazil nut, was used. Saalbach et al. (1996) reported previously that recombinant 2S albumin was correctly processed and accumulated in the vacuoles of mesophyll cells when expressed in transgenic tobacco cells. Deletion of the four carboxy-terminal amino acids IAGF resulted in the secretion of the truncated 2S albumin from the cells (construct 2SΔ4), indicating that the tetrapeptide is an essential part of the targeting sequence and is necessary for the proper sorting of this protein to the vacuoles of plant cells.
Based on these results, we used both constructs, 2S albumin and 2SΔ4, which also possess the original ER signal peptide to construct C-terminal fusion proteins with two different peptides comprising the last nine or four amino acids of RNase LX, respectively. Constructs, which are schematically shown in Figure 5, were introduced into the binary plant transformation vector pBinAR and transferred into tobacco using Agrobacterium tumefaciens-mediated gene transfer. Protein constructs were expressed under the control of the 35S promoter. Transgenic tobacco plants of the different lines were analyzed for expression of 2S albumin by western blots using total protein extracts from leaves probed with anti-2S albumin antiserum. Plants from transgenic lines that produce moderate amounts of the reporter protein were chosen for further experiments.
Figure 5.
Schematic representation of the 2S albumin-derived constructs used in this study. 2S construct localizes to vacuoles in tobacco protoplasts, whereas truncated 2SΔ4 albumin missing the C-terminal amino acids IAGF is secreted (Saalbach et al., 1996). The other constructs synthesized carry C-terminal amino acid extensions (-HDEF or -STNDDHDEF) derived from RNase LX in different combinations as indicated.
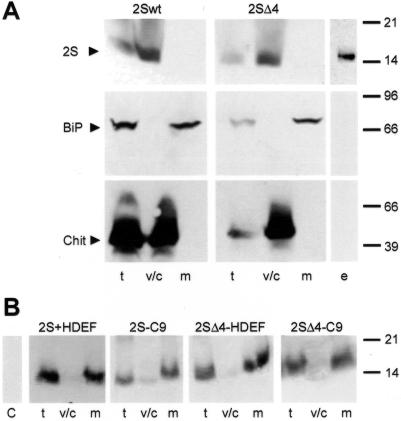
Discontinuous Suc gradients, as already described for the localization studies in cultivated tomato cells, were performed using total leaf extracts. We analyzed the distribution of wild type (2S) and truncated form of 2S albumin (2SΔ4) between the microsomal fraction and the soluble fraction, which contains cytoplasmic, vacuolar, and soluble extracellular proteins of mesophyll cells. Aliquots of all fractions were separated on SDS gels, blotted, and 2S protein was immunochemically detected using 2S albumin antibodies. Additionally, two antisera directed against the vacuolar protein chitinase from tobacco (Kaletta et al., 1998) and the lumenal ER protein BiP (Denecke et al., 1991) were used to check the proper separation of microsomal from cytoplasmic, vacuolar, and extracellular proteins in the gradients (Fig. 6A). In both lines harboring 2S albumin or truncated 2S albumin, vacuolar chitinase is detectable in total leaf extracts and in the soluble fraction, but not in the microsomal fraction. The opposite is seen when anti-BiP antiserum is used to probe the filters. The total leaf extract and the microsomal fraction contain BiP. Neither chitinase nor BiP are found in the extracellular fluid that was collected by vacuum infiltration of leaf material (Fig. 6A). In agreement with former studies, wild-type 2S albumin is found in the soluble fraction corresponding to the vacuolar compartment but is not found in the extracellular fluid (Fig. 6A), whereas the truncated construct 2SΔ4, not detectable in the microsomal fraction, is found in the soluble fraction and in the extracellular fluid, suggesting that it is secreted.
Figure 6.
The C-terminal peptide HDEF is able to retain marker protein constructs in microsomal fractions. A, Analysis of transgenic tobacco leaves for the distribution of the 2S albumin and the 2SΔ4 construct between the soluble and the microsomal fraction in a two-step gradient and analysis of secreted protein 2SΔ4. Leaves were homogenized, centrifuged, and fractionated in a discontinuous Suc gradient (13%/50%, w/v). Note that the soluble fraction of leaves contains cytoplasmic, vacuolar, and soluble extracellular proteins. Intercellular fluid was collected by vacuum infiltration and tested for extracellular proteins. Analyses were done with protein gel blotting by using anti-2S albumin (2S), anti-BiP (BiP), and anti-chitinase (Chit) antibodies. Lanes: t, total cell extract; v/c, soluble fraction; m, microsomal fraction; e, extracellular fluid. B, Analysis of transgenic tobacco leaves for the distribution of 2S albumin constructs carrying RNase LX derived C termini (2S-HDEF, 2S-C9, 2SΔ4-HDEF, 2SΔ4-C9) between the soluble and the microsomal fraction in a two-step gradient. Fractionations were performed with homogenates from transgenic tobacco lines. Aliquots of fractions were separated on SDS gels and analyzed by protein gel blotting by using anti-2S albumin (2S) antibodies.
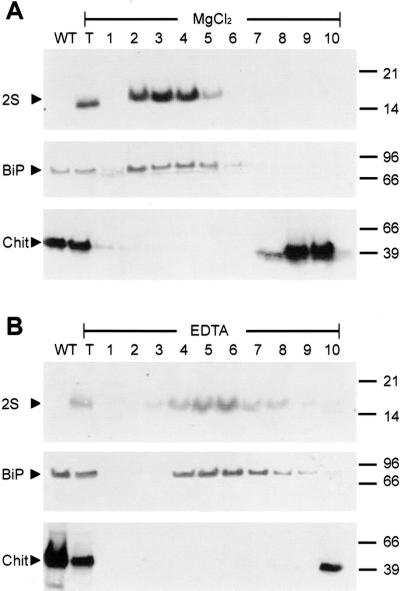
In the following experiments, we analyzed immunochemically the 2S albumin distribution in all tobacco lines carrying RNase LX C-terminal sequences (2S-HDEF, 2S-C9, 2SΔ4-HDEF, 2SΔ4-C9). As shown in Figure 6B, unlike the constructs 2S and 2SΔ4, which were found in the vacuole and in the extracellular space, respectively, we found all HDEF- and STNDDHDEF-tagged 2S albumins in microsomal fractions. It is highly probable that the tagged proteins are retained in the ER. To determine in which microsomal compartment these modified proteins are localized, linear Suc gradients were performed. Homogenization and fractionation steps were done in the presence of either magnesium ions or in the presence of EDTA as described for tomato cells. Proteins of the gradient fractions were separated by SDS-PAGE followed by immunodetection on blots with anti-2S albumin, anti-BiP, and anti-chitinase antisera. As shown in Figure 7A, the reporter protein construct 2S-HDEF, which carries the vacuolar sorting signal followed by the HDEF extension, was found exclusively in Suc gradient fractions, which also contained BiP. In gradients performed in the presence of EDTA instead of magnesium ions, both 2S-HDEF and BiP were found in fractions of lighter density supporting the assumed colocalization in the ER (Fig. 7B). The vacuolar protein chitinase is detected in the upper part of the gradient, mainly in fractions 9 and 10 containing cytosolic as well as vacuolar and extracellular proteins. These fractions are well separated from ER-containing fractions and do not contain 2S-HDEF protein.
