Abstract
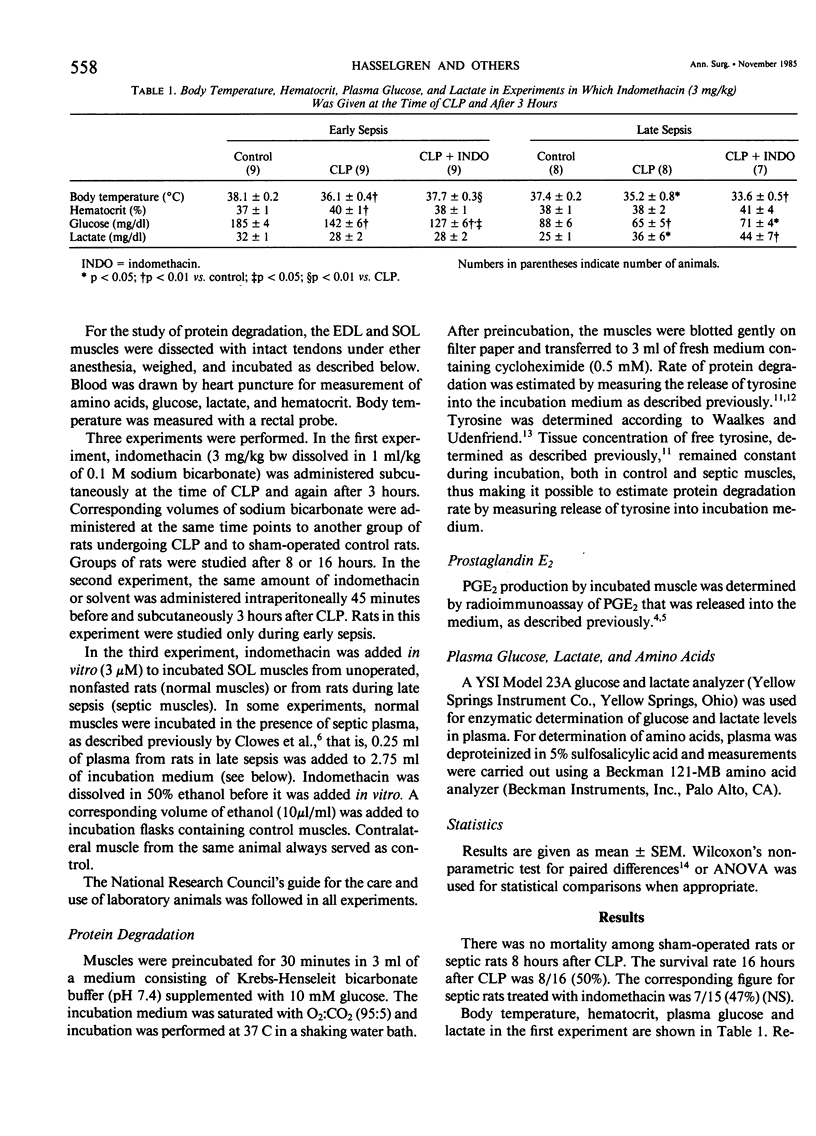
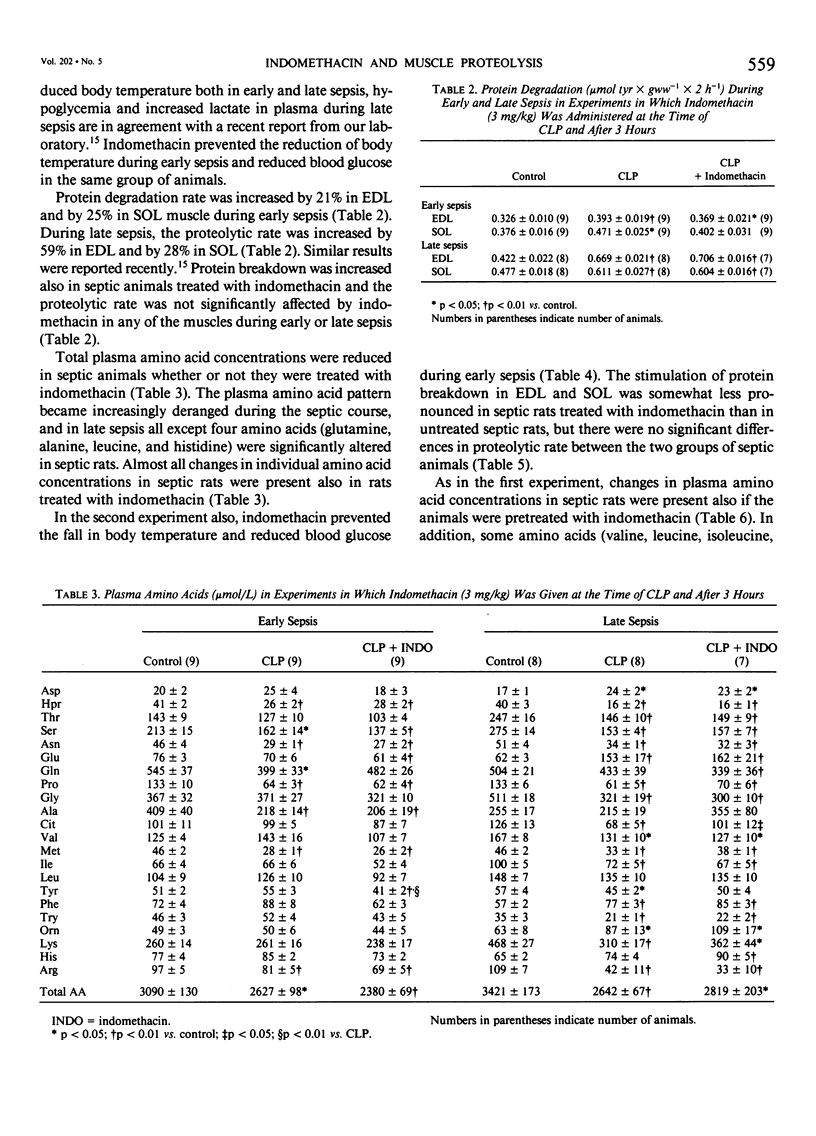
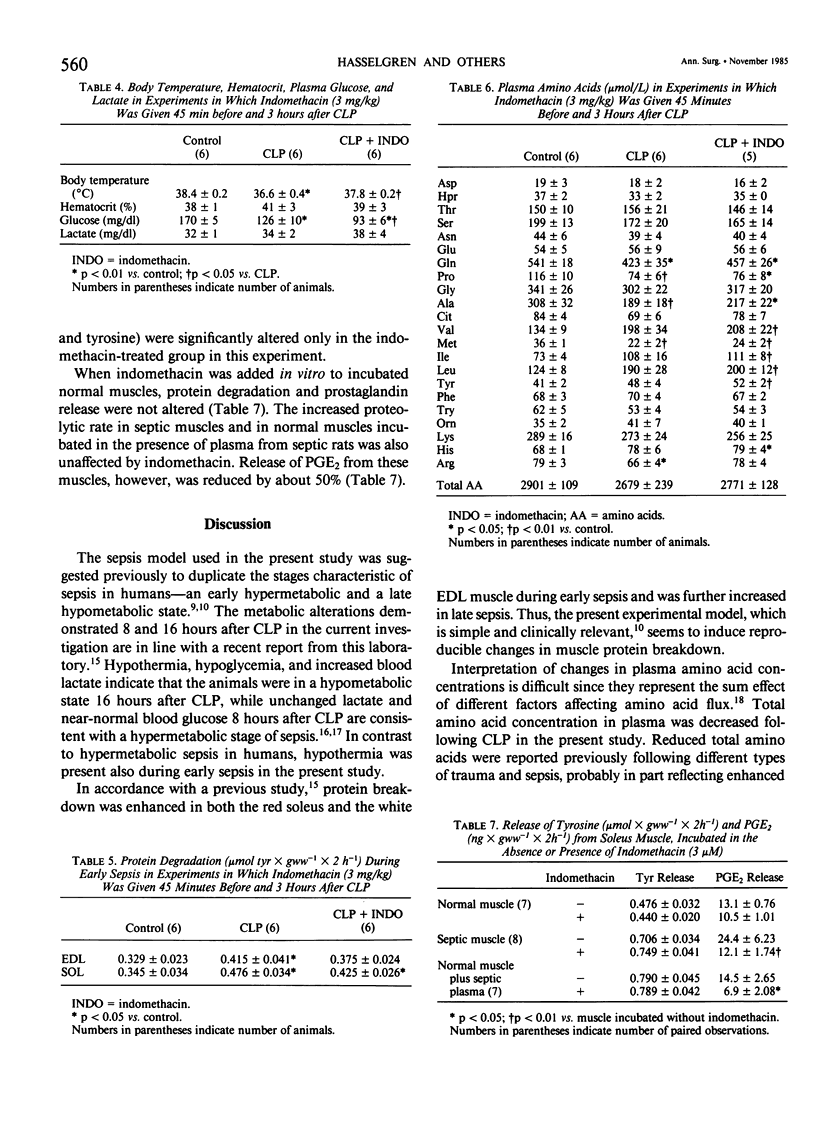
The effect of indomethacin on protein degradation in skeletal muscle from septic rats was investigated. Sepsis was induced by cecal ligation and puncture (CLP). Control rats were sham-operated. Protein degradation rate was estimated by measuring release of tyrosine from incubated soleus (SOL) and extensor digitorum longus (EDL) muscles. Three experiments were performed. In the first experiment, indomethacin was administered subcutaneously (3 mg/kg) at the time of CLP and again after 3 hours. Control rats received corresponding volumes of solvent. Groups of rats were studied after 8 hours (early sepsis) or 16 hours (late sepsis). In the second experiment, the animals were pretreated 45 minutes before induction of sepsis with indomethacin (3 mg/kg) and again 3 hours after CLP and were studied during early sepsis. In the third experiment, indomethacin was added in vitro (3 microM) to incubated normal or septic muscle or to normal muscle incubated in the presence of plasma from septic animals, and release of prostaglandin E2 (PGE2) by incubated muscle was measured in addition to protein degradation. There was no mortality in early sepsis. Survival rate 16 hours after CLP was 8/16 (50%) in rats receiving control injections and 7/15 (47%) in indomethacin-treated rats (NS). Proteolytic rate in incubated EDL and SOL was increased by 20-25% during early sepsis and by 30-50% during late sepsis. The increased proteolytic rate was not affected by administration of indomethacin, neither in the first nor in the second experiment. When indomethacin was added in vitro, release of PGE2 by septic muscles and by normal muscles incubated in the presence of septic plasma was reduced by about 50%, but the increased proteolytic rate in these muscles was not affected. In normal muscle, neither release of PGE2 nor protein