Abstract
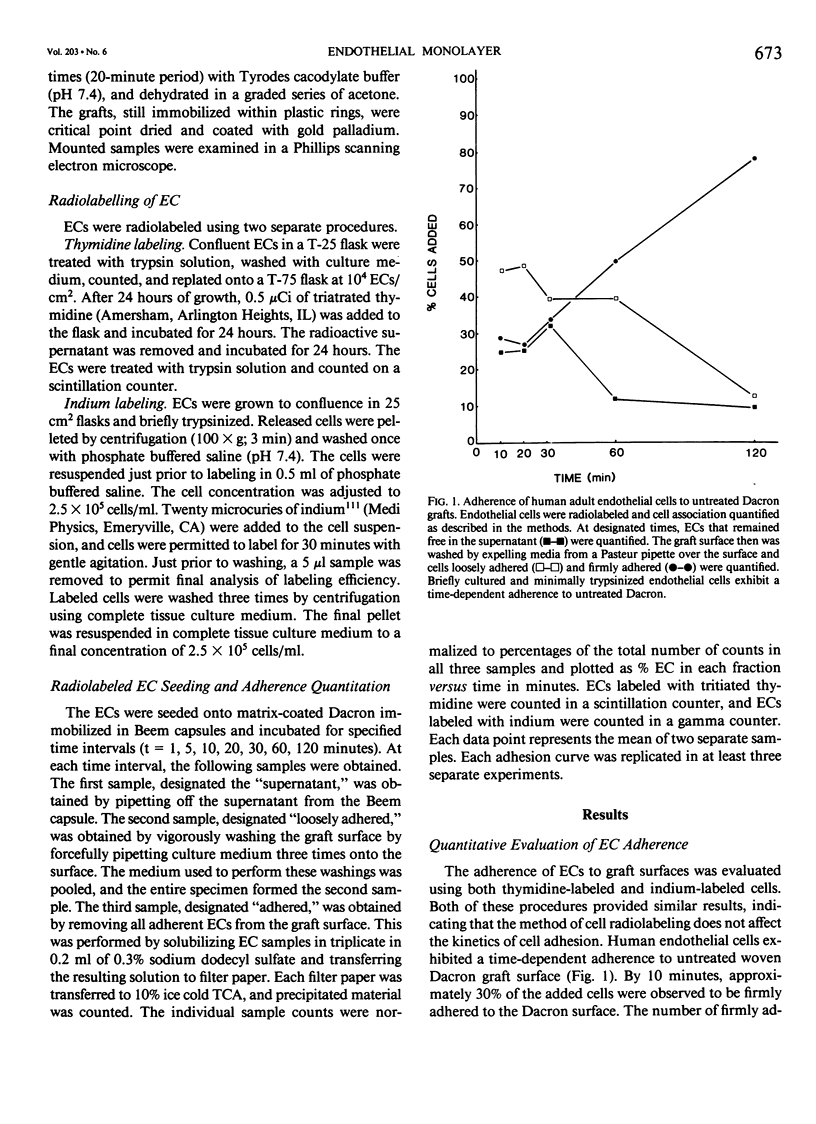
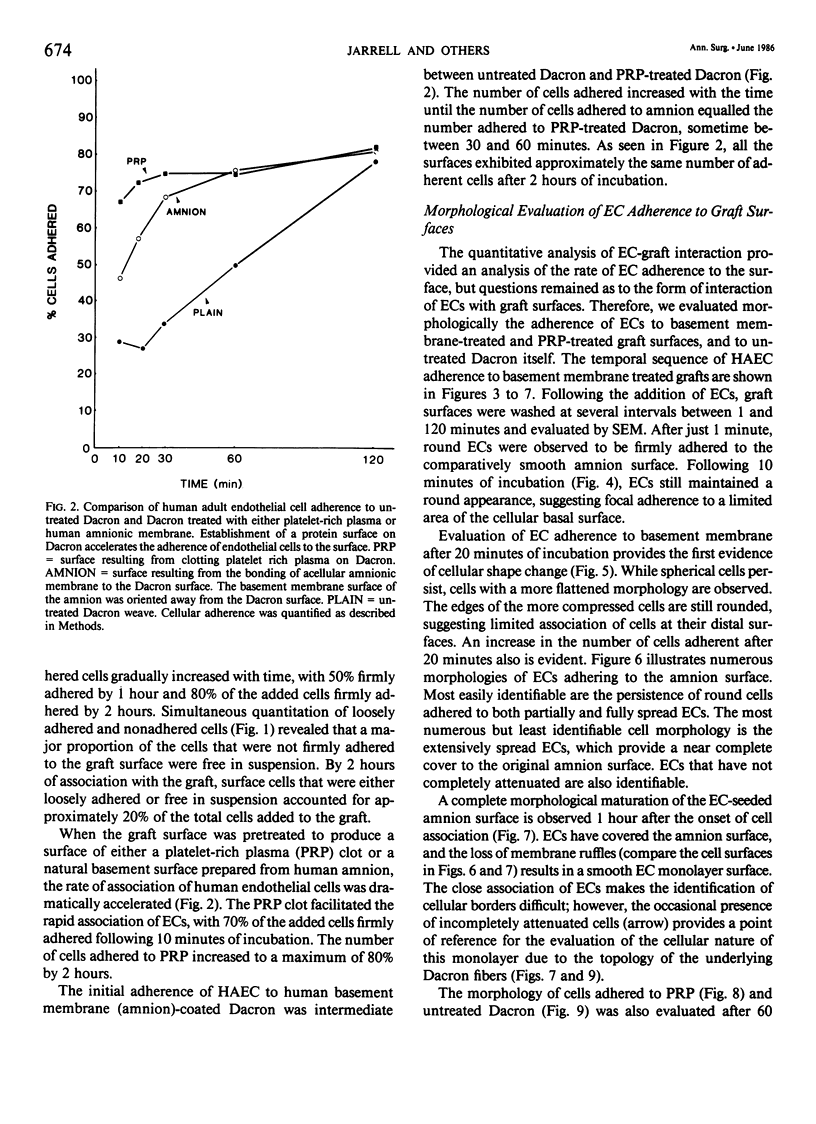
The temporal sequence of events was examined from initial contact of endothelial cells (ECs) to Dacron until the establishment of a monolayer. Cultured human adult ECs were radiolabeled, seeded onto Dacron, and adherence was quantified after vigorous washing. Firm adherence of 70% of the seeded ECs was seen by 2 hours to untreated Dacron, by 30 minutes to Dacron pretreated with a combination of interstitial type I/III collagen and an amnion-derived basement membrane (Type IV) collagen surface, and by 10 minutes to plasma-coated Dacron. Parallel samples were examined morphologically by scanning electron microscopy (SEM) to evaluate the adherence of ECs to surfaces. ECs seeded onto plain Dacron exhibited limited adherence, while cells on plasma-treated Dacron exhibited limited cell-cell associations. On basement membrane-treated Dacron, by 30 minutes the ECs exhibited a flat attenuated morphology, completely covering the graft surface. This time-frame is compatible with most vascular procedures, making an immediately endothelialized graft feasible.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker K. S., Williams S. K., Jarrell B. E., Koolpe E. A., Levine E. Endothelialization of human collagen surfaces with human adult endothelial cells. Am J Surg. 1985 Aug;150(2):197–200. doi: 10.1016/0002-9610(85)90118-7. [DOI] [PubMed] [Google Scholar]
- Belden T. A., Schmidt S. P., Falkow L. J., Sharp W. V. Endothelial cell seeding of small-diameter vascular grafts. Trans Am Soc Artif Intern Organs. 1982;28:173–177. [PubMed] [Google Scholar]
- Berger K., Sauvage L. R., Rao A. M., Wood S. J. Healing of arterial prostheses in man: its incompleteness. Ann Surg. 1972 Jan;175(1):118–127. doi: 10.1097/00000658-197201000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkel W. E., Ford J. W., Vinter D. W., Kahn R. H., Graham L. M., Stanley J. C. Fate of knitted dacron velour vascular grafts seeded with enzymatically derived autologous canine endothelium. Trans Am Soc Artif Intern Organs. 1982;28:178–184. [PubMed] [Google Scholar]