Figure 7.
Subcellular colocalization of the chimeric protein 2S-HDEF with the ER marker protein BiP using different procedures of ER preparation. A, Leaf homogenates were prepared in the presence of MgCl2-containing buffer using a transgenic tobacco line expressing 2S-HDEF protein. B, Leaf homogenates were prepared with EDTA-containing buffer using the same transgenic tobacco plant as described in the experiment (A). In both cases, homogenates were fractionated on isopycnic linear Suc gradients (10%–50%, w/v), and fractions were collected and numbered consecutively from the bottom to the top of the gradients (1–10). Total leaf protein from a wild-type tobacco plant (lane WT) and from the transgenic tobacco plant (lane T) expressing 2S-HDEF construct were also analyzed. Aliquots of gradient fractions were separated by SDS-PAGE and analyzed by protein gel blotting and immunodetection with anti-2S albumin (2S) and anti-BiP antiserum (BiP) as described in “Materials and Methods.” Immunodetection of chitinase with anti-chitinase antiserum (Chit) was used to determine fractions in which vacuolar proteins accumulate. HDEF-tagged 2S albumin is found colocalized with the ER-marker protein BiP. Compared with preparations in the presence of magnesium ions, EDTA treatment results in a shift of ER fractions to lighter density in Suc gradients. Both marker construct 2S-HDEF and BiP were shifted into fractions of lighter Suc density indicating the same localization.
Biochemical analysis of the other constructs, 2S-C9, 2SΔ4-HDEF, and 2SΔ4-C9, resulted in a colocalization of proteins in ER fractions as described for the construct 2S-HDEF (results not shown). Since the analysis of the chimeric proteins fused to the nine-amino acid-long C-terminal sequence of RNase LX results in the same cellular location as already determined for the HDEF-constructs, it is assumed that the pentapeptide STNDD is not necessary for the retention process. Taken together, these results clearly indicate that RNase LX is associated with ER.
Expression of RNase LX in Tomato Plants Is Detected in Tissues Undergoing Programmed Cell Death
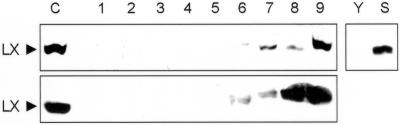
The understanding of the physiological role of RNase LX in tomato plants is limited, especially when we consider the subcellular location in an ER compartment. To identify processes in which RNase LX is involved, we screened tomato plants for RNase LX enzyme activity and used western blots for protein identification. Not unexpectedly, in mature tomato plants, especially in leaves, stems, roots, and fruits, enzyme activity as well as RNase LX protein is virtually undetectable (data not shown). Nevertheless, this result does not rule out the expression of RNase LX in defined cell types since the activity assay is very sensitive and the protein amount might be too low to detect. In contrast to this, western blot analysis revealed a remarkable increase in the amount of RNase LX protein in senescing leaves (Fig. 8A). This result is consistent with results of Lers et al. (1998), who reported increasing transcript levels during leaf senescence. In addition, a similar and drastic increase of RNase LX protein could be detected in flowers after anthesis, especially in petals, with highest yield in late senescence (Fig. 8B).
Figure 8.
RNase LX protein accumulates in senescing leaves and flowers. Protein gel-blot analysis in extracts from whole buds, flowers, and leaves (upper) and from isolated petals (lower) by using anti-RNase LX antiserum (LX). On the left, presence of RNase LX is shown in total cellular extract from cultivated tomato cells (C). RNase LX protein is also present in senescing leaves (S) but not in young leaves (Y) of tomato plants. Aliquots of soluble protein extracts were loaded (20 μg protein lane−1). Stages were defined as follows: 1 through 5, buds <4 mm, 4 to 6 mm, 6 to 9 mm, >9 mm, and bud with open sepals; 6, open flower; and 7 through 9, senescing flowers.