degradation was affected by indomethacin in vitro. The present results do not support a role for prostaglandins in the enhancement of muscle proteolysis during sepsis. Since neither survival rate nor protein breakdown was affected by indomethacin, recent suggestions to use this substance in the treatment of septic patients might be questioned.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abumrad N. N., Miller B. The physiologic and nutritional significance of plasma-free amino acid levels. JPEN J Parenter Enteral Nutr. 1983 Mar-Apr;7(2):163–170. doi: 10.1177/0148607183007002163. [DOI] [PubMed] [Google Scholar]
- Aulick L. H., Wilmore D. W. Increased peripheral amino acid release following burn injury. Surgery. 1979 May;85(5):560–565. [PubMed] [Google Scholar]
- Baracos V. E., Wilson E. J., Goldberg A. L. Effects of temperature on protein turnover in isolated rat skeletal muscle. Am J Physiol. 1984 Jan;246(1 Pt 1):C125–C130. doi: 10.1152/ajpcell.1984.246.1.C125. [DOI] [PubMed] [Google Scholar]
- Baracos V., Rodemann H. P., Dinarello C. A., Goldberg A. L. Stimulation of muscle protein degradation and prostaglandin E2 release by leukocytic pyrogen (interleukin-1). A mechanism for the increased degradation of muscle proteins during fever. N Engl J Med. 1983 Mar 10;308(10):553–558. doi: 10.1056/NEJM198303103081002. [DOI] [PubMed] [Google Scholar]
- Chaudry I. H., Wichterman K. A., Baue A. E. Effect of sepsis on tissue adenine nucleotide levels. Surgery. 1979 Feb;85(2):205–211. [PubMed] [Google Scholar]
- Clark A. S., Kelly R. A., Mitch W. E. Systemic response to thermal injury in rats. Accelerated protein degradation and altered glucose utilization in muscle. J Clin Invest. 1984 Sep;74(3):888–897. doi: 10.1172/JCI111506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowes G. H., Jr, George B. C., Villee C. A., Jr, Saravis C. A. Muscle proteolysis induced by a circulating peptide in patients with sepsis or trauma. N Engl J Med. 1983 Mar 10;308(10):545–552. doi: 10.1056/NEJM198303103081001. [DOI] [PubMed] [Google Scholar]
