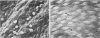Abstract
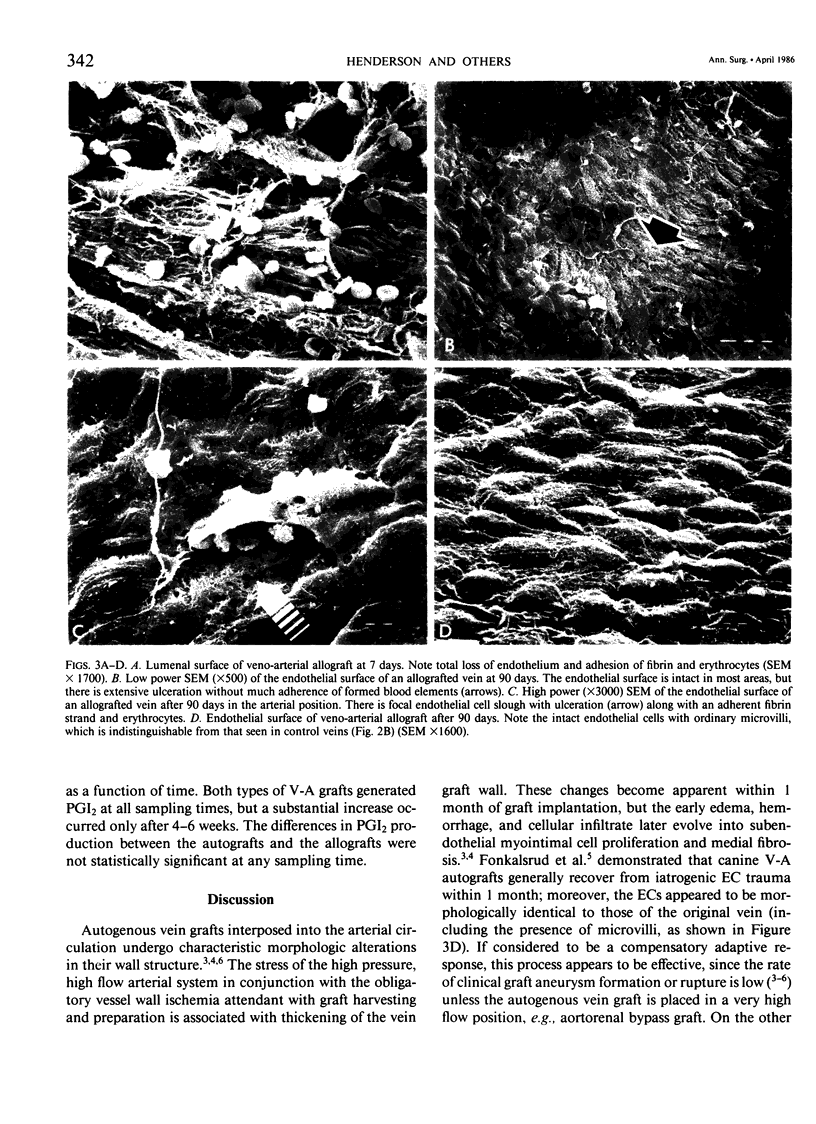
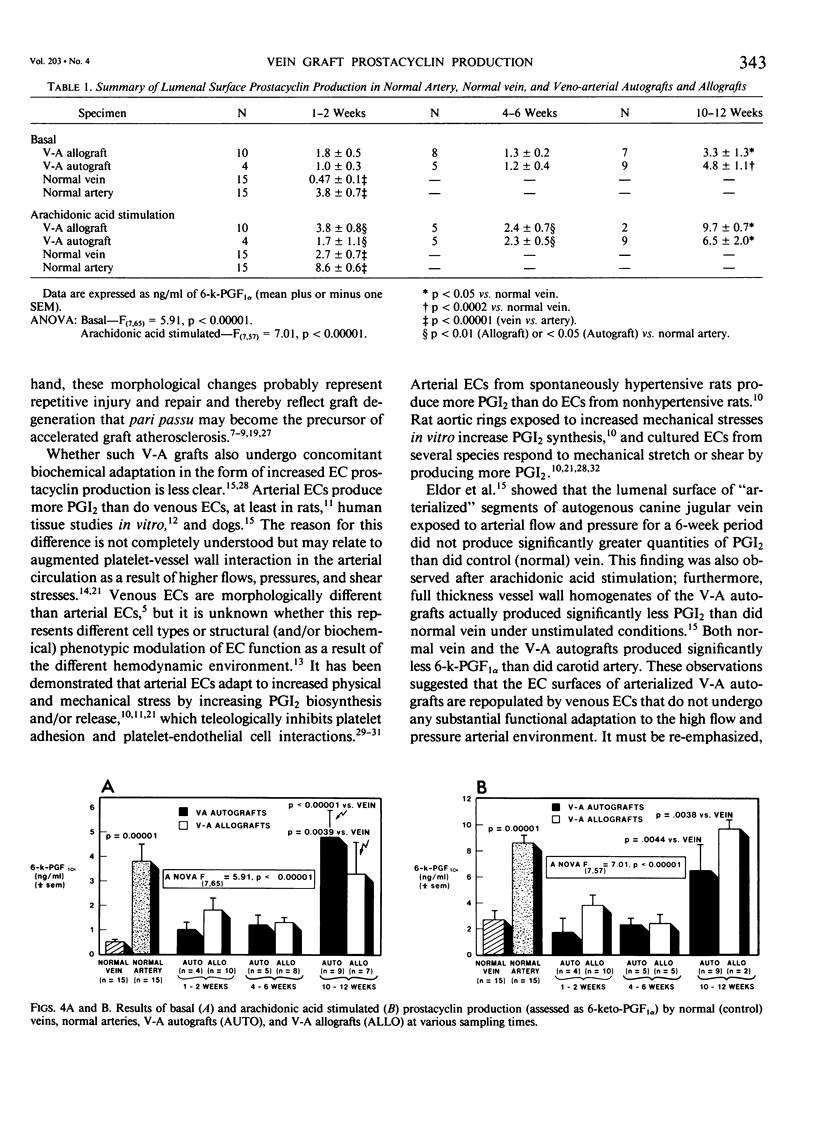
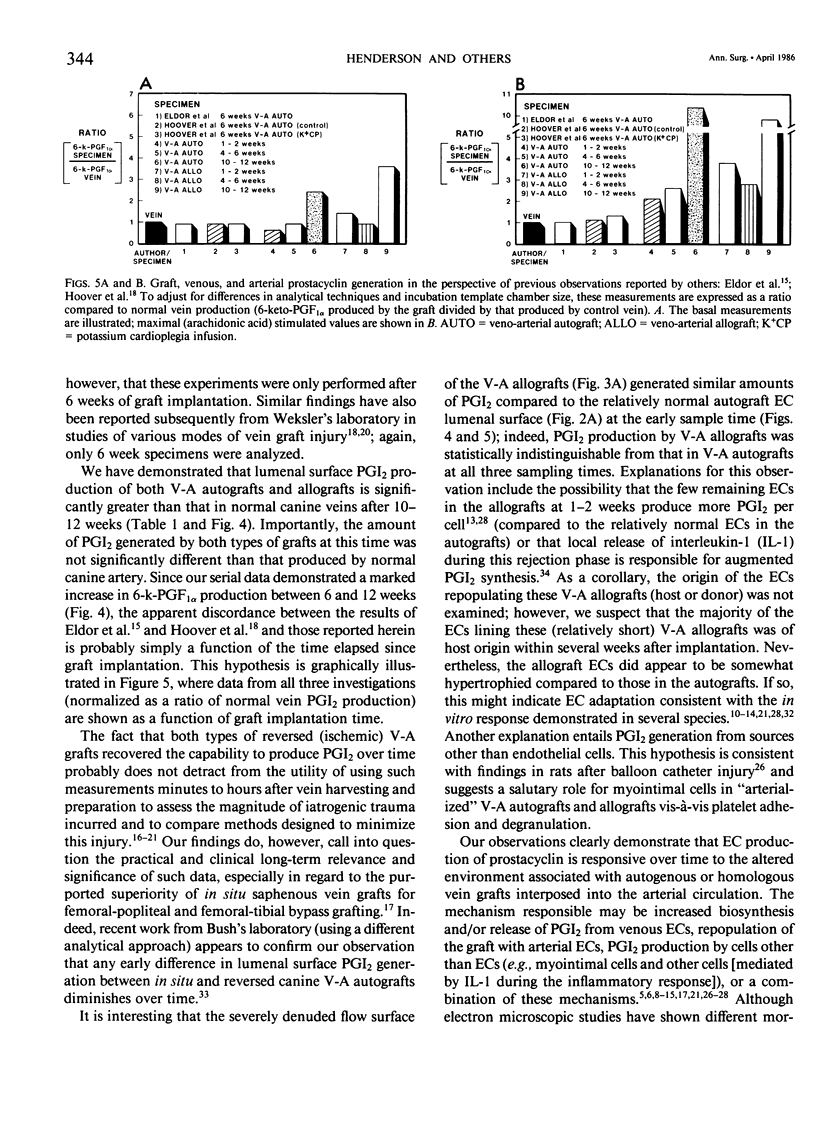
Canine venous autografts and allografts were interposed in the femoral and carotid arterial positions in 29 dogs; grafts were harvested at three postoperative intervals (1-2 weeks, 4-6 weeks, and 8-10 weeks) for light and scanning electron (SEM) microscopy and lumenal surface prostacyclin (PGI2) production. Normal veins and arteries were used as controls. Radioimmunoassay for tritiated 6-k-PGF1 alpha, the stable metabolite of PGI2, was performed using a flow surface template incubation chamber during basal and arachidonic acid stimulated conditions. Using SEM, the autografts revealed normal endothelial cell (EC) surfaces at all time intervals; conversely, allografts exhibited extensive EC loss at 1-2 weeks with gradual reparation by 10-12 weeks (such that the EC surface was virtually indistinguishable from that of control veins or autografts). PGI2 production was significantly greater in control arteries than veins (p = 0.0001). At 1-2 weeks and 4-6 weeks, lumenal production of PGI2 in both the autografts and allografts was not significantly different from control vein; however, PGI2 production after 10-12 weeks was identical to normal arterial levels (and significantly [p less than 0.0044] higher than venous levels) in both basal and stimulated conditions. Although the mechanisms responsible for this functional (biochemical) "arterialization" process remain conjectural, increased biosynthesis and/or release of PGI2 by endothelial cells, acute phase inflammatory cells (allografts) mediated by interleukin-1 or myointimal cells seems most likely. Further elucidation of these sources of PGI2 is necessary, but these data demonstrate for the first time that venous grafts placed in the arterial circulation undergo complete functional adaptation (in addition to the well known morphological changes).