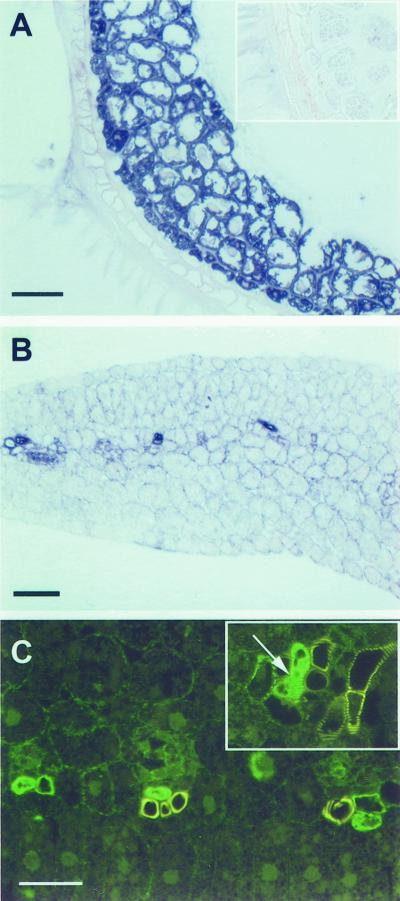
We also investigated the early phases of plant development. To examine the spatial and temporal expression pattern we harvested tomato seeds every 24 h from d 1 to 5 after imbibition. Using western blots on the basis of equal loading of total protein, accumulation of RNase LX protein in germinating seeds can be detected beginning with d 3; protein amount increases to high levels at d 4 and 5 (Fig. 9). Dissection of seeds into seedlings and endosperm revealed that RNase LX is exclusively synthesized in endosperm tissue; no signal can be detected with seedling protein (Fig. 9). RNase LX transcript accumulation precedes LX protein accumulation as revealed by northern analysis (data not shown). To show the distribution of RNase LX in endosperm tissue, we carried out microscopic analysis following immunodetection of RNase LX protein. As shown in Figure 10A, all cells of the endosperm contain large amounts of RNase LX. Further microscopic analysis of seedlings elucidated the occurrence of RNase LX expression in the vascular tissue of cotyledons (Fig. 10B). Using immunofluorescence we can clearly detect RNase LX in distinct cells of the xylem. Mature tracheary elements, which show yellow autofluorescence, are not labeled (Fig. 10C). To characterize the immunolabeled cells that were identified in cross sections, longitudinal sections were analyzed. As shown in Figure 10C (inset), the labeled cells already possess reinforced secondary cell walls seen as spiral and reticulate thickenings (arrow). Processes of secondary cell wall formation precede the initiation of cell death and cellular autolysis. Since only cells which become tracheary elements synthesize a secondary cell wall, we can conclude that these cells represent immature and differentiating xylem cells. After completion of autolysis, proteins and other constituents are removed from mature tracheary elements and, therefore, RNase LX is no longer detectable in mature tracheary elements.
Figure 9.
RNase LX protein accumulates in tomato endosperm during germination. Protein gel-blot analysis of extracts from whole seeds (upper), from isolated seedlings (central), and isolated endosperm including testa (lower) by using anti-RNase LX antiserum (LX). Seedlings of defined stages were collected and aliquots of soluble protein extracts were loaded (80 μg total protein lane−1). Emergence of radicula (d 2–3) is regarded as transition point between germinative and postgerminative phase.
Figure 10.
Localization of RNase LX in tomato endosperm and vascular tissue. A and B, Immunochemical detection of RNase LX in a cross-section of endosperm at d 4 (A) and in vascular tissue of cross-sectioned cotyledons (B). RNase LX protein was visualized by immunodecoration with the rabbit anti-RNase LX antibody followed by a goat anti-rabbit IgG antibody conjugated with alkaline phosphatase. Inset in A, Control, incubation of cross-sectioned endosperm with the same secondary antibody. C, Localization of RNase LX in cross-sections as well as longitudinal sections (inset in C) of tomato cotyledons by immunolabeling with anti-RNase LX antibody and visualization by BODIPY-conjugated secondary antibodies. Note the green fluorescence signal in differentiating tracheary elements, which are characterized by spiral and reticulate thickenings (arrow), whereas mature tracheary elements show yellow autofluorescence of lignin (C). A and B, Bars = 50 μm; C, bar = 25 μm.
Taken together, expression of RNase LX is correlated with defined developmental processes like senescence, endosperm mobilization, and xylem differentiation. From the point of view of RNase LX function, it seems important that these processes involve cell death on a local or large scale.
DISCUSSION
In this paper, we describe the subcellular localization of the RNase LX protein in tomato cells using a combination of biochemical and immunological methods. The evidence that RNase LX is a resident of the ER is supported by an independent approach to show the important and decisive role of the peptide motif HDEF in the retention process. One prerequisite to conduct these studies was the successful generation of a specific antiserum against RNase LX that does not detect the closely related RNase LE protein as shown in Figure 1. To avoid cross-reactivity, only a partial sequence of RNase LX was used. The antiserum detects RNase LX in western blots after SDS gel electrophoresis as well as in microscopic immunodetection. In addition, we can clearly distinguish tomato acid RNases using native PAGE followed by determination of enzyme activity in gels. This very sensitive technique, used formerly throughout the purification procedure of intracellular RNases (Löffler et al., 1992; Abel and Köck, 2001), was applied in localization studies accompanying the immunological detection of RNase LX. Our studies reveal that RNase LX is always found to co-purify with the ER markers NADH cytochrome C reductase and binding protein in cultured tomato cells. Neither do isolated and highly purified vacuoles contain the complete RNase LX protein (Löffler et al., 1992) nor can RNase LX be detected extracellularly (Fig. 1). The immunochemical detection of both RNase LX protein and BiP in the ER of tomato cells strongly supports the biochemical data presented. Our findings are the first report about the actual intracellular location of an S-like RNase in the ER of plant cells.
It has been assumed previously that the C-terminal peptide HDEF of RNase LX is responsible for the ER location because of its high similarity to known ER retention signals K/HDEL (Köck et al., 1995). In this study, we attempted to show that this short motif acts as signal in the plant system. Chimeric gene constructs of 2S albumin, a seed storage protein from Brazil nut, sorted to vacuoles or the cell surface were used as reporter proteins for targeting studies. It is worth noting that 2S albumin contains its complete vacuolar targeting signal, which has to compete with the putative ER retention signal HDEF (Saalbach et al., 1996). We conclude from our data (a) that the last four amino acids HDEF at the C terminus are necessary and sufficient to accumulate 2S albumin in the ER, and (b) that protein constructs of both vacuolar and extracellular final destination can be efficiently retained in the ER by virtue of this motif. These results provide compelling evidence that the C-terminal peptide HDEF is a novel ER retention signal in plants. Together, these results confirm our localization studies and the conclusion that RNase LX is associated with the ER.