- Clowes G. H., Jr, O'Donnell T. F., Blackburn G. L., Maki T. N. Energy metabolism and proteolysis in traumatized and septic man. Surg Clin North Am. 1976 Oct;56(5):1169–1184. doi: 10.1016/s0039-6109(16)41036-4. [DOI] [PubMed] [Google Scholar]
- Clowes G. H., Jr, Randall H. T., Cha C. J. Amino acid and energy metabolism in septic and traumatized patients. JPEN J Parenter Enteral Nutr. 1980 Mar-Apr;4(2):195–205. doi: 10.1177/014860718000400225. [DOI] [PubMed] [Google Scholar]
- Duke J. H., Jr, Jørgensen S. B., Broell J. R., Long C. L., Kinney J. M. Contribution of protein to caloric expenditure following injury. Surgery. 1970 Jul;68(1):168–174. [PubMed] [Google Scholar]
- Fletcher J. R., Ramwell P. W., Herman C. M. Prostaglandins and the hemodynamic course of endotoxin shock. J Surg Res. 1976 Jun;20(6):589–594. doi: 10.1016/0022-4804(76)90095-0. [DOI] [PubMed] [Google Scholar]
- Fletcher J. R., Ramwell P. W. Lidocaine or indomethacin improves survival in baboon endotoxin shock. J Surg Res. 1978 Mar;24(3):154–160. doi: 10.1016/0022-4804(78)90168-3. [DOI] [PubMed] [Google Scholar]
- Fletcher J. R., Ramwell P. W. Modification, by aspirin and indomethacin, of the haemodynamic and prostaglandin releasing effects of E. coli endotoxin in the dog. Br J Pharmacol. 1977 Oct;61(2):175–181. doi: 10.1111/j.1476-5381.1977.tb08402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher J. R. The role of prostaglandins in sepsis. Scand J Infect Dis Suppl. 1982;31:55–60. [PubMed] [Google Scholar]
- Fulks R. M., Li J. B., Goldberg A. L. Effects of insulin, glucose, and amino acids on protein turnover in rat diaphragm. J Biol Chem. 1975 Jan 10;250(1):290–298. [PubMed] [Google Scholar]
- Goldberg A. L., Baracos V., Rodemann P., Waxman L., Dinarello C. Control of protein degradation in muscle by prostaglandins, Ca2+, and leukocytic pyrogen (interleukin 1). Fed Proc. 1984 Apr;43(5):1301–1306. [PubMed] [Google Scholar]