- Cranley J. J., Hafner C. D. Newer prosthetic material compared with autogenous saphenous vein for occlusive arterial disease of the lower extremity. Surgery. 1981 Jan;89(1):2–7. [PubMed] [Google Scholar]
- Dewey C. F., Jr, Bussolari S. R., Gimbrone M. A., Jr, Davies P. F. The dynamic response of vascular endothelial cells to fluid shear stress. J Biomech Eng. 1981 Aug;103(3):177–185. doi: 10.1115/1.3138276. [DOI] [PubMed] [Google Scholar]
- Edwards W. H., Mulherin J. L., Jr The role of graft material in femorotibial bypass grafts. Ann Surg. 1980 Jun;191(6):721–726. doi: 10.1097/00000658-198006000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskin S. G., Navarro L. T., O'Bannon W., DeBakey M. E. Behavior of endothelial cells cultured on Silastic and Dacron velour under flow conditions in vitro: implications for prelining vascular grafts with cells. Artif Organs. 1983 Feb;7(1):31–37. doi: 10.1111/j.1525-1594.1983.tb04156.x. [DOI] [PubMed] [Google Scholar]
- Graham L. M., Burkel W. E., Ford J. W., Vinter D. W., Kahn R. H., Stanley J. C. Expanded polytetrafluoroethylene vascular prostheses seeded with enzymatically derived and cultured canine endothelial cells. Surgery. 1982 May;91(5):550–559. [PubMed] [Google Scholar]
- Herring M., Baughman S., Glover J. Endothelium develops on seeded human arterial prosthesis: a brief clinical note. J Vasc Surg. 1985 Sep;2(5):727–730. [PubMed] [Google Scholar]
- Herring M., Gardner A., Glover J. A single-staged technique for seeding vascular grafts with autogenous endothelium. Surgery. 1978 Oct;84(4):498–504. [PubMed] [Google Scholar]
- Ives C. L., Eskin S. G., McIntire L. V., DeBakey M. E. The importance of cell origin and substrate in the kinetics of endothelial cell alignment in response to steady flow. Trans Am Soc Artif Intern Organs. 1983;29:269–274. [PubMed] [Google Scholar]
- Jarrell B., Levine E., Shapiro S., Williams S., Carabasi R. A., Mueller S., Thornton S. Human adult endothelial cell growth in culture. J Vasc Surg. 1984 Nov;1(6):757–764. doi: 10.1067/mva.1984.avs0010757. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Lee C. W., Morakis D. J. New method for preparing large surfaces of intact human basement membrane for tumor invasion studies. Cancer Lett. 1980 Dec;11(2):141–152. doi: 10.1016/0304-3835(80)90105-6. [DOI] [PubMed] [Google Scholar]