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barboriak J. J., Pintar K., Van Horn D. L., Batayias G. E., Korns M. E. Pathologic findings in the aortocoronary vein grafts. A scanning electron microscope study. Atherosclerosis. 1978 Jan;29(1):69–80. doi: 10.1016/0021-9150(78)90095-3. [DOI] [PubMed] [Google Scholar]
- Bieber C. P., Stinson E. B., Shumway N. E., Payne R., Kosek J. Cardiac transplantation in man. VII. Cardiac allograft pathology. Circulation. 1970 May;41(5):753–772. doi: 10.1161/01.cir.41.5.753. [DOI] [PubMed] [Google Scholar]
- Brody W. R., Angeli W. W., Kosek J. C. Histologic fate of the venous coronary artery bypass in dogs. Am J Pathol. 1972 Jan;66(1):111–130. [PMC free article] [PubMed] [Google Scholar]
- Brody W. R., Kosek J. C., Angell W. W. Changes in vein grafts following aorto-coronary bypass induced by pressure and ischemia. J Thorac Cardiovasc Surg. 1972 Dec;64(6):847–854. [PubMed] [Google Scholar]
- Bush H. L., Jr, Graber J. N., Jakubowski J. A., Hong S. L., McCabe M., Deykin D., Nabseth D. C. Favorable balance of prostacyclin and thromboxane A2 improves early patency of human in situ vein grafts. J Vasc Surg. 1984 Jan;1(1):149–159. [PubMed] [Google Scholar]
- Bush H. L., Jr, McCabe M. E., Nabseth D. C. Functional injury of vein graft endothelium. Role of hypothermia and distention. Arch Surg. 1984 Jul;119(7):770–774. doi: 10.1001/archsurg.1984.01390190014003. [DOI] [PubMed] [Google Scholar]
- De Bono D. P. Host repopulation of endothelium in human kidney transplants. Transplantation. 1972 Oct;14(4):438–441. doi: 10.1097/00007890-197210000-00005. [DOI] [PubMed] [Google Scholar]
- De Caterina R., Weksler B. B., Alonso D. R., Cruz-Bracho M. R., Subramanian V. A. Functional endothelial damage by high-potassium cardioplegic solutions to saphenous vein bypass grafts. Surgery. 1985 Sep;98(3):465–471. [PubMed] [Google Scholar]
- Eldor A., Falcone D. J., Hajjar D. P., Minick C. R., Weksler B. B. Recovery of prostacyclin production by de-endothelialized rabbit aorta. Critical role of neointimal smooth muscle cells. J Clin Invest. 1981 Mar;67(3):735–741. doi: 10.1172/JCI110090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldor A., Hoover E. L., Pett S. B., Jr, Gay W. A., Jr, Alonso D. R., Weksler B. B. Prostacyclin production by arterialized autogenous venous grafts in dogs. Prostaglandins. 1981 Sep;22(3):485–498. doi: 10.1016/0090-6980(81)90108-8. [DOI] [PubMed] [Google Scholar]
- Fonkalsrud E. W., Sanchez M., Zerubavel R. Morphological evaluation of canine autogeneous vein grafts in the arterial circulation. Surgery. 1978 Aug;84(2):253–264. [PubMed] [Google Scholar]
- Frangos J. A., Eskin S. G., McIntire L. V., Ives C. L. Flow effects on prostacyclin production by cultured human endothelial cells. Science. 1985 Mar 22;227(4693):1477–1479. doi: 10.1126/science.3883488. [DOI] [PubMed] [Google Scholar]
- Fry G. L., Czervionke R. L., Hoak J. C., Smith J. B., Haycraft D. L. Platelet adherence to cultured vascular cells: influence of prostacyclin (PGI2). Blood. 1980 Feb;55(2):271–275. [PubMed] [Google Scholar]
- Fuchs R. M., Brin K. P., Brinker J. A., Guzman P. A., Heuser R. R., Yin F. C. Augmentation of regional coronary blood flow by intra-aortic balloon counterpulsation in patients with unstable angina. Circulation. 1983 Jul;68(1):117–123. doi: 10.1161/01.cir.68.1.117. [DOI] [PubMed] [Google Scholar]