- Goldberg A. L., Martel S. B., Kushmerick M. J. In vitro preparations of the diaphragm and other skeletal muscles. Methods Enzymol. 1975;39:82–94. doi: 10.1016/s0076-6879(75)39012-5. [DOI] [PubMed] [Google Scholar]
- Li J. B., Goldberg A. L. Effects of food deprivation on protein synthesis and degradation in rat skeletal muscles. Am J Physiol. 1976 Aug;231(2):441–448. doi: 10.1152/ajplegacy.1976.231.2.441. [DOI] [PubMed] [Google Scholar]
- O'Donnel T. F., Clowes G. H., Jr, Blackburn G. L., Ryan N. T., Benotti P. N., Miller J. D. Proteolysis associated with a deficit of peripheral energy fuel substrates in septic man. Surgery. 1976 Aug;80(2):192–200. [PubMed] [Google Scholar]
- Rodemann H. P., Goldberg A. L. Arachidonic acid, prostaglandin E2 and F2 alpha influence rates of protein turnover in skeletal and cardiac muscle. J Biol Chem. 1982 Feb 25;257(4):1632–1638. [PubMed] [Google Scholar]
- Ryan N. T. Metabolic adaptations for energy production during trauma and sepsis. Surg Clin North Am. 1976 Oct;56(5):1073–1090. doi: 10.1016/s0039-6109(16)41032-7. [DOI] [PubMed] [Google Scholar]
- Short B. L., Gardiner M., Walker R. I., Jones S. R., Fletcher J. R. Indomethacin improves survival in gram-negative sepsis. Adv Shock Res. 1981;6:27–36. [PubMed] [Google Scholar]
- WAALKES T. P., UDENFRIEND S. A fluorometric method for the estimation of tyrosine in plasma and tissues. J Lab Clin Med. 1957 Nov;50(5):733–736. [PubMed] [Google Scholar]
- Wannemacher R. W., Jr Key role of various individual amino acids in host response to infection. Am J Clin Nutr. 1977 Aug;30(8):1269–1280. doi: 10.1093/ajcn/30.8.1269. [DOI] [PubMed] [Google Scholar]
- Wichterman K. A., Baue A. E., Chaudry I. H. Sepsis and septic shock--a review of laboratory models and a proposal. J Surg Res. 1980 Aug;29(2):189–201. doi: 10.1016/0022-4804(80)90037-2. [DOI] [PubMed] [Google Scholar]