- Madri J. A., Williams S. K. Capillary endothelial cell cultures: phenotypic modulation by matrix components. J Cell Biol. 1983 Jul;97(1):153–165. doi: 10.1083/jcb.97.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols W. K., Gospodarowicz D., Kessler T. R., Olsen D. B. Increased adherence of vascular endothelial cells to Biomer precoated with extracellular matrix. Trans Am Soc Artif Intern Organs. 1981;27:208–212. [PubMed] [Google Scholar]
- Robison J. G., Brewster D. C., Abbott W. M., Darling R. C. Femoropopliteal and tibioperoneal artery reconstruction using human umbilical vein. Arch Surg. 1983 Sep;118(9):1039–1042. doi: 10.1001/archsurg.1983.01390090029006. [DOI] [PubMed] [Google Scholar]
- Sauvage L. R., Berger K. E., Wood S. J., Yates S. G., 2nd, Smith J. C., Mansfield P. B. Interspecies healing of porous arterial prostheses: observations, 1960 to 1974. Arch Surg. 1974 Nov;109(5):698–705. doi: 10.1001/archsurg.1974.01360050092020. [DOI] [PubMed] [Google Scholar]
- Schmidt S. P., Hunter T. J., Hirko M., Belden T. A., Evancho M. M., Sharp W. V., Donovan D. L. Small-diameter vascular prostheses: two designs of PTFE and endothelial cell-seeded and nonseeded Dacron. J Vasc Surg. 1985 Mar;2(2):292–297. [PubMed] [Google Scholar]
- Thornton S. C., Mueller S. N., Levine E. M. Human endothelial cells: use of heparin in cloning and long-term serial cultivation. Science. 1983 Nov 11;222(4624):623–625. doi: 10.1126/science.6635659. [DOI] [PubMed] [Google Scholar]
- Weisel R. D., Johnston K. W., Baird R. J., Drezner A. D., Oates T. K., Lipton I. H. Comparison of conduits for leg revascularization. Surgery. 1981 Jan;89(1):8–15. [PubMed] [Google Scholar]
- Williams S. K., Jarrell B. E., Friend L., Radomski J. S., Carabasi R. A., Koolpe E., Mueller S. N., Thornton S. C., Marinucci T., Levine E. Adult human endothelial cell compatibility with prosthetic graft material. J Surg Res. 1985 Jun;38(6):618–629. doi: 10.1016/0022-4804(85)90084-8. [DOI] [PubMed] [Google Scholar]






