Abstract
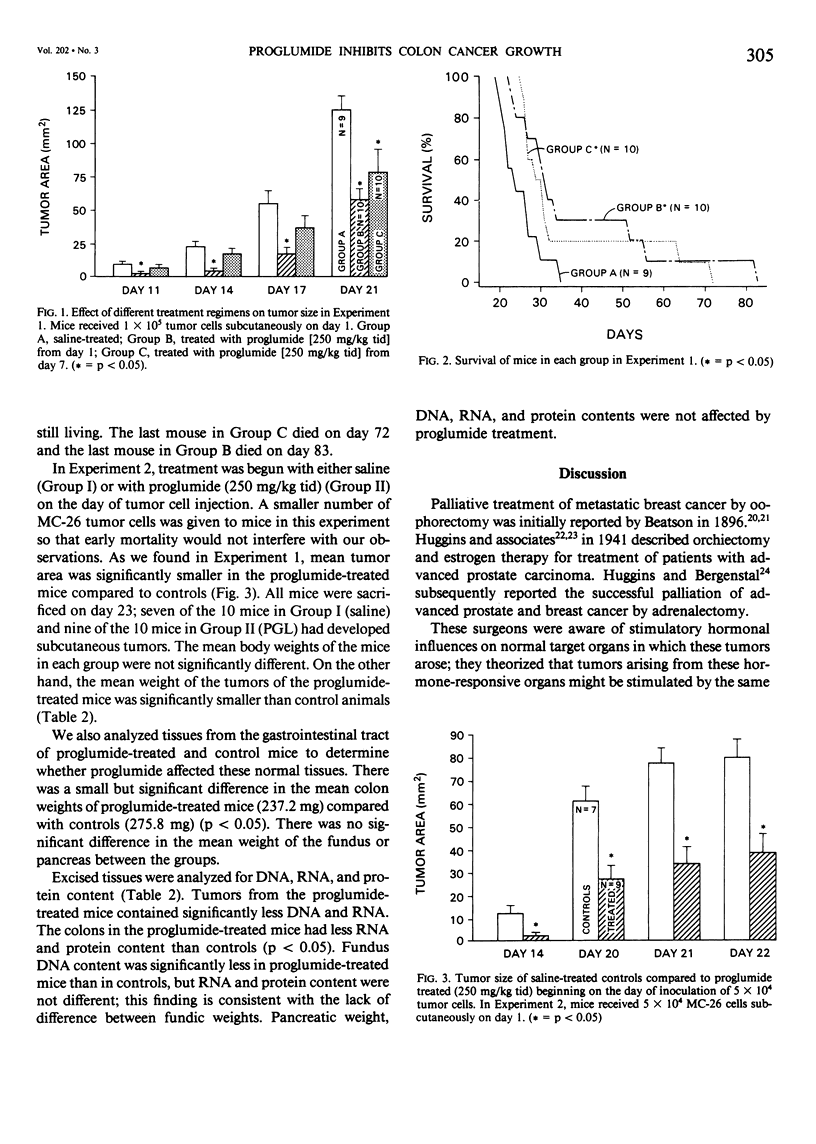
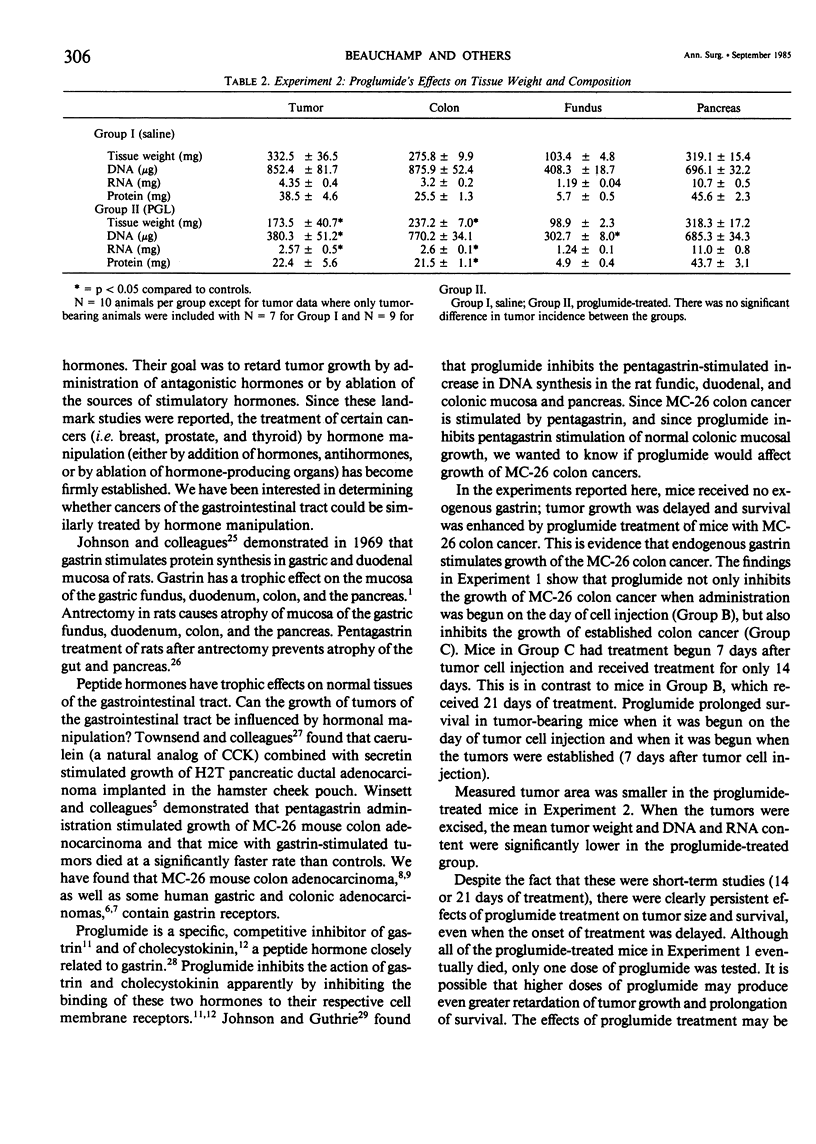
Some tumors are responsive to hormone manipulation. Some gastric and colonic adenocarcinomas from both humans and animals have specific gastrin receptors. A transplantable mouse colon adenocarcinoma cell line (MC-26) contains gastrin receptors; growth of MC-26 colon cancer in vivo is stimulated by pentagastrin (PG). The purpose of this study was to determine whether a gastrin-receptor antagonist, proglumide (PGL), would inhibit growth of MC-26 colon cancer and prolong survival in tumor-bearing mice. Subcutaneous tumors were induced by injecting single-cell suspensions of MC-26 cells into 50 mice divided into 10/group. In Experiment 1, all mice received 1 X 10(5) tumor cells and treatment groups were divided as follows: Group A received intraperitoneal (IP) saline (0.2 ml tid beginning on day 1); B, IP, PGL (250 mg/kg tid) from day of tumor cell inoculation; and C, IP PGL (250 mg/kg tid) from day 7 after tumor implantation. In Experiment 2, mice were inoculated with half the number of tumor cells. Group I mice received saline and Group II received PGL in the same manner starting on day 1. Tumors were measured and all mice were sacrificed on day 23. In Experiment 1, mean tumor area in Group B (PGL-treated) was significantly smaller than Group A on days 11, 14, 17, and 21. Tumors of Group C were significantly smaller than controls on day 21. Survival of PGL-treated mice was significantly prolonged. In Experiment 2, mean tumor area, mean tumor weight, and tumor DNA and RNA content were significantly less in the PGL-treated group than control. It was concluded that growth of a gastrin-responsive colon cancer was inhibited and host survival was enhanced by treatment with a gastrin-receptor antagonist. Hormone manipulation may be a useful treatment for gastrointestinal cancers.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DISCHE Z. Qualitative and quantitative colorimetric determination of heptoses. J Biol Chem. 1953 Oct;204(2):983–997. [PubMed] [Google Scholar]