All known S-like RNase genes encode N-terminal signal sequences enabling the co-translational transfer into the ER, the first compartment of the secretory pathway. Although it is assumed that S-like RNases are proteins mostly targeted to the extracellular space, it seems very likely that among them are enzymes which are targeted to the same intracellular compartment as tomato RNase LX. Recently, Bariola et al. (1999) reported that RNS2, the only of three acid RNases from Arabidopsis, is an intracellular enzyme as well. An obvious feature of the RNS2 sequence is a C-terminal extension with the most terminal amino acids REAL, which bears some resemblance to known ER retention motifs. Two other S-like RNases were identified that harbor a similar or the same C-terminal motif as RNase LX. Unfortunately, data about localization studies are not known. ZRNase I from Zinnia elegans encodes a polypeptide of 215 amino acids and carries the peptide RDEL at its C terminus (Ye and Droste, 1996). The sequence RDEL, having Arg at the first position instead of Lys or His compared with known ER retention signals, has been tested independently and was found to function as ER retention signal in plants (Denecke et al., 1992), supporting our results. The primary structure of RNase NGR3 from Nicotiana glutinosa (EMBL accession no. AB032257) comprises 213 amino acids and ends C-terminally with amino acids HDEF like the tomato enzyme. From these data, there is evidence that a group of intracellular S-like RNases exists that is targeted to the ER. Moreover, we assume that gene families of acid S-like RNases from different plant species consist at least of one extracellular and one intracellular enzyme, which are represented in tomato by RNase LE and RNase LX (Köck et al., 1995).
We have demonstrated recently (Kaletta et al., 1998) and in this report that the alternative ER retention signal HDEF very efficiently retains reporter proteins in the ER of yeast and plant cells, also suggesting that other proteins having this signal accumulate in the ER. Searching protein databases with the C-terminal HDEF pattern revealed several entries. Apart from tomato RNase LX and RNase NGR3, only two animal protein groups from different species and two bacterial proteins were found. One group is comprised of Ca2+-binding proteins designated calumenins that were cloned from man, rat, and mouse. Recently, the subcellular location of calumenin from mouse was determined by immunostaining revealing an ER staining pattern. Although the authors did not investigate the functional capacity of the motif HDEF in the animal system, it was shown that calumenin lacking the peptide HDEF is secreted into the medium of transfected COS cells (Yabe et al., 1997). These findings are consistent with experiments presented in our report. DNA supercoiling factors representing the other animal group were identified in silkworm and fruit fly. Compartmentalization studies were not undertaken with these proteins, which also contain Ca2+-binding domains (Ohta et al., 1995).
It is expected that an RNase LX construct lacking the HDEF motif is deposited in vacuoles or is secreted. Indeed, truncated RNase LX protein that misses the last four amino acids was identified in vacuoles of tomato cells. The RNase form designated RNase LV2 is present in cultivated tomato cells starved for phosphate. It and a second vacuolar form, LV1, lacking even more amino acids, could be purified and protein-sequenced (Löffler et al., 1992; Köck et al., 1995). Since these RNase forms can easily be distinguished by electrophoretic methods, we analyzed the temporal and spatial occurrence under conditions where RNase LX is expressed, e.g. during phosphate starvation of cultivated tomato cells and in senescing flowers of tomato plants. Complete RNase LX always represents the major intracellular RNase form. Conditions in which only RNase LV2 (LV1) is present or is the dominating form could not be found (data not shown). Therefore, it is assumed that RNase LX is the original gene product, whereas the vacuolar forms may result from proteolytic cleavage after partial escape of RNase LX from the ER. It is possible that the hydrophilic C terminus unique to RNase LX (Köck et al., 1995) is more accessible to proteolytic attack than the folded protein core.
Only recently, a similar behavior has been reported from experiments investigating the efficiency of HDEL C-terminal extension in retaining sporamin constructs in the ER of tobacco cells (Gomord et al., 1997). Noteworthy are results showing that HDEL retains sporamin in the ER very efficiently but that any sporamin-HDEL that escapes this compartment is transported to lytic vacuoles for degradation, regardless of the presence (or absence) of the vacuolar targeting signal. Additionally, sporamin constructs are transported with HDEL motif to the vacuole, where a small peptide containing the HDEL epitope is cleaved. These results are consistent with the data presented here. Nevertheless, it is not ruled out that at least the HDEL sequence is cleaved off prior to arrival in vacuoles. Whether the expression pattern described for tomato RNase LX is unique or also exists for RNases in other plants is not known.
Our data indicate a differential expression pattern of RNase LX, which is related to plant development. Using activity assay and immunodetection, we were able to show that the enzyme is highly active in tomato seed endosperm during germination and in senescing tomato leaves and senescing flowers, especially in petals. Considering the subcellular location in an ER compartment, an acid RNase like RNase LX appears to be an unusual constituent of the ER. Nevertheless, there are a few other plant proteins known that also possess the KDEL motif. These proteins, a group of papain-type Cys endoproteases, have a similar expression pattern as RNase LX. Recently, a Cys endopeptidase was isolated from germinating endosperm of castor beans (Schmid et al., 1998). This enzyme is exclusively located in ricinosomes, a lytic compartment also appearing during senescence of daylily petals. Electron micrographs revealed that ricinosomes are derived from the ER. In addition, different lumenal and membrane proteins characteristic for the ER were found in purified ricinosomes (Schmid et al., 1999). It is possible that specific hydrolytic enzymes like Cys protease and acid RNase accumulate in distinct ER domains or even ER-derived structures like ricinosomes, which are assumed to play an important role in controlled degradation processes, e.g. endosperm mobilization and senescence. Evidence was shown that ricinosomes are leaking and thus liberate enzymes. This finding is consistent with our assertion that RNase LX accumulates in an ER-derived compartment and is released by membrane rupture into the cytoplasma. Proteases and RNases are thought to be involved with the mobilization of cellular macromolecules during germination and senescence, proteins, and ribonucleic acids.
We have shown that RNase LX is also expressed during xylem differentiation, another process of controlled degradation of plant cells, which is characterized by secondary wall formation and cellular autolysis. It has been recently shown that after the disruption of the tonoplast, organelles with a single membrane, such as Golgi bodies and the ER, become swollen and then rupture. This process causes hydrolytic enzymes as RNases, DNases, and proteases enriched in lytic compartments to invade the soluble cell content for degradation (Fukuda, 1997). These results could explain that the acid tomato RNase LX, although accumulating in the ER, participates in these processes.
We assume (a) that the destruction of the integrity of intracellular ER- or ER-derived membranes is a prerequisite to bring together the RNase and its substrates (RNA), and (b) that the accumulation of RNase LX occurs in the ER before cellular lysis without detrimental effects to cells. Our data support a physiological function of RNase LX in selective cell death processes that are also thought to involve programmed cell death (Pennell and Lamb, 1997; Groover and Jones, 1999). It can be expected that this type of intracellular acid RNase is also expressed during other controlled degradation processes on the cellular level in plants.
