Abstract
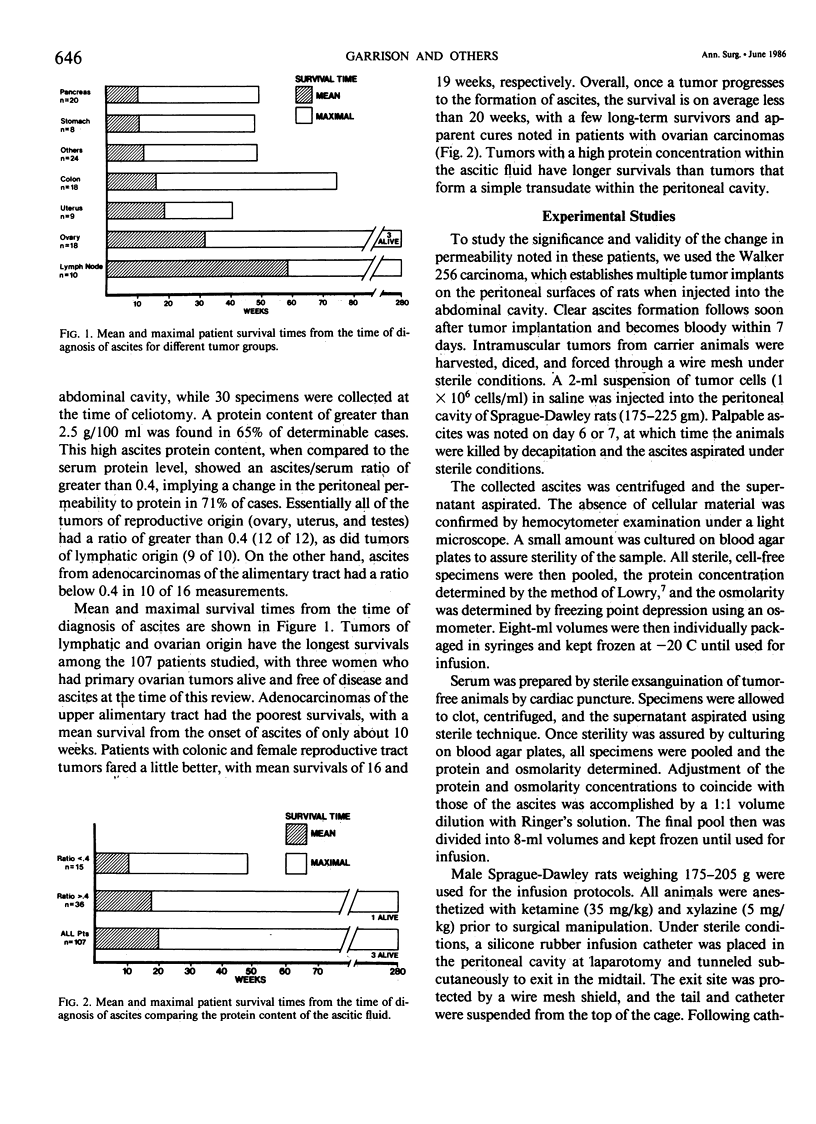
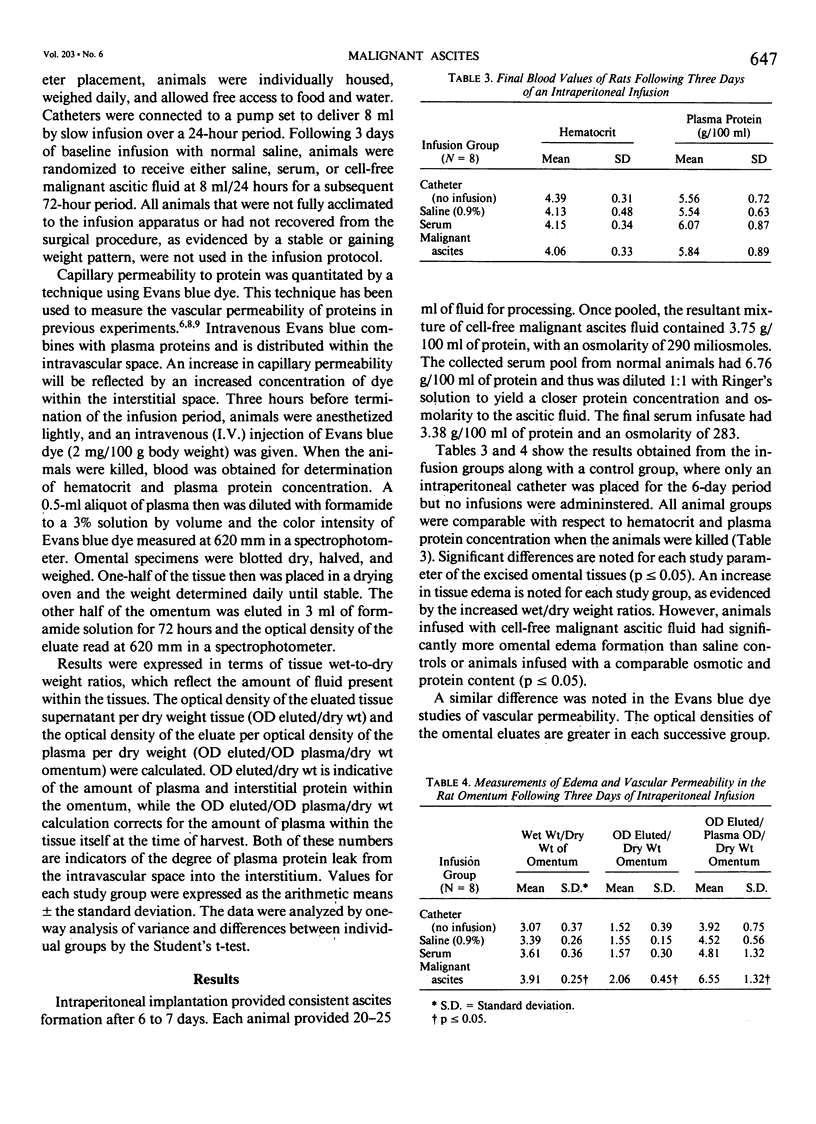
Malignant ascites formation is a grave prognostic sign, but palliative efforts seem justified in some patients. Lack of knowledge concerning the natural history of this process hinders the choice of therapeutic options. Over 5 years, 107 patients with untreated malignant ascites were reviewed to define their survival. Pancreas (20), ovary (18), and colon (18) were the most frequent tumors, with 52% of patients presenting with ascites at the time of the initial cancer diagnosis. Cytology evaluation of the ascitic fluid was positive for tumor cells in 57% of cases and a high protein content was noted in 65%. Mean survival of the entire series was only 20 weeks from the time of diagnosis of ascites, with tumors of ovarian and lymphatic origin having better mean survivals of 32 and 58 weeks, respectively. Patients with high ascitic protein levels fared better than those with low levels. In an effort to explain this correlation of elevated protein levels and a favorable survival rate, a hypothesis was proposed that certain tumors secrete a factor, which alters vascular permeability and causes fluid accumulation in the absence of lymphatic obstruction. In an experimental rat model of malignant ascites, the intraperitoneal infusion of cell-free malignant ascitic fluid caused an increase in edema formation and a significant increase in capillary permeability to protein in the omentum. This demonstrated change in the leak of protein explains the formation of ascites by some tumors in the absence of tumor obstruction of the draining lymphatics of the peritoneal cavity and suggests another important mechanism in the genesis of malignant ascites.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman N. B. Differences in vascular permeability between normal and tumor vessels produced by vasoactive agents. Surg Forum. 1973;24:105–107. [PubMed] [Google Scholar]
- Appelqvist P., Silvo J., Salmela L., Kostiainen S. On the treatment and prognosis of malignant ascites: is the survival time determined when the abdominal paracentesis is needed? J Surg Oncol. 1982 Aug;20(4):238–242. doi: 10.1002/jso.2930200411. [DOI] [PubMed] [Google Scholar]