- Dembinski A. B., Johnson L. R. Growth of pancreas and gastrointestinal mucosa in antrectomized and gastrin-treated rats. Endocrinology. 1979 Sep;105(3):769–773. doi: 10.1210/endo-105-3-769. [DOI] [PubMed] [Google Scholar]
- Fölsch U. R. Regulation of pancreatic growth. Clin Gastroenterol. 1984 Sep;13(3):679–699. [PubMed] [Google Scholar]
- GEHAN E. A. A GENERALIZED WILCOXON TEST FOR COMPARING ARBITRARILY SINGLY-CENSORED SAMPLES. Biometrika. 1965 Jun;52:203–223. [PubMed] [Google Scholar]
- HUGGINS C., BERGENSTAL D. M. Inhibition of human mammary and prostatic cancers by adrenalectomy. Cancer Res. 1952 Feb;12(2):134–141. [PubMed] [Google Scholar]
- Johnson L. R., Aures D., Yuen L. Pentagastrin-induced stimulation of protein synthesis in the gastrointestinal tract. Am J Physiol. 1969 Jul;217(1):251–254. doi: 10.1152/ajplegacy.1969.217.1.251. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lamers C. B., Jansen J. B. The effect of a gastrin-receptor antagonist on gastric acid secretion and serum gastrin in the Zollinger-Ellison syndrome. J Clin Gastroenterol. 1983 Feb;5(1):21–24. doi: 10.1097/00004836-198302000-00005. [DOI] [PubMed] [Google Scholar]
- Magous R., Bali J. P. Evidence that proglumide and benzotript antagonize secretagogue stimulation of isolated gastric parietal cells. Regul Pept. 1983 Nov;7(3):233–241. doi: 10.1016/0167-0115(83)90016-2. [DOI] [PubMed] [Google Scholar]
- OGUR M., ROSEN G. The nucleic acids of plant tissues; the extraction and estimation of desoxypentose nucleic acid and pentose nucleic acid. Arch Biochem. 1950 Feb;25(2):262–276. [PubMed] [Google Scholar]
- Thompson J. C., Marx M. Gastrointestinal hormones. Curr Probl Surg. 1984 Jun;21(6):1–80. doi: 10.1016/0011-3840(84)90005-4. [DOI] [PubMed] [Google Scholar]
- Williams J. A., Bailey A., Steigerwalt R. W. Effects of proglumide on pancreatic acinar cell function. Digestion. 1983;27(4):227–233. doi: 10.1159/000198957. [DOI] [PubMed] [Google Scholar]