MATERIALS AND METHODS
Plant Materials
Cell suspension cultures of tomato (Lycopersicon esculentum Mill. cv Lukullus) were propagated in a modified Murashige and Skoog medium and phosphate starvation (−Pi) was induced as described by Köck et al. (1995). Tomato seeds were surface-sterilized, washed with water, and germinated in darkness at 28°C. After 3 d, seedlings were shifted into light with a 16-h/8-h light regime.
Cell Fractionation
Homogenates
Cultured tomato cells were harvested at indicated time points by centrifugation, washed twice with buffer (150 mm Na-acetate buffer, pH 5.6), homogenized with mortar and pestle in liquid nitrogen, and resuspended in the same buffer. After centrifugation (12,000g, 10 min, 4°C), the supernatants were collected. Equal protein amounts were loaded on gels for separation and immunodetection.
Tomato Cell Fractionation
To facilitate gentle lysis of cells for fractionation studies, cell walls of cultured tomato cells were partially digested. For the digestion, an aliquot of cells taken from a −Pi culture was incubated at 28°C under shaking (100 rpm) for 2 h in 10 mL of cell protoplast washing solution with 0.5 m sorbitol containing 1% (w/v) Driselase (Sigma, Deisenhofen, Germany), 1% (w/v) cellulase Onozuka R-10 (Serva, Heidelberg), and 0.01% (w/v) HUPc-cellulase (Glund et al., 1984). The protoplasts were washed three times in cell protoplast washing solution with 0.4 m sorbitol by sedimentation at 100g for 5 min and were kept at 4°C. Protoplasts were resuspended in 0.2 mL of 50% Suc and carefully mixed with 1.6 mL of lysis buffer {10 mm HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid], pH 7.5; 1 mm β-mercaptoethanol} on ice. The lysis was mechanically assisted by pressing the dense solution through a needle several times and was confirmed by microscopic inspection. Suc gradient preparations were made according to Sticher et al. (1992). After centrifugation at 1500g for 5 min, pooled supernatants (3 mL) were loaded on a discontinuous step gradient of 7 mL of 13% and 1.5 mL of 50% (w/v) Suc, which was centrifuged (100,000g, 2 h at 4°C) in an SW 41 Ti rotor (Beckman Instruments, Munich, Germany). The turbid fraction at the interphase (total microsomal fraction) was collected. For further fractionation, the total microsomal fraction was diluted to 12% (w/v) Suc with 10 mm HEPES (pH 7.5), 1 mm β-mercaptoethanol, and loaded onto a 9-mL linear density gradient of 13% to 50% (w/v) Suc in gradient buffer (50 mm HEPES, pH 7.5, 10 mm MgCl2, 2.5 mm EDTA, 5 mm β-mercaptoethanol, 10% [v/v] glycerol), which was centrifuged (100,000g, 16–18 h at 4°C) in an SW 41 Ti rotor. One-milliliter fractions were collected from the bottom to the top of the tubes. Suc concentrations were determined refractometrically.
For separation of endomembrane organelles based on their densities, total protoplasts were lysed in 10 mm HEPES (pH 7.5) containing 10 mm MgCl2 or 2.5 mm Na-EDTA without β-mercaptoethanol (Pedrazzini et al., 1997). The resulting supernatant collected after centrifugation (1,500g, 10 min) was loaded on a 9-mL linear density gradient of 13% to 50% (w/v) Suc in modified gradient buffer (50 mm HEPES, pH 7.5, 10% [v/v] glycerol) containing either 10 mm MgCl2 or 2.5 mm EDTA. Centrifugation was carried out at 100,000g for 3 h at 4°C using an SW 41 Ti rotor.
Tobacco (Nicotiana tabacum Samsun NN) Plants
Intracellular distribution of 2S albumin in transgenic tobacco plants was analyzed using the discontinuous Suc gradient already described (see above). One gram of leaf material from transgenic lines was homogenized with a mortar and pestle in 3 mL of gradient buffer (50 mm HEPES, pH 7.5, 10 mm MgCl2, 2.5 mm EDTA, 5 mm β-mercaptoethanol, 10% [v/v] glycerol) containing 13% (w/v) Suc at room temperature. After filtering through cheesecloth, the supernatant was collected by centrifugation (1,500g, 10 min, 4°C) and was layered on top of the gradient (3 mL). Besides the turbid fraction at the interphase (total microsomal fraction), the soluble fraction (soluble cytosolic, vacuolar, and extracellular components) at the top of the gradient was collected (Sticher et al., 1992).
For localization experiments in endomembrane organelles, leaf material of selected transgenic tobacco lines expressing modified 2S albumins was ground with mortar and pestle in the EDTA- or magnesium-containing buffer as described for cultivated tomato cells (Pedrazzini et al., 1997). Homogenates collected after centrifugation by 1,500g for 10 min were fractionated by centrifugation through the linear Suc gradient (13%–50%, w/v) with modified gradient buffer including EDTA or MgCl2. Fractions (1 mL) were analyzed.
To collect intercellular fluid, leaves were cut into 2-cm-wide strips and were rinsed in water. Strips were vacuum infiltrated for 15 min at room temperature with buffer (25 mm Tris-HCl, pH 7.8, 0.5 m Suc, 10 mm MgCl2, 10 mm CaCl2, 5 mm β-mercaptoethanol, 0.5 mm PMSF). Material was blotted dry and put into the barrel of a syringe. The syringe was placed in an appropriate size centrifuge tube and centrifuged at 800g for 10 min.