- Grondin C. M., Campeau L., Lespérance J., Solymoss B. C., Vouhé P., Castonguay Y. R., Meere C., Bourassa M. G. Atherosclerotic changes in coronary vein grafts six years after operation. Angiographic aspect in 110 patients. J Thorac Cardiovasc Surg. 1979 Jan;77(1):24–31. [PubMed] [Google Scholar]
- Hahn G. L., Polgar P. R. Prostaglandin production in phenotypically distinct cultured bovine pulmonary artery endothelium. Atherosclerosis. 1984 Apr;51(1):143–150. doi: 10.1016/0021-9150(84)90150-3. [DOI] [PubMed] [Google Scholar]
- Haudenschild C. C., Schwartz S. M. Endothelial regeneration. II. Restitution of endothelial continuity. Lab Invest. 1979 Nov;41(5):407–418. [PubMed] [Google Scholar]
- Higgs E. A., Moncada S., Vane J. R., Caen J. P., Michel H., Tobelem G. Effect of prostacyclin (PGI2) on platelet adhesion to rabbit arterial subendothelium. Prostaglandins. 1978 Jul;16(1):17–22. doi: 10.1016/0090-6980(78)90197-1. [DOI] [PubMed] [Google Scholar]
- Johnson A. R. Human pulmonary endothelial cells in culture. Activities of cells from arteries and cells from veins. J Clin Invest. 1980 Apr;65(4):841–850. doi: 10.1172/JCI109736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann R. L., Larson R. M., Mitchener J. S., 3rd, Fuchs J. C., Hagen P. O. Intimal thickening and hyperlipidemia in experimental primate vascular autografts. Ann Surg. 1979 Jan;189(1):62–67. doi: 10.1097/00000658-197901000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Herman A. G., Higgs E. A., Vane J. R. Differential formation of prostacyclin (PGX or PGI2) by layers of the arterial wall. An explanation for the anti-thrombotic properties of vascular endothelium. Thromb Res. 1977 Sep;11(3):323–344. doi: 10.1016/0049-3848(77)90185-2. [DOI] [PubMed] [Google Scholar]
- Pace-Asciak C. R., Carrara M. C., Rangaraj G., Nicolaou K. C. Enhanced formation of PGI2, a potent hypotensive substance, by aortic rings and homogenates of the spontaneously hypertensive rat. Prostaglandins. 1978 Jun;15(6):1005–1012. doi: 10.1016/0090-6980(78)90043-6. [DOI] [PubMed] [Google Scholar]
- Qvarfordt P. G., Reilly L. M., Lusby R. J., Effeney D. J., Ferrell L. D., Price D. C., Fuller J., Ehrenfeld W. K., Stoney R. J., Goldstone J. Prostacyclin production in regions of arterial stenosis. Surgery. 1985 Sep;98(3):484–491. [PubMed] [Google Scholar]
- Rossi V., Breviario F., Ghezzi P., Dejana E., Mantovani A. Prostacyclin synthesis induced in vascular cells by interleukin-1. Science. 1985 Jul 12;229(4709):174–176. doi: 10.1126/science.2409598. [DOI] [PubMed] [Google Scholar]
- Schwartz S. I., Kutner F. R., Neistadt A., Barner H., Resnicoff S., Vaughan J. Antigenicity of homografted veins. Surgery. 1967 Mar;61(3):471–477. [PubMed] [Google Scholar]
- Skidgel R. A., Printz M. P. PGI2 production by rat blood vessels: diminished prostacyclin formation in veins compared to arteries. Prostaglandins. 1978 Jul;16(1):1–16. doi: 10.1016/0090-6980(78)90196-x. [DOI] [PubMed] [Google Scholar]
- Stemerman M. B., Spaet T. H., Pitlick F., Cintron J., Lejnieks I., Tiell M. L. Intimal healing. The pattern of reendothelialization and intimal thickening. Am J Pathol. 1977 Apr;87(1):125–142. [PMC free article] [PubMed] [Google Scholar]
- Weksler B. B., Marcus A. J., Jaffe E. A. Synthesis of prostaglandin I2 (prostacyclin) by cultured human and bovine endothelial cells. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3922–3926. doi: 10.1073/pnas.74.9.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G. M., ter Haar A., Krajewski C., Parks L. C., Roth J. Rejection and repair of endothelium in major vessel transplants. Surgery. 1975 Dec;78(6):694–706. [PubMed] [Google Scholar]