- Bronskill M. J., Bush R. S., Ege G. N. A quantitative measurement of peritoneal drainage in malignant ascites. Cancer. 1977 Nov;40(5):2375–2380. doi: 10.1002/1097-0142(197711)40:5<2375::aid-cncr2820400554>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Cattau E. L., Jr, Benjamin S. B., Knuff T. E., Castell D. O. The accuracy of the physical examination in the diagnosis of suspected ascites. JAMA. 1982 Feb 26;247(8):1164–1166. [PubMed] [Google Scholar]
- Cheung D. K., Raaf J. H. Selection of patients with malignant ascites for a peritoneovenous shunt. Cancer. 1982 Sep 15;50(6):1204–1209. doi: 10.1002/1097-0142(19820915)50:6<1204::aid-cncr2820500631>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Coates G., Bush R. S., Aspin N. A study of ascites using lymphoscintigraphy with 99m Tc-sulfur colloid. Radiology. 1973 Jun;107(3):577–583. doi: 10.1148/107.3.577. [DOI] [PubMed] [Google Scholar]
- Fastaia J., Dumont A. E. Pathogenesis of ascites in mice with peritoneal carcinomatosis. J Natl Cancer Inst. 1976 Mar;56(3):547–550. doi: 10.1093/jnci/56.3.547. [DOI] [PubMed] [Google Scholar]
- Heuser L. S., Miller F. N. Differential macromolecular leakage from the vasculature of tumors. Cancer. 1986 Feb 1;57(3):461–464. doi: 10.1002/1097-0142(19860201)57:3<461::aid-cncr2820570310>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Heuser L. S., Taylor S. H., Folkman J. Prevention of carcinomatosis and bloody malignant ascites in the rat by an inhibitor of angiogenesis. J Surg Res. 1984 Mar;36(3):244–250. doi: 10.1016/0022-4804(84)90094-5. [DOI] [PubMed] [Google Scholar]
- Jackson G. L., Blosser N. M. Intracavitary chromic phosphate (32P) colloidal suspension therapy. Cancer. 1981 Dec 15;48(12):2596–2598. doi: 10.1002/1097-0142(19811215)48:12<2596::aid-cncr2820481210>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Reinhold R. B., Lokich J. J., Tomashefski J., Costello P. Management of malignant ascites with peritoneovenous shunting. Am J Surg. 1983 Apr;145(4):455–457. doi: 10.1016/0002-9610(83)90039-9. [DOI] [PubMed] [Google Scholar]
- Torisu M., Katano M., Kimura Y., Itoh H., Takesue M. New approach to management of malignant ascites with a streptococcal preparation, OK-432. I. Improvement of host immunity and prolongation of survival. Surgery. 1983 Mar;93(3):357–364. [PubMed] [Google Scholar]
- Yamada S., Takeda T., Matsumoto K. Prognostic analysis of malignant pleural and peritoneal effusions. Cancer. 1983 Jan 1;51(1):136–140. doi: 10.1002/1097-0142(19830101)51:1<136::aid-cncr2820510127>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]