Antibodies, SDS-PAGE and Immunoblotting
Comparing mature protein sequences, the C-terminal part of RNase LX shows a lower similarity to a related tomato RNase, and therefore it was chosen for the production of the polyclonal antiserum (142 amino acids). Synthesis and purification of the partial RNase LX protein were performed using the glutathione S-transferase gene fusion system following the instructions of the manufacturer (pGEX vector, Amersham Pharmacia Biotech, Freiburg, Germany). Using gene-specific primers (corresponding to amino acids 72–77 and 208–213 of the mature RNase LX protein, accession no. X79338) extended by BamHI and SalI recognition sites, a 448-bp-long PCR product was generated using the RNase LX cDNA clone as template. The product was ligated into the BamHI/SalI digested vector pGEX-4T-2 using standard recombinant DNA techniques (Sambrook et al., 1989). The product encoded a 40-kD fusion protein consisting of glutathione S-transferase (26 kD) and truncated RNase LX (14.4 kD), which was expressed under inducing conditions (0.5 mm isopropylthio-β-galactoside). Purified fusion protein was cleaved with thrombin, and the RNase LX fragment was recovered by electroelution (80 mA) in 10 mm NH4HCO3 at 4°C for 4 h, after separation by SDS gel electrophoresis. Rabbits were immunized according to a standard protocol by three injections with truncated RNase LX protein (first injection, 1 mg; second and third injections, 800 μg each) at intervals of 4 weeks. The use of rabbit antisera against Brazil nut (Bertholletia excelsa) 2S albumin (Saalbach et al., 1996), the binding protein BiP from tobacco (Denecke et al., 1991), and class I chitinase of tobacco (Kaletta et al., 1998) have been described previously.
Proteins were quantified according to Lowry et al. (1951) using bovine serum albumin (BSA) as a standard. Protein Mr standards were purchased from Roche Diagnostics (Mannheim, Germany). Protein probes were separated on either 12% SDS-PAGE under reducing conditions (Laemmli, 1970) or on SDS-PAGE according to Schägger and von Jagow (1987) to investigate 2S albumin containing probes. Proteins were transferred onto nitrocellulose membranes in 25 mm Tris, 250 mm Gly, 0.1% (w/v) SDS, 20% (v/v) methanol using the semi-dry blotting procedure, and transfer was controlled by staining with Ponceau S solution. Membranes were incubated with blocking solution (5% [w/v] milk powder in 1× TBS [50 mm Tris-HCl, pH 7.5, 150 mm NaCl] and 0.1% [v/v] Tween 20) followed by incubation with primary antibodies overnight at 4°C in 2.5% (w/v) milk powder in 1× TBS and 0.02% (w/v) NaN3 (dilutions of antisera: RNase LX 1:5,000; 2S albumin 1:1,000; BiP 1:2,500; chitinase 1:2,000) and then reacted with secondary antibody conjugated to horseradish peroxidase. Enhanced chemiluminescence immunodetection was used as recommended by the manufacturer (Amersham Pharmacia Biotech).
Immunocytochemistry
Suspension cells, tomato seedlings, and isolated endosperm were fixed with 3% (w/v) paraformaldehyde in phosphate-buffered saline (PBS; 135 mm NaCl, 3 mm KCl, 1.5 mm KH2PO4, 8 mm Na2HPO4). Suspension cells were collected in sieves and immobilized with 3% (w/v) agar/1% (w/v) gelatin in PBS. After dehydration by a graded series of ethanol, material was embedded in polyethylene glycol and cut as described (Hause et al., 1996). Sections of 2-μm thickness were labeled with the rabbit anti-RNase LX antibody raised against recombinant tomato RNase LX (diluted 1:500 in PBS containing 1% [w/v] BSA) or with the rabbit anti-BiP antibody (diluted 1:500 in PBS containing 1% [w/v] BSA, kindly provided by Jürgen Denecke, University of Leeds, UK), respectively. Subsequently, an anti-rabbit-IgG antibody conjugated with BODIPY (Molecular Probes, Leiden, The Netherlands) or alkaline phosphatase (Roche Diagnostics) was used as indicated according to the supplier's instructions. Alkaline phosphatase was visualized by staining with p-nitroblue tetrazolium chloride/5-bromo-4-chloro-3-indolylphosphate and analyzed using bright field microscopy, whereas BODIPY-immuno-decorated sections were analyzed by fluorescence microscopy with an epifluorescence microscope (Axioskop, Zeiss, Jena, Germany) equipped with a CCD camera (Sony, Tokyo).
In order to visualize ER in living cells, suspension cells of tomato were stained with 1 μg mL−1 hexyl rhodamine B for 10 min according to Grabski et al. (1993). Optical sections of cells were obtained by confocal laser scanning microscopy using a Zeiss LSM 410 equipped with a He/Ne laser (543 nm).
Enzyme Assays
Enzyme activities of antimycin A-insensitive NADH cytochrome C reductase (ER) and of inosine diphosphatase (Golgi apparatus) were assayed as described by Shore and MacLachlan (1975). The marker enzymes α-mannosidase (vacuole), ethanol dehydrogenase (cytosol), and malate dehydrogenase (mitochondria/cytosol) were also measured as described previously (Glund et al., 1984). Using RNA as substrate, total RNase activity was estimated from the release of ethanol-soluble A260 materials (Abel and Köck, 2001). The enzyme unit is defined as the amount of enzyme causing an increase in A260 of 1.0 x mL−1. Soluble protein concentrations were measured according to the method of Lowry et al. (1951) with BSA as the standard.
Disc gel electrophoresis on native polyacrylamide slab gels (15% [w/v] acrylamide) was carried out without SDS using the buffer system according to Laemmli (1970). Detection of RNase activity was performed by washing the gels in buffer (150 mm Na-acetate, pH 5.6) for 2 × 10 min, followed by incubating gels in substrate solution (150 mm Na-acetate, pH 5.6; 2.5 mm EDTA, 0.4% [w/v] yeast RNA) at 37°C for 30 min. After rinsing off adhering substrate solution with buffer, gels were stained in 0.2% (w/v) toluidine blue, 0.5% (v/v) acetic acid for 5 min and were destained in 0.5% acetic acid (Abel and Köck, 2001).
Chimeric Gene Constructs of 2S Albumin
The DNA fragments encoding the modified 2S albumin precursors from Brazil nut were generated by PCR using the following oligonucleotides given in 5′ to 3′ direction. The forward primer matching at the 5′ end of the coding sequence, 5′-GGATCCATGGCGAAGATT-3′, was combined with each of the four reverse primers: 2S-HDEFrev/2SΔ4-HDEFrev are 5′-TTAAAATTCATCATGGAACCCGGCAATGGAGCCACCCAT-3′ and primers 2S-C9rev/2SΔ4-C9rev are 5′-TAAAATTCATCATGGTCATCATTTGTGGAGAACCCGGCAATGGAGCCACCCAT-3′ (nucleotides encoding amino acids IAGF are underlined and are omitted in primers of both Δ4-constructs of 2S albumin). The clone utilized as template (50–100 pg) was described previously and originates from in vitro assembled synthetic oligonucleotides (Saalbach et al., 1994). For all amplifications, 10 cycles of 1 min at 94°C, 2 min at 60°C, 3 min at 71°C, following 20 cycles of 1 min at 94°C, 65°C for 2 min, 3 min at 72°C were done in a 100-μL reaction volume using 1 unit Taq polymerase, the recommended buffer (Eurogentech, Seraing, Belgium), and 50 pmol of each oligonucletide. PCR products of the appropriate size were ligated into the vector pCRII (TA Cloning Kit, Invitrogen, Groningen, The Netherlands). Recombinant plasmids were isolated using commercial plasmid purification kits (Qiagen, Hilden, Germany). All four constructs were sequenced. Ligations, transformations, and analysis of recombinants were done according to standard recombinant DNA techniques (Sambrook et al., 1989). The BamHI-XbaI fragments were subcloned into the binary plant transformation vector BinAR, and resulting plasmids were transformed in Agrobacterium tumefaciens strain C58C1 according to Höfgen and Willmitzer (1988). Leaf discs of sterile tobacco plants cultivated in vitro were used for transformation as described (Saalbach et al., 1996). Wild-type and truncated form of 2S albumin, 2S and 2SΔ4, were a generous gift of Gerhard Saalbach and Mario Rosso (IPK Gatersleben, Germany). Transformants expressing 2S albumin were selected from the regenerated and kanamycin-resistant plants by immunodetection of 2S albumin in leaf extracts.
ACKNOWLEDGMENTS
We are indebted to Gerhard Saalbach and Mario Rosso (IPK Gatersleben) for providing clones for 2S albumin constructs (2S and 2SΔ4), tobacco line for transformation, and anti-2S albumin antiserum, as well as Jürgen Denecke (University of Leeds) and Irene Kunze (IPK Gatersleben) for providing immune serum directed against BiP and chitinase, respectively. We thank Silvia Hertel (Biocenter, Universität Halle) for transformation of tobacco plants with 2S albumin constructs. Technical assistance of Kerstin Eichhorst (Biocenter, Universität Halle) is gratefully acknowledged. We express our thanks to Jon Page (IPB Halle, Germany) for comments on and carefully reading of the manuscript.
Footnotes
This work was supported by the Deutsche Forschungsgemeinschaft (grant no. B9SFB 363 to M.K.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010362.
LITERATURE CITED
- Abel S, Köck M. Secretory acid ribonucleases from tomato (Lycopersicon esculentum Mill.) In: Nicholson AW, editor. Ribonucleases, Methods in Enzymology. New York: Academic Press; 2001. 341: 351–368. [DOI] [PubMed] [Google Scholar]
- Bariola PA, MacIntosh GC, Green PJ. Regulation of S-like ribonuclease levels in Arabidopsis: antisense inhibition of RNS1 or RNS2 elevates anthocyanin accumulation. Plant Physiol. 1999;119:331–342. doi: 10.1104/pp.119.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosse D, Köck M. Influence of phosphate starvation on phosphohydrolases during development of tomato seedlings. Plant Cell Environ. 1998;21:325–332. [Google Scholar]
- D'Alessio G, Riordan JF. Ribonucleases: structures and functions. New York: Academic Press; 1997. [Google Scholar]
- Denecke J, De Rycke R, Botterman J. Plant and mammalian sorting signals for protein retention in the endoplasmic reticulum contain a conserved epitope. EMBO J. 1992;11:2345–2355. doi: 10.1002/j.1460-2075.1992.tb05294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denecke J, Ek B, Caspers M, Sinjorgo KMC, Palva ET. Analysis of sorting signals responsible for the accumulation of soluble reticuloplasmins in the plant endoplasmic reticulum. J Exp Bot. 1993;44:213–221. [Google Scholar]
- Denecke J, Goldman MHS, Demolder J, Seurinck J, Botterman J. The tobacco luminal binding protein is encoded by a multigene family. Plant Cell. 1991;3:1025–1035. doi: 10.1105/tpc.3.9.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda H. Tracheary element differentiation. Plant Cell. 1997;9:1147–1156. doi: 10.1105/tpc.9.7.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glund K, Tewes A, Abel S, Leinhos V, Walther R, Reinbothe H. Vacuoles from cell suspension cultures of tomato (Lycopersicon esculentum): isolation and characterization. Z Pflanzenphysiol. 1984;113:151–161. [Google Scholar]
- Gomord V, Denmat L-A, Fitchette-Lainé A-C, Satiat-Jeunemaitre B, Hawes C, Faye L. The C-terminal HDEL sequence is sufficient for retention of secretory proteins in the endoplasmic reticulum (ER) but promotes vacuolar targeting of proteins that escape the ER. Plant J. 1997;11:313–325. doi: 10.1046/j.1365-313x.1997.11020313.x. [DOI] [PubMed] [Google Scholar]
- Gomord V, Faye L. Signals and mechanisms involved in intracellular transport of secreted proteins in plants. Plant Physiol Biochem. 1996;34:165–181. [Google Scholar]
- Grabski S, de Feijter AW, Schindler M. Endoplasmic reticulum forms a dynamic continuum for lipid diffusion between contiguous soybean root cells. Plant Cell. 1993;5:25–38. doi: 10.1105/tpc.5.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green PJ. The ribonucleases of higher plants. Annu Rev Plant Physiol Mol Biol. 1994;45:421–445. [Google Scholar]
- Groover A, Jones AM. Tracheary element differentiation uses a novel mechanism coordinating programmed cell death and secondary cell wall synthesis. Plant Physiol. 1999;119:375–384. doi: 10.1104/pp.119.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hause B, Demus U, Teichmann C, Parthier B, Wasternack C. Developmental and tissue-specific expression of JIP-23, a jasmonate-inducible protein of barley. Plant Cell Physiol. 1996;37:641–649. doi: 10.1093/oxfordjournals.pcp.a028993. [DOI] [PubMed] [Google Scholar]
- Höfgen R, Willmitzer L. Storage of competent cells for Agrobacterium transformation. Nucleic Acids Res. 1988;16:9877. doi: 10.1093/nar/16.20.9877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie M. Structure-function relationship of acid ribonucleases: lysosomal, vacuolar, and periplasmic enzymes. Pharmacol Ther. 1999;81:77–89. doi: 10.1016/s0163-7258(98)00035-7. [DOI] [PubMed] [Google Scholar]
- Kaletta K, Kunze I, Kunze G, Köck M. The peptide HDEF as a new retention signal is necessary and sufficient to direct proteins to the endoplasmic reticulum. FEBS Lett. 1998;434:377–381. doi: 10.1016/s0014-5793(98)01013-8. [DOI] [PubMed] [Google Scholar]
- Köck M, Löffler A, Abel S, Glund K. Structural and regulatory properties of a family of starvation induced ribonucleases from tomato. Plant Mol Biol. 1995;27:477–485. doi: 10.1007/BF00019315. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lers A, Khalchitski A, Lomaniec E, Burd S, Green PJ. Senescence-induced RNases in tomato. Plant Mol Biol. 1998;36:439–449. doi: 10.1023/a:1005993024161. [DOI] [PubMed] [Google Scholar]
- Löffler A, Abel S, Jost W, Beintema JJ, Glund K. Phosphate-regulated induction of intracellular ribonucleases in cultured tomato (Lycopersicon esculentum) cells. Plant Physiol. 1992;98:1472–1478. doi: 10.1104/pp.98.4.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löffler A, Glund K, Irie M. Amino acid sequence of an intracellular, phosphate-starvation-induced ribonuclease from cultured tomato (Lycopersicon esculentum) cells. Eur J Biochem. 1993;214:627–633. doi: 10.1111/j.1432-1033.1993.tb17962.x. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough AL, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- McCubbin AG, Kao T-H. Molecular recognition and response in pollen and pistil interactions. Annu Rev Cell Dev Biol. 2000;16:333–364. doi: 10.1146/annurev.cellbio.16.1.333. [DOI] [PubMed] [Google Scholar]
- Ohta T, Kobayashi M, Hirose S. Cloning of a cDNA for DNA supercoiling factor reveals a distinctive Ca2+-binding protein. J Biol Chem. 1995;270:15571–15575. doi: 10.1074/jbc.270.26.15571. [DOI] [PubMed] [Google Scholar]
- Pedrazzini E, Giovinazzo G, Bielli A, de Virgilio M, Frigerio L, Pesca M, Faoro F, Bollini R, Ceriotti A, Vitale A. Protein quality control along the route to the plant vacuole. Plant Cell. 1997;9:1869–1880. doi: 10.1105/tpc.9.10.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennell RI, Lamb C. Programmed cell death in plants. Plant Cell. 1997;9:1157–1168. doi: 10.1105/tpc.9.7.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalbach G, Rosso M, Schumann U. The vacuolar targeting signal of the 2S albumin from Brazil nut resides at the C terminus and involves the C-terminal propeptide as an essential element. Plant Physiol. 1996;112:975–985. doi: 10.1104/pp.112.3.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalbach I, Pickardt T, Machemehl F, Saalbach G, Schieder O, Müntz K. A chimeric gene encoding the methionine-rich 2S albumin of the Brazil nut (Bertholletia excelsa H.B.K.) is stably expressed and inherited in transgenic grain legumes. Mol Gen Genet. 1994;242:226–236. doi: 10.1007/BF00391017. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Schägger H, von Jagow GS. Tricine-sodium dodecyl sulfate-polyacrylamide gel elektrophoresis for the separation of proteins in the range from 1 to 100 kD. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Schmid M, Simpson D, Gietl C. Programmed cell death in castor bean endosperm is associated with the accumulation and release of a cysteine endopeptidase from ricinosomes. Proc Natl Acad Sci USA. 1999;96:14159–14164. doi: 10.1073/pnas.96.24.14159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid M, Simpson D, Kalousek F, Gietl C. A cysteine endopeptidase with a C-terminal KDEL motif isolated from castor bean endosperm is a marker enzyme for the ricinosome, a putative lytic compartment. Planta. 1998;206:466–475. doi: 10.1007/s004250050423. [DOI] [PubMed] [Google Scholar]
- Shore G, MacLachlan GA. The side of cellulose synthesis. J Cell Biol. 1975;64:557–571. doi: 10.1083/jcb.64.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin LA. The plant ER: a dynamic organelle composed of a large number of discrete functional domains. Plant J. 1997;11:1151–1165. doi: 10.1046/j.1365-313x.1997.11061151.x. [DOI] [PubMed] [Google Scholar]
- Sticher L, Hinz U, Meyer AD, Meins F., Jr Intracellular transport and processing of a tobacco vacuolar β-1,3-glucanase. Planta. 1992;188:559–565. doi: 10.1007/BF00197049. [DOI] [PubMed] [Google Scholar]
- Vitale A, Ceriotti A, Denecke J. The role of the endoplasmic reticulum in protein synthesis, modification and intracellular transport. J Exp Bot. 1993;44:1417–1444. [Google Scholar]
- Vitale A, Denecke J. The endoplasmic reticulum: gateway of the secretory pathway. Plant Cell. 1999;11:615–628. doi: 10.1105/tpc.11.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabe D, Nakamura T, Kanazawa N, Tashiro K, Honjo T. Calumenin, a Ca2+-binding protein retained in the endoplasmic reticulum with a novel carboxyl-terminal sequence, HDEF. J Biol Chem. 1997;272:18232–18239. doi: 10.1074/jbc.272.29.18232. [DOI] [PubMed] [Google Scholar]
- Ye Z-H, Droste DL. Isolation and characterization of cDNAs encoding xylogenesis-associated and wounding-induced ribonucleases in Zinnia elegans. Plant Mol Biol. 1996;30:697–709. doi: 10.1007/BF00019005. [DOI] [PubMed] [Google Scholar